Abstract
The synthetic double-stranded RNA poly(I:C) is commonly used as an adjuvant to boost CD8 T-cell function; however, polyinosinic:polycytidylic acid [poly(I:C)] can also suppress autoimmune disease. The mechanism by which a single adjuvant achieves two distinct immunoregulatory roles is unknown. Although it is clear that coadministration of poly(I:C) with antigen elicits strong adjuvant effects in mice, we found that poly(I:C) injection before antigen substantially reduced antigen-dependent CD8 T-cell responses. Notably, CD8 T cells sensitized in poly(I:C)-pretreated mice failed to fully up-regulate IL-33R (ST2), which led to impaired T-cell receptor-independent responses to IL-33. In contrast, nonsensitized effector CD8 T cells responded robustly to IL-33 using a two-signal cytokine mechanism. During an acute lung response to Staphylococcus aureus enterotoxin, peripheral injection of poly(I:C) manifested a suppressive process by inhibiting the differentiation of both antigen- and IL-33–responsive CD8 effectors systemically. These findings highlight that early exposure to double-stranded RNA reverses its role as an adjuvant and, importantly, prevents IL-33R up-regulation on CD8 effector T cells to dampen inflammation.
Keywords: superantigens, pathogen associated molecular patterns
Polyinosinic:polycytidylic acid [poly(I:C)] is a synthetic double-stranded RNA (dsRNA) that is widely used to mimic the biological effects of dsRNA viruses and viral replication products. Immune responses to poly(I:C) are mediated by several known dsRNA receptors, some of which reside intracellularly (MDA5, RIG-I, and LGP2) and others of which are found on the endosomal membrane (TLR3) (reviewed in ref. 1). Poly(I:C) activates TLR3 and MDA5, which trigger the production of proinflammatory cytokines and IFN responses (2, 3). The potent effects of the synthetic dsRNA poly(I:C) on CD8 T-cell activation is well appreciated and used for tumor eradication (4). Poly(I:C) induces dendritic cell maturation, and it facilitates antigen cross-priming through the TLR3 pathway (5). Furthermore, cytokine production in response to poly(I:C) plays a crucial role in priming the T-cell response. In this regard, multiple studies have demonstrated that induction of type I IFN is indispensable for the poly(I:C)-dependent augmentation of CD8 T-cell function (6–8).
The immune-enhancing aspects of TLR ligands, including poly(I:C), have been extensively investigated (reviewed in ref. 9); however, evidence suggests that TLR ligands can also block responses. Poly(I:C) was shown to inhibit autoimmune diseases (10, 11). Additionally, when given prophylactically, poly(I:C) can prevent autoimmune diabetes in mice and in rat strains that are genetically susceptible to the disease (12, 13). In retrospect, these results are fascinating considering that CD8 T cells are pathological mediators in autoimmune diabetes (14). In contrast, other reports using different models of diabetes found that poly(I:C) potentiates or exacerbates disease, consistent with poly(I:C)’s known adjuvant effects (15–18). On the basis of these reports, we reasoned that the seemingly contradictory roles of poly(I:C) during autoimmune diabetes development may be related to its differential influence on effector CD8 T cells.
We demonstrate that poly(I:C) could promote or suppress the T-cell receptor (TCR) response to a cognate antigen depending on the timing of poly(I:C) injection. Prophylactic poly(I:C) stimulation weakened CD8 T-cell expansion and effector differentiation, dampening sensitivity to antigen restimulation. The weakened effector CD8 T cells did not up-regulate the IL-33 receptor α (ST2) chain as did conventional effector CD8 T cells. Even though the IL-33 pathway has been historically associated with Th2 responses, we found that the effector CD8 T cells responded vigorously to IL-33, IL-2, or IL-12 by synthesizing prodigious IFNγ levels. The mechanism of this process involved a two-signal sequence that first required IL-12 followed by IL-33 for the induction of IFNγ synthesis. Finally, systemic injection of poly(I:C) manifested suppression in response to acute lung injury by limiting local IL-5 and the differentiation of antigen- and IL-33–responsive effector CD8 T cells in the periphery. These findings suggest that responses to poly(I:C) before onset of inflammatory-based diseases may be beneficial by preventing excessive T-cell activation and that one point of control is the IL-33 receptor pathway.
Results
Systemic Pretreatment with the dsRNA Poly(I:C) Suppresses Antigen-Specific CD8 T-Cell Responses.
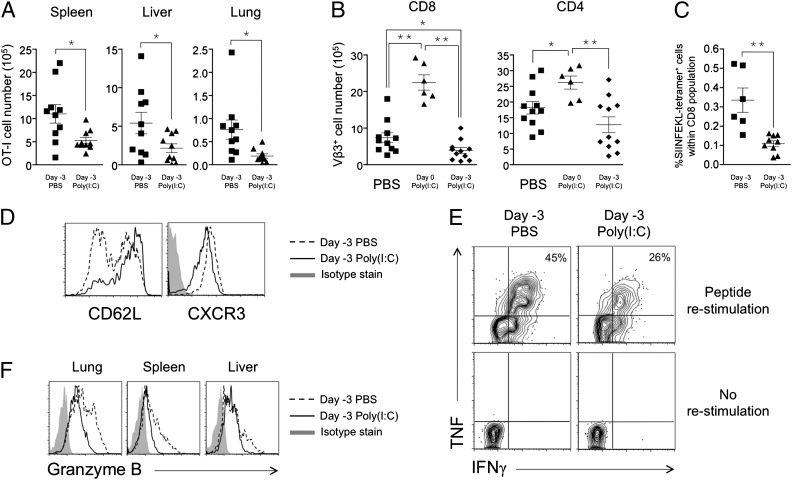
Poly(I:C) coadministration with antigen elicits potent adjuvant effects on CD8 T cells (7, 19). In contrast, we observed that poly(I:C) injected before antigen encounter [poly(I:C) 3 d before antigen immunization] can suppress CD8 T-cell expansion (Fig. 1 A–C). This effect was first observed using adoptively transferred CD8 T cells (OT-I cells) that can be traced by costaining the congenic marker CD45.1 and the TCR Vα2 chain (Fig. S1A). In the absence of adjuvant, the response of OT-I CD8 T cells toward their cognate antigen, SIINFEKL (an ovalbumin-derived, class I-restricted peptide), constituted ∼16% of the total splenic CD8 T-cell population on day 3 (Fig. S1A). Overall numbers of activated OT-I CD8 T cells decreased significantly in spleen, liver, and lung of poly(I:C)-pretreated recipients, indicating a systemic effect on CD8 T-cell expansion in both secondary and tertiary lymphoid organs (Fig. 1A). Phenotypic analysis revealed that the majority of the OT-I CD8 T cells primed in poly(I:C)-pretreated mice remained CD62L-high and had slightly lower CXCR3 expression compared with their counterparts in control-treated mice (Fig. 1D), indicating a less activated status for these cells. Specifically, poly(I:C) pretreatment resulted in only 36 ± 7% of CD62Llo OT-I CD8 T cells compared with 59 ± 6% in control-treated mice (P = 0.041, n > 6 from four independent experiments).
Fig. 1.
Systemic pretreatment with poly(I:C) suppresses CD8 T-cell clonal expansion and Ag-dependent effector responses. (A) C57BL/6 mice were injected with PBS or 1 μg poly(I:C) on day −3, with OT-I cells on day −1, and with 50 μg SIINFEKL peptide on day 0. The numbers of tissue OT-I cells were enumerated on day 3. (B) C57BL/6 mice were injected with PBS or 1 μg poly(I:C) on day −3 followed by 1 μg SEA on day 0 or SEA together with poly(I:C) on day 0. Splenic Vβ3+ cells were enumerated on day 3. (C) C57BL/6 mice were injected with 100 μg poly(I:C) or PBS on day −3 and immunized with 100 μg SIINFEKL peptide, 50 μg CpG, and 5 μg anti–4-1BB agonist antibody on day 0. Splenic SIINFEKL-tetramer+ cells were analyzed on day 6. (D) Phenotypes of splenic OT-I cells from A. (E) Splenocytes from A were restimulated with SIINFEKL peptide to determine the frequency of effectors. (F) Ex vivo granzyme B expression on OT-I cells in A. Data are representative of three to four experiments.
To test the robustness of this finding, we adapted this to a system that uses staphylococcal enterotoxin A (SEA) to activate endogenous T cells. In this system, the fate of antigen-specific CD8 or CD4 T cells is followed by staining for Vβ3+ cells (Fig. S1B). Poly(I:C) treatment before SEA immunization significantly decreased the number of CD8+Vβ3+ T cells compared with control-treated animals (Fig. 1B, Left) whereas coadministration of poly(I:C) with SEA on day 0 demonstrated its adjuvant effect (Fig. 1B, Left). A similar trend was observed with SEA-specific CD4 T cells (Fig. 1B, Right).
Suppression by poly(I:C) pretreatment was further demonstrated with endogenous ovalbumin-specific CD8 T cells. Endogenous CD8 T cells responding to SIINFEKL peptide coadministered with the adjuvant CpG and the agonist antibody targeting the 4–1BB costimulatory molecule can be detected by tetramer-staining on day 6 post immunization (Fig. S1C). Mice that were pretreated with poly(I:C) demonstrated significantly weaker CD8 T-cell expansion (Fig. 1C). Altogether, we showed that pre- exposure to poly(I:C) resulted in suboptimal expansion of both adoptively transferred (Fig. 1A) and endogenous (Fig. 1 B and C) CD8 T cells. This observation is true for CD8 T cells activated by antigen alone (Fig. 1A), with CD4 T-cell help (Fig. 1B), or in the presence of adjuvant with costimulation (Fig. 1C).
We next investigated if poly(I:C) pretreatment impacts differentiation of CD8 T cells into cytotoxic effectors. Effector differentiation (indicated by the frequency of IFNγ and TNF double producers within OT-I cells) was reduced by close to half in the poly(I:C)-pretreated hosts (Fig. 1E, Upper Right). Enumeration of IFNγ and TNF double producers in spleen and liver revealed that the total number of such CD8 effectors decreased by 4.4- and 6.5-fold, respectively (Fig. S2A). Granzyme B was substantially reduced in poly(I:C)-pretreated mice (Fig. 1F). Throughout spleen, liver, and lung, the mean fluorescence intensity of granzyme B staining decreased by 42 ± 6%. Importantly, OT-I CD8 T cells from poly(I:C)-pretreated mice displayed significantly less degranulation, as measured by CD107 expression when restimulated with peptide ex vivo compared with control-treated mice (Fig. S2B). Hence, poly(I:C) could suppress both the magnitude of T-cell expansion and the quality of the cytotoxic effectors formed.
Poly(I:C) Pretreatment Suppresses TCR-Independent Effector Function of Activated CD8 T cells by Impeding the IL-33 Pathway.
To uncover differences between CD8 T cells primed in control and poly(I:C)-pretreated mice, OT-I CD8 T cells were sorted from spleens and subjected to genechip analysis. Results from two separate experiments showed that ST2, which is the IL-33 receptor α-chain that binds IL-33 as its ligand (20), was not up-regulated on OT-I CD8 T cells from poly(I:C)-pretreated mice (Fig. S3). IL-33/ST2 signaling is known to drive Th2 responses (20), induce effector cytokine secretion from human mast cells and eosinophils (21, 22), and synergize with IL-18, IL-12, and IL-23 to induce IFNγ from NK cells (23) and is required for protective antiviral responses (24).
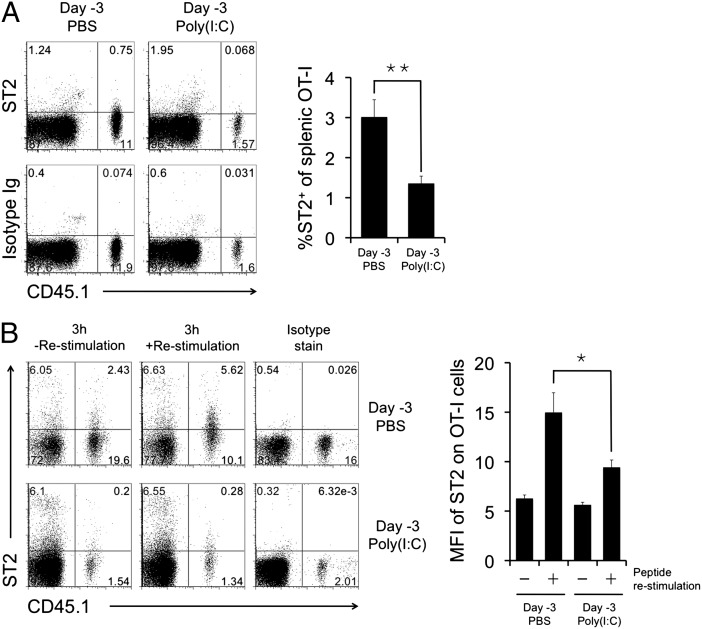
To validate our genechip data, surface ST2 staining was tested on activated CD8 T cells (Fig. 2A, Left). After in vivo immunization with SIINFEKL peptide, an average of 3% of day 3-activated OT-I CD8 T cells (CD45.1+) express surface ST2 (Fig. 2A, Right). Consistent with the genechip data, ST2 protein expression was significantly reduced when the OT-I CD8 T cells were primed in poly(I:C)-pretreated mice (Fig. 2A). Importantly, upon ex vivo restimulation with peptide antigen, day 3-activated OT-I CD8 T cells that were previously not ST2+ rapidly up-regulated ST2 (Fig. 2B). OT-I CD8 T cells primed in poly(I:C)-pretreated mice have much reduced capacity to express ST2 upon restimulation (Fig. 2B).
Fig. 2.
Poly(I:C) pretreatment inhibits IL-33 receptor (ST2) up-regulation on CD8 T cells during primary responses and after restimulation. C57BL/6 mice were injected with PBS or 50 μg poly(I:C) on day −3 and with OT-I cells on day −1 and immunized with SIINFEKL peptide on day 0. (A) Splenocytes were harvested on day 3, and ST2 expression was analyzed by gating on total CD8 T cells. (B) Splenocytes were restimulated ex vivo for 3 h, and ST2 expression on OT-I was analyzed by gating on total CD8 T cells. Results were obtained from three to four experiments.
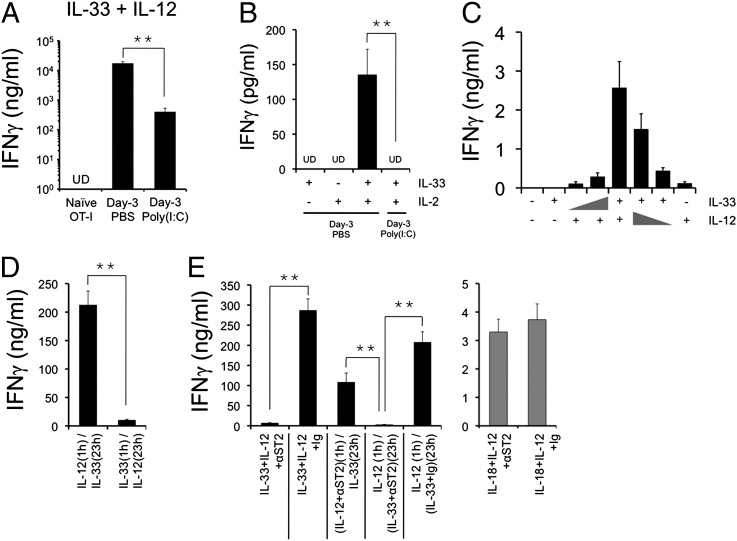
We reasoned that ST2 expression on activated CD8 T cells might enable these cells to respond to IL-33 stimulation. It was recently reported that in vitro-differentiated type I cytotoxic T cells (Tc1 cells) express ST2 and respond synergistically to IL-33 and IL-12 stimulation to produce IFNγ (25). Extending this observation to in vivo-activated CD8 T cells, we found that purified day 3 OT-I CD8 T cells responded to the combined action of IL-33 and IL-12 to produce prodigious levels of IFNγ (Fig. 3A). On the other hand, naive OT-I CD8 T cells did not respond to the combined action of these cytokines (Fig. 3A). In a different set of experiments, day 3-activated OT-I CD8 T cells were FACS-sorted to prevent inclusion of contaminating cells that might respond to IL-33 (Fig. S4A). We discovered that, in addition to IL-12, IL-33 also synergized with IL-2 to induce IFNγ from activated CD8 T cells (Fig. 3B). Under both conditions, OT-I CD8 T cells activated in poly(I:C)-pretreated mice responded poorly to IL-33 + IL-12 or IL-2 stimulation (Fig. 3 A and B), consistent with the down-regulation of ST2 on these T cells. To further validate that IFNγ was indeed produced by CD8 T cells, brefeldin A was added during the last 5 h of the 24-h ex vivo culture to trap the cytokine intracellularly (Fig. S4B). We observed that ∼2% of OT-I CD8 T cells are the source of IFNγ whereas TNF, another effector cytokine that is commonly induced alongside IFNγ during TCR restimulation, was not induced by IL-33 + IL-12 (Fig. S4B). As a negative control, IL-18 was tested, and we showed that IL-33 displayed no synergistic effects when cocultured with IL-18 (Fig. S4B). Therefore, IL-33 selectively cooperates with certain cytokines to trigger the TCR-independent effector function of CD8 T cells. Poly(I:C) pretreatment in the host impairs this aspect of T-cell effector differentiation by inhibiting ST2 expression on the activated CD8 T cells.
Fig. 3.
Poly(I:C) pretreatment impairs CD8 T-cell responses to IL-33. (A and B) C57BL/6 mice were injected with PBS or 50 μg poly(I:C) on day −3 and with OT-I cells on day −1 and immunized with SIINFEKL peptide on day 0. Lymph nodes were harvested on day 3, and OT-I cells were purified by magnetic beads (A) or FACS sorting (B) before a 24-h culture with different cytokines. (C) OT-I CD8 T cells were activated in vivo by SIINFEKL peptide and purified from lymph nodes 3 d post immunization. IFNγ release by OT-I CD8 T cells in response to log-fold titration of IL-33 and IL-12 was measured. (D and E) OT-I CD8 T cells were activated in vivo by SIINFEKL peptide, anti–4-1BB, and anti-OX40 agonist antibodies. OT-I cells were purified from lymph nodes 3 d post immunization and used for ex vivo culture. UD, undetected. Data shown were obtained from at least three independent experiments.
Mechanistic Analysis of IL-33 and IL-12 Synergy on IFNγ Production by CD8 T Cells.
We conducted a detailed analysis of the T-cell response to IL-33 and IL-12 stimulation. IFNγ production by in vivo-activated OT-I CD8 T cells was strictly dependent on the dosage of both IL-33 and IL-12 because reduction of either cytokine decreased IFNγ secretion (Fig. 3C). However, a log-fold reduction of IL-33 concentration appeared to have a more dramatic effect on decreasing IFNγ production compared with that of IL-12 (Fig. 3C). Next, to identify the order of action by each cytokine, 4–1BB and OX40-activated OT-I CD8 T cells (26) were first cultured with IL-12 followed by IL-33 or vice versa. We found that a 1-h incubation with IL-12 was sufficient to prime the CD8 T-cell response to IL-33 in the following 23 h (Fig. 3D). Reversing the order of these cytokines failed to induce IFNγ production, suggesting that the bulk of IFNγ production was triggered by IL-33 stimulation (Fig. 3D). Furthermore, the presence of a blocking antibody against ST2 diminished IFNγ production during OT-I CD8 T-cell culture with IL-33 + IL-12 (Fig. 3E, Left, first two columns), confirming that contact between IL-33 and its receptor is necessary for synergy. Specifically, the ST2 blockade achieved its effects during the IL-33 incubation time in an assay of 1 h for IL-12 followed by 23 h for IL-33 (Fig. 3E, Left, last three columns). Neither the addition of an isotype antibody nor the use of the anti-ST2 antibody with a different cytokine combination (IL-12 + IL-18) affected IFNγ production (Fig. 3E, Right), confirming the specificity of the blockade. Thus, much like a T-cell costimulatory signal, IL-33 requires a prior signaling event in CD8 T cells to manifest its proinflammatory function.
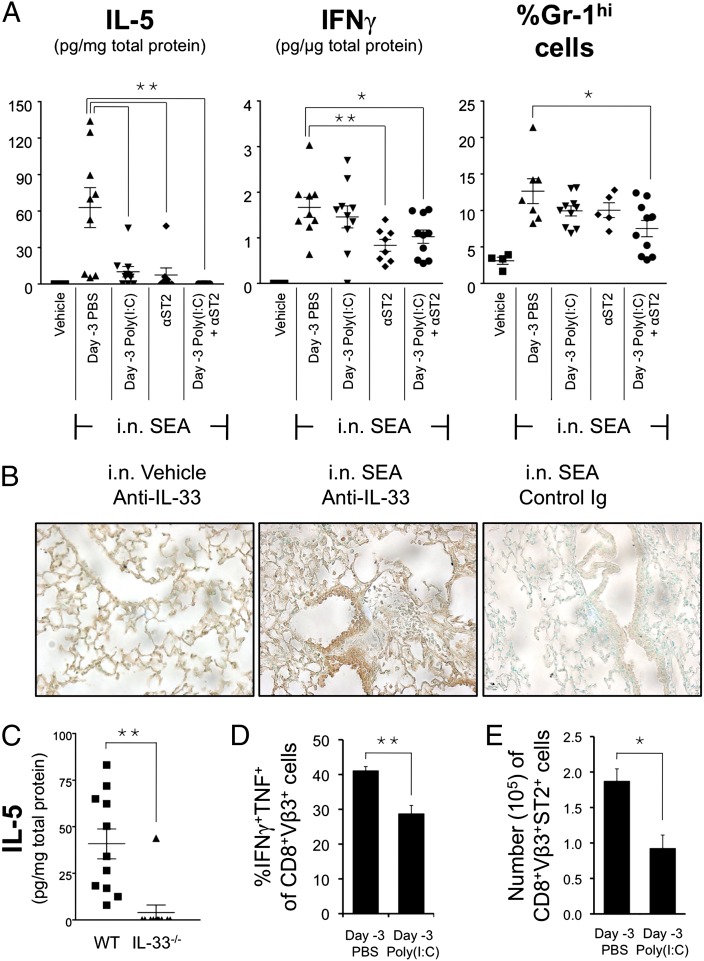
Poly(I:C) Pretreatment and Blocking the ST2 Pathway Reduces Local and Systemic Immune Effectors During Acute Lung Injury.
Colonization of Staphylococcus aureus in nasal polyps and their production of enterotoxins are associated with allergic rhinitis and chronic sinusitis (27). Our laboratory showed that intranasal (i.n.) SEA results in acute lung injury that depends on the activation of CD8 T cells and IFNγ, and importantly, this lung injury model also presents systemic effects (28). We tested if systemic pretreatment with poly(I:C) could mitigate the local and systemic responses to i.n. SEA challenge.
Two of the major cytokines found in bronchial-alveolar lavage (BAL) fluid at 2 d post i.n. SEA challenge were IL-5 and IFNγ (Fig. 4A and Fig. S5). On the basis of our ex vivo data (Figs. 1 and 3), the source of IFNγ was likely to be SEA-triggered T cells, through either TCR activation or IL-33 stimulation. On the contrary, the source of IL-5 is less clear. It is unlikely to be from Th2 cells because SEA elicits mainly Th1 responses (29). However, we observed IL-4, IL-5, IL-13, and IL-10 in the BAL fluid after i.n. SEA (Fig. S5), indicating that this route of SEA inoculation may induce Th2-type responses. Other airway allergy models indicate that IL-5 could be induced by IL-33 following antigen challenge (30). We performed immunohistochemical staining of IL-33 on lung tissue 2 d after i.n. SEA and found that, indeed, IL-33 was expressed (Fig. 4B). IL-33 may be responsible for IL-5 production in our model because IL-5 was not detected in IL-33−/− mice after i.n. SEA challenge (Fig. 4C). The presence of IL-33 and IL-5 could exacerbate airway inflammation by recruiting eosinophils and other innate immune cells (31, 32). These and our previous reports suggest that considerable lung damage might be caused by both effector cytokines IL-5 and IFNγ. We showed here that either poly(I:C) pretreatment or blocking ST2 was sufficient to inhibit IL-5 production, but the administration of both proved most effective (Fig. 4A, Left). Next, whereas poly(I:C) pretreatment did not significantly reduce IFNγ secretion into the BAL fluid, ST2 blocking was able to do so (Fig. 4A, Center). Interestingly, when examining the influx of leukocytes into the BAL fluid, we observed that poly(I:C) and anti-ST2 antibody worked additively to reduce the presence of Gr-1hi cells (Fig. 4A, Right). Importantly, mice pretreated with poly(I:C) showed reduced systemic accumulation of effector T cells (Fig. 4 D and E), suggesting that it could potentially limit bystander damage in the periphery caused by a septic response originating in the lung. Overall, these data indicate that poly(I:C), especially when aided by blocking the ST2 pathway, can reduce local and systemic toxicity.
Fig. 4.
The effects of poly(I:C) pretreatment and ST2 blockade on local and systemic responses during i.n. S. aureus enterotoxin-induced lung injury. C57BL/6 mice were i.p. injected with PBS or 200 μg poly(I:C) on day –3 followed by i.n. inoculation with 1 μg SEA on day 0. Responses were analyzed 2 d post SEA challenge. To block ST2 signaling, two doses of 500 μg anti-ST2 antibody were i.p. injected at −24 h and +6 h, respectively, in relation to SEA challenge. (A) Cytokines in the BAL fluid and influx of Gr-1hi cells. (B) Immunohistochemical staining of IL-33 in paraffin-embedded lung sections (20× magnification). (C) BAL fluid IL-5 level in C57BL/6 versus IL-33−/− mice. (D) Splenocytes were restimulated with SEA ex vivo, and the frequency of effector CD8 T cells was analyzed. (E) Enumeration of ST2+ effector cells in the spleens. Data shown were obtained from at least three independent experiments.
Discussion
Like many other TLR ligands, poly(I:C) has routinely been used as an adjuvant, but others (33, 34) and this study (Fig. 1) have shown that prior exposure to poly(I:C) can inhibit CD8 T-cell responses. Two distinct mechanisms were put forth to explain these findings: First, naive CD8 T cells that were exposed to poly(I:C)-induced type I IFNs became refractory to Ag stimulation later (33), and, second, TLR ligands can inhibit antigen cross presentation (34), thereby impairing CD8 T-cell priming. In addition, the Welsh group showed that exposure to poly(I:C) affects CD8 effector differentiation and impacts the memory T cell pool (35, 36). These studies highlight the danger of constant exposure to TLR ligands, in particular during chronic infection, coinfection, or sepsis, in mounting a robust CD8 T-cell response when the host encounters viral infection. However, a recent report using a panel of TLR ligands to suppress asthma and autoimmune diabetes suggested that microbial stimulation (typically with systemic administration of TLR ligands) could prevent allergies and autoimmunity, providing a plausible explanation for the hygiene hypothesis (37). Hence, TLR ligands can have dual immuno-modulating properties, and the suppressive nature of them could be damaging (impairing antiviral responses) or beneficial (preventing immune disorders). Historical data demonstrating the dual immuno-modulating properties of poly(I:C) include a classic model of graft-versus-host disease (38, 39) and autoimmune diabetes (13, 18). Collectively, these results suggest that poly(I:C), and perhaps many other TLR ligands, may be able to both promote and dampen an immune response based on the timing, the magnitude, or the type of inflammation induced. Consequently, T-cell priming is affected. Our results show that one potential mechanism by which poly(I:C) dampens the immune response may be by restricting CD8 T-cell responses to IL-33.
It is known that IFNγ transcription in CD8 T effector cells can be activated by TCR stimulation (CD3 + CD28) and proinflammatory cytokines (IL-18 + IL-12 or IL-33 + IL-12) (25, 40). Although synthesis of IFNγ after TCR triggering tends to be rapid (within 5 h), that from the IL-33/ST2 pathway requires more time (>20 h) (Fig. 1E and Fig. S4B). It will be interesting to determine if these are the same subset of effector cells or different subpopulations. We found that the IL-33–dependent effector function of CD8 T cells was down-modulated by poly(I:C) pretreatment (Fig. 3A). This finding is consistent with the observation that CD8 T cells expressed less ST2 after poly(I:C) pretreatment (Fig. 2A). Because both IL-33 and IL-18 are members of the IL-1 cytokine family and IL-33 is phylogenetically related to IL-18 (20), it is possible that the synergy between IL-33 and IL-12 uses the same intracellular pathway as IL-18 and IL-12 to induce IFNγ production. Indeed, the signaling protein GADD45β, which is responsible for IL-18 and IL-12 synergy on CD4 T cells (41), is involved in IL-33 and IL-12 synergy as well (25). Further analysis using the IL-33R blockade showed that IL-33 + IL-12 synergy was achieved in a sequential manner, with IL-12 acting ahead of IL-33 (Fig. 3D). Hence, during secondary TCR-independent stimulation of effector CD8 T cells, IL-12 acted as signal 1 and IL-33 as signal 2 for the induction of IFNγ synthesis. This sequence-dependent synergy on IFNγ release uncovers an aspect of the regulation of T-cell biology that awaits further investigation. Specifically, a thorough understanding of IL-12–triggered intracellular events within effector CD8 T cells is instrumental in deciphering their response to IL-33.
Altogether, we are beginning to recognize that once CD8 T cells are activated, their effector function is dictated not only by the availability of antigen, but also very much by the cytokine milieu. In addition to the above-mentioned inflammatory cytokines, which are often produced by innate immune cells, we further demonstrated that a T-cell–derived cytokine IL-2 is also capable of synergizing with IL-33 to stimulate CD8 T cells (Fig. 3B). Similarly, this synergistic effect is impaired for T cells activated in poly(I:C)-pretreated mice (Fig. 3B). These results suggested that, in a physiological setting, previously activated CD8 T cells may be stimulated nonspecifically by multiple cytokines during inflammation or secondary infections. Consequently, T cells may mediate tissue pathology when IL-33 is present, even when cognate antigen is absent. However, recent data demonstrated that, during primary antiviral responses, IL-33 mediates beneficial effects that support CD8 effector responses (24). In the future, it will be important to examine the physiological impact of the CD8 T-cell response to IL-33 in different immunological settings to fully appreciate the benefits or damage related to this pathway.
Our findings showing that the biology of effector CD8 T cells can be fundamentally altered by a TLR ligand adds a perspective to the interpretation of the hygiene hypothesis. In particular, it contributes to the understanding of why CD8 T-cell–mediated autoimmune disease such as diabetes can be prevented by TLR ligands before disease onset in experimental animals (12). On the other hand, our ST2 expression data on CD8 T cells (Fig. 2) and their responses to IL-33, IL-12, and IL-2 (Fig. 3) pinpointed a potential role of these cells in exacerbating tissue damage during proinflammatory responses where such cytokines are abundant. For example, IL-33 is associated with exacerbation of asthma, a widely studied airway inflammatory disorder (42). Extrapolating from our results with CD8 T cells, it would be interesting to test if pre-exposure to TLR ligands could dampen ST2 expression on other inflammatory cells (such as Th2 cells, eosinophils, and mast cells) and, by extension, their IL-33–mediated effector function during airway inflammation.
The superantigen-mediated acute lung injury model that we used in this study shares some common features with other inflammatory airway diseases. SEA induces IL-33 expression in the lung and IL-5 production in the BAL fluid (Fig. 4 A–C), and both cytokines are linked to pulmonary disease models (30, 31). In addition, we also observed increased Gr-1+ cells in the BAL fluid, an event that associates with augmentation of airway allergy and recruitment of T helper cells to the airways (43). Systemic pre-exposure to poly(I:C) or ST2 blockade suppressed IL-5 levels in BAL fluid (Fig. 4A, Left), and the combination of both inhibited the accumulation of Gr1hi cells in BAL (Fig. 4A, Right).
On the other hand, the pulmonary response to SEA is unique because effector T cells activated after insult in the lung could spread systemically. It may be argued that these effector T cells can instigate damage elsewhere in the body because they are now able to respond to the inflammatory cytokines IL-33 and IL-12. Here we observed that the effector responses outside the lung were dampened in the poly(I:C)-pretreated mice (Fig. 4 D and E), demonstrating a systemic immunoregulatory effect of poly(I:C) during an ongoing mucosal immune response.
In conclusion, we uncovered an unappreciated role of poly(I:C) and, on reflection, of double-stranded RNA, in the immune system. The suppressive effects presented here are what we would term an “exjuvant” effect of poly(I:C). Unlike its well-studied adjuvant effects, we showed that pretreatment with poly(I:C) could be exploited to thwart rather than to promote not only TCR-related effector T-cell responses, but also, and importantly, the newly discovered IL-33/ST2 responses that are widely implicated in mucosal inflammatory diseases. Thus, exploiting the timing of TLR ligand exposure is likely to provide much-needed clinical benefit for human diseases.
Materials and Methods
General sources of reagents, description of spleen, liver, lung, BAL fluid processing, flow cytometry, and immunohistochemical analysis can be found in SI Materials and Methods. IL-33−/− mice were produced at Amgen using standard methodology and provided by D.E.S., whereas the wild-type controls were purchased from Taconic Farms Inc. The ST2-blocking monoclonal antibody and the rat IgG1 isotype control (anti-KLH, monoclonal) were also provided by D.E.S. (44). The immunization procedures are described in SI Materials and Methods and include a description of in vivo stimulation of transferred OT-I cells and stimulation of endogenous T cells using SEA and SIINFEKL. The IL-33 ex vivo culture system, statistical analysis, and costimulation procedure are also described in the SI Materials and Methods.
Supplementary Material
Acknowledgments
Due to space constraints, we regret that we could not cite the important contributions by others. This work was supported by National Institutes of Health Grants R01 CA109339 (to A.J.A. and A.T.V.) and P01 AI056172 Project 3 (to A.T.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202607109/-/DCSupplemental.
References
- 1.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 4.Currie AJ, et al. Targeting the effector site with IFN-alphabeta-inducing TLR ligands reactivates tumor-resident CD8 T cell responses to eradicate established solid tumors. J Immunol. 2008;180:1535–1544. doi: 10.4049/jimmunol.180.3.1535. [DOI] [PubMed] [Google Scholar]
- 5.Schulz O, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 6.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngoi SM, Tovey MG, Vella AT. Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta. J Immunol. 2008;181:7670–7680. doi: 10.4049/jimmunol.181.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 10.Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;177:7505–7509. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- 11.Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. 2007;178:2204–2211. doi: 10.4049/jimmunol.178.4.2204. [DOI] [PubMed] [Google Scholar]
- 12.Serreze DV, Hamaguchi K, Leiter EH. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989;2:759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 13.Sobel DO, et al. Low dose poly I:C prevents diabetes in the diabetes prone BB rat. J Autoimmun. 1998;11:343–352. doi: 10.1006/jaut.1998.0203. [DOI] [PubMed] [Google Scholar]
- 14.Pinkse GG, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devendra D, et al. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- 16.Ewel CH, Sobel DO, Zeligs BJ, Bellanti JA. Poly I:C accelerates development of diabetes mellitus in diabetes-prone BB rat. Diabetes. 1992;41:1016–1021. doi: 10.2337/diab.41.8.1016. [DOI] [PubMed] [Google Scholar]
- 17.Moriyama H, et al. Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci USA. 2002;99:5539–5544. doi: 10.1073/pnas.082120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobel DO, et al. Poly I:C induces development of diabetes mellitus in BB rat. Diabetes. 1992;41:515–520. doi: 10.2337/diab.41.4.515. [DOI] [PubMed] [Google Scholar]
- 19.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: Evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Iikura M, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 22.Suzukawa M, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 23.Smithgall MD, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 24.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 27.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Curr Allergy Asthma Rep. 2002;2:252–258. doi: 10.1007/s11882-002-0027-9. [DOI] [PubMed] [Google Scholar]
- 28.Muralimohan G, Rossi RJ, Guernsey LA, Thrall RS, Vella AT. Inhalation of Staphylococcus aureus enterotoxin A induces IFN-gamma and CD8 T cell-dependent airway and interstitial lung pathology in mice. J Immunol. 2008;181:3698–3705. doi: 10.4049/jimmunol.181.5.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arad G, Hillman D, Levy R, Kaempfer R. Superantigen antagonist blocks Th1 cytokine gene induction and lethal shock. J Leukoc Biol. 2001;69:921–927. [PubMed] [Google Scholar]
- 30.Kurowska-Stolarska M, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 31.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, et al. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–185. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Marshall HD, Urban SL, Welsh RM. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J Virol. 2011;85:5929–5939. doi: 10.1128/JVI.02516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson NS, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 35.Bahl K, Huebner A, Davis RJ, Welsh RM. Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to Toll-like receptor agonists. J Virol. 2010;84(10):4866–4877. doi: 10.1128/JVI.02571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185(3):1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aumeunier A, et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE. 2010;5:e11484. doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peres A, Amlani S, Kornbluth M, Seemayer TA, Lapp WS. The effects of polyinosinic:polycytidylic acid on the graft-versus-host reaction. III: Increased severity of the reaction with delayed pI:C treatment. Transplantation. 1989;48:80–84. [PubMed] [Google Scholar]
- 39.Peres A, Seemayer TA, Lapp WS. The effects of polyinosinic: polycytidylic acid (pI:C) on the GVH reaction: Immunopathological observations. Clin Immunol Immunopathol. 1986;39:102–111. doi: 10.1016/0090-1229(86)90209-6. [DOI] [PubMed] [Google Scholar]
- 40.Balasubramani A, et al. Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli. Immunity. 2010;33:35–47. doi: 10.1016/j.immuni.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat Immunol. 2001;2:157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 42.Borish L, Steinke JW. Interleukin-33 in asthma: How big of a role does it play? Curr Allergy Asthma Rep. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung YW, Zindl CL, Lai JF, Weaver CT, Chaplin DD. MMP induced by Gr-1+ cells are crucial for recruitment of Th cells into the airways. Eur J Immunol. 2009;39:2281–2292. doi: 10.1002/eji.200838985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer G, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.