Abstract
The plant hormone abscisic acid (ABA) is produced in response to abiotic stresses and mediates stomatal closure in response to drought via recently identified ABA receptors (pyrabactin resistance/regulatory component of ABA receptor; PYR/RCAR). SLAC1 encodes a central guard cell S-type anion channel that mediates ABA-induced stomatal closure. Coexpression of the calcium-dependent protein kinase 21 (CPK21), CPK23, or the Open Stomata 1 kinase (OST1) activates SLAC1 anion currents. However, reconstitution of ABA activation of any plant ion channel has not yet been attained. Whether the known core ABA signaling components are sufficient for ABA activation of SLAC1 anion channels or whether additional components are required remains unknown. The Ca2+-dependent protein kinase CPK6 is known to function in vivo in ABA-induced stomatal closure. Here we show that CPK6 robustly activates SLAC1-mediated currents and phosphorylates the SLAC1 N terminus. A phosphorylation site (S59) in SLAC1, crucial for CPK6 activation, was identified. The group A PP2Cs ABI1, ABI2, and PP2CA down-regulated CPK6-mediated SLAC1 activity in oocytes. Unexpectedly, ABI1 directly dephosphorylated the N terminus of SLAC1, indicating an alternate branched early ABA signaling core in which ABI1 targets SLAC1 directly (down-regulation). Furthermore, here we have successfully reconstituted ABA-induced activation of SLAC1 channels in oocytes using the ABA receptor pyrabactin resistant 1 (PYR1) and PP2C phosphatases with two alternate signaling cores including either CPK6 or OST1. Point mutations in ABI1 disrupting PYR1–ABI1 interaction abolished ABA signal transduction. Moreover, by addition of CPK6, a functional ABA signal transduction core from ABA receptors to ion channel activation was reconstituted without a SnRK2 kinase.
Keywords: Arabidopsis, chloride channel
The perception of the phytohormone abscisic acid (ABA) is achieved by the recently discovered 14-member START protein family of ABA receptors named pyrabactin resistance (PYR), or regulatory component of ABA receptor (RCAR) (1, 2). PYR/RCARs have been shown to bind to clade A PP2Cs and inhibit the activity of these PP2Cs in the presence of ABA (1–5). Structural studies show that PYR1, PYL1, and PYL2 function as ABA receptors, with ABA binding in a protein cavity that locks down the ABA molecule (6–10).
ABA reduces transpirational water loss of plants by inducing stomatal closure (11). ABA can cause an increase in guard cell intracellular Ca2+ concentration (12–17), which leads to the down-regulation of inward-rectifying K+ channels and activation of both slow-sustained (S-type) and rapid-transient (R-type) anion channels (18–20). Previous findings have led to the model that S-type anion channels play a key role in controlling stomatal closure (18, 21, 22). slac1 mutant plants have greatly reduced S-type anion channel activity (23) and display impaired stomatal closure in response to ABA, elevated CO2, ozone, reactive oxygen species, calcium, and reduced humidity, underlining that SLAC1 represents a key component functioning downstream of these signals and is crucial for stomatal closure (23, 24).
The subclass III SnRK2 kinase OST1 (Open Stomata 1), also known as SnRK2.6, is required for ABA- and CO2-induced stomatal closure (25–27). Several members of the PP2C family have been shown to directly interact with (3, 28, 29) and deactivate subclass III SnRK2 protein kinases (SnRK2.2, SnRK2.3, and SnRK2.6/OST1) through dephosphorylation in vitro (28–30).
S-type anion channels in guard cells are activated by phosphorylation events (31, 32). Xenopus oocyte electrophysiology experiments demonstrated that SLAC1 in the presence of the protein kinases OST1, calcium-dependent protein kinase 23 (CPK23), and, to a lesser extent, CPK21 can generate S-type anion channel activity (33–35). An S120A mutation in SLAC1 prevents channel activation by OST1, but CPK23 is still able to induce SLAC1 S120A-mediated currents in Xenopus oocytes (33, 34). SLAC1-mediated anion currents are down-regulated by the PP2C-type phosphatases ABI1, ABI2 (33, 34), and PP2CA (35). However, whether ABA activation of anion channels can be reconstituted in Xenopus oocytes with these core signaling components remains unknown (33–36).
The role and function of CPKs in the regulation of SLAC1 are still ambiguous. In vivo, CPK6 functions as a positive regulator of ABA control of S-type anion channels in guard cells (37). In cpk3cpk6 double knockout mutant Arabidopsis plants, ABA- and Ca2+-induced stomatal closure, as well as activation of S-type anion channel activity, was impaired in vivo, with CPK6 playing a more prominent role than CPK3 (37). CPK21 and CPK23 activate SLAC1 in Xenopus laevis oocytes (34), but cpk21 and cpk23 mutants show enhanced drought and salt tolerance (38, 39) rather than drought sensitivity. Furthermore, CPK6 was reported to weakly interact with SLAC1 in Xenopus oocytes (34). Here we functionally reconstitute activation of SLAC1 channels by ABA with either CPK6 or OST1 in the ABA signaling core and identify mechanisms of this signaling pathway. Interestingly, these findings further show that an alternate early ABA signaling core can be functionally reconstituted for ABA activation of a plant ion channel without inclusion of a SnRK2 kinase. Furthermore, in addition to the known dephosphorylation of OST1 by the ABI1 PP2C, we demonstrate that ABI1 directly dephosphorylates the N terminus of SLAC1, enabling tight control of anion channel activity.
Results
CPK6 Activates SLAC1 in Xenopus Oocytes and Phosphorylates the N Terminus of SLAC1.
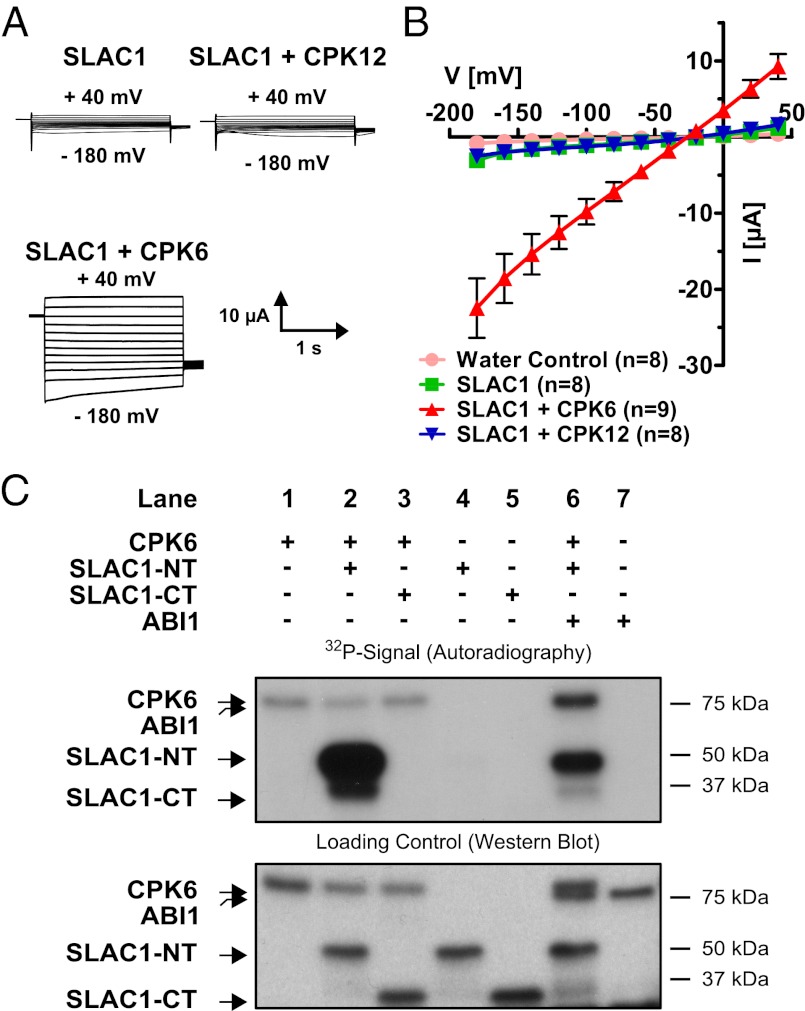
Two-electrode voltage-clamp experiments in X. laevis oocytes were performed to analyze SLAC1 regulation. When SLAC1 cRNA was injected into oocytes alone, average inward currents were similar to those of water-injected control oocytes (Fig. 1 A and B). We investigated whether CPK6, which functions in S-type anion channel activation in vivo (37), can activate SLAC1 channels. Coexpression of SLAC1 and individual protein kinases, including CPK6 and CPK23, significantly activated SLAC1-dependent ion currents compared with those of SLAC1 alone (Fig. 1 A and B and Fig. S1A; P = 0.001 and P = 0.04 for CPK6 and CPK23, respectively; Mann–Whitney U test at −180 mV). It was also confirmed that CPK6 was able to activate SLAC1 consistently throughout six of the tested batches of oocytes (P < 0.001; two-way ANOVA). Reversal potentials of SLAC1 and kinase-activated ion currents were close to the equilibrium potential for Cl−, consistent with the anion selectivity of S-type anion channels. Robust CPK6 activation of SLAC1 channels (Fig. 1 A and B and Fig. S1A) did not require experimentally imposed linkage of SLAC1 to the protein kinase using split yellow fluorescent protein (YFP-BiFC) fusions, as shown for OST1 (33). The calcium dependent protein kinases closely related to CPK6 and CPK23 [CPK12 and CPK31, respectively (40)] were not able to stimulate SLAC1 activity (Fig. 1 A and B and Fig. S1A; CPK12, P = 0.305; Student’s t test; CPK31, P = 0.234; Mann–Whitney U test at −180 mV). Coexpressing the kinase-inactive variant of CPK6 (D209A) together with SLAC1 did not activate SLAC1 (Fig. S1B), indicating a kinase activity-dependent event. Comparison of SLAC1 activity in the presence of the tested kinases suggests that SLAC1 activations by CPK6 and CPK23 are statistically comparable (Fig. S1A; P = 0.561; Mann–Whitney U test at −180 mV), whereas CPK3 and CPK31 showed no significant activation of SLAC1 (Fig. S1A; P = 0.363 and P = 0.321 for CPK3 and CPK31, respectively; Mann–Whitney U test at −180 mV).
Fig. 1.
Strong activation of SLAC1 channels by the CPK6 protein kinase in Xenopus oocytes and in vitro. (A) Whole-cell voltage-clamp recordings for oocytes expressing SLAC1, in the presence or absence of CPKs, show that coexpression of SLAC1 with CPK6 results in large Cl− currents, as opposed to SLAC1 with CPK12. (B) The average steady-state current–voltage relationships are shown in water-injected (pink circles), SLAC1-injected (green squares), and SLAC1 + CPK-injected oocytes. The protein kinase CPK6 (red triangles) stimulates SLAC1 activity, whereas CPK12 (blue triangles), a homolog of CPK6, did not increase SLAC1 activity in oocytes. Representative data from one batch of oocytes are shown (of >3 batches tested). Error bars represent SEM. (C) Autoradiograph of in vitro kinase assays using recombinant proteins (Upper) and Western blot analysis detecting GST as loading control for the individual proteins (Lower). The kinase CPK6 (lane 1) is able to transphosphorylate the N terminus of SLAC1 (lane 2; SLAC1-NT) but not the C terminus (lane 3; SLAC1-CT). No signal could be detected upon incubation of SLAC1-NT (lane 4) or SLAC1-CT alone (lane 5). Coincubation of CPK6 with ABI1 decreased SLAC1-NT transphosphorylation (lane 6). Assaying ABI1 alone did not result in a 32P signal (lane 7).
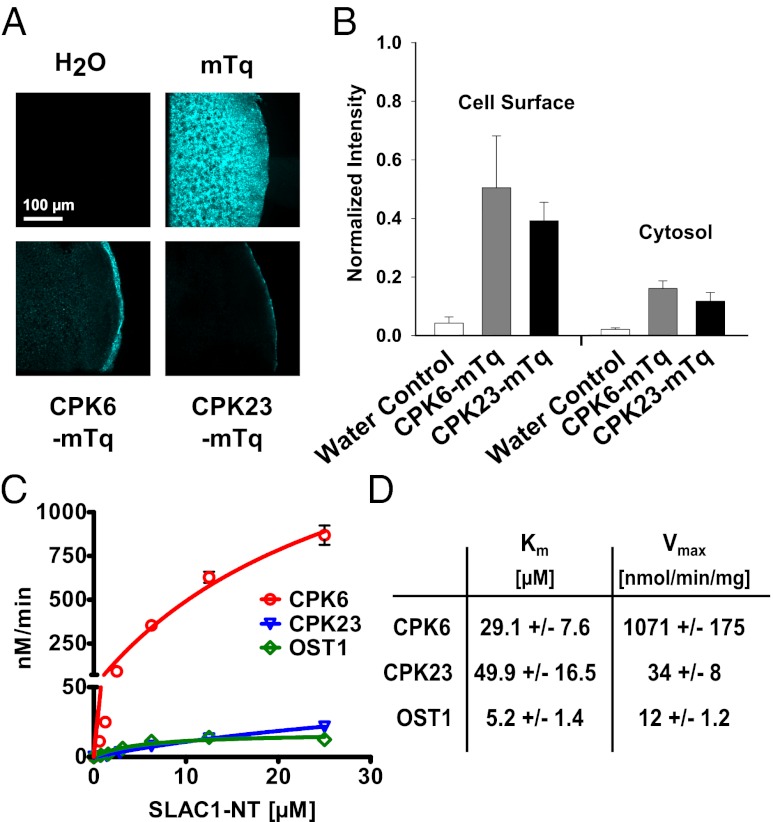
Localizations and abundances of CPK6 and CPK23 were analyzed in Xenopus oocytes. Confocal laser microscopy of cryomicrodissected CPK fusion protein (mTurquoise; mTq)-expressing Xenopus oocytes revealed that a significant proportion of the CPK6 and CPK23 proteins are located at the cell surface, with an additional weak fluorescence distributed throughout the cytoplasm (Fig. 2 A and B). Quantification of the fluorescence intensities showed that CPK6-mTq and CPK23-mTq abundance did not significantly differ (Fig. 2B; cell surface, P = 0.474; cytosol, P = 0.344; Student’s t test). To estimate a saturation point of CPK6 and CPK23-dependent SLAC1 activation, defined amounts of cRNA encoding the protein kinases CPK6 and CPK23 were injected into oocytes: a standard [CPK6/23] cRNA amount (∼25 ng; [1.0]), an intermediate cRNA amount (∼12.5 ng CPK6/23; [0.5]), or one-tenth cRNA amount (∼2.5 ng CPK6/23; [0.1]). For these experiments, oocytes were coinjected with 25 ng SLAC1 cRNA. Halving the injected cRNA amount resulted in no significant change in SLAC1 channel activity (Fig. S2; P > 0.685 at −180 mV; Student’s t test). However, injecting one-tenth of the amount of cRNA resulted in a strong reduction of SLAC1-mediated anion currents (Fig. S2; P < 0.01 at −180 mV; Mann–Whitney U test), suggesting that the injected CPK6/23 cRNA amount at 25 ng was near the saturation point when 25 ng SLAC1 cRNA was coinjected.
Fig. 2.
CPK6 is expressed at the cell surface and high in vitro CPK6 activity toward SLAC1-NT phosphorylation. (A) Kinases CPK6 and CPK23 are localized at the cell surface, whereas mTurquoise (mTq) alone is uniformly spread throughout oocytes. (B) Analysis of fluorescence intensity (normalized to mTq) highlights the strong cell-surface localization of both CPK6 and CPK23 (data points represent mean ± SE; n > 3). (C) P81 filter-based protein kinase assays show kinetics of SLAC1-NT phosphorylation by CPK6 (red circles), CPK23 (blue triangles), and OST1 (green diamonds). Data points represent mean values ± SE (n = 3). (D) Km values are given in μM SLAC1-NT, and maximum specific activities are in nmol PO4 incorporated⋅min−1⋅mg−1 kinase.
In vitro protein kinase assays were performed to assess whether CPK6 can phosphorylate cytosolic domains of the SLAC1 channel. Wild-type recombinant CPK6 protein showed weak autophosphorylation (Fig. 1C, Upper, lane 1). The N terminus of SLAC1 (SLAC1-NT; amino acids 1–186), but not the C terminus (SLAC1-CT; amino acids 497–556), was strongly phosphorylated by CPK6 (Fig. 1C, Upper, lanes 2 and 3). The SLAC1 termini incubated alone did not show any signal (Fig. 1C, Upper, lanes 4 and 5). The strong CPK6-mediated SLAC1-NT phosphorylation was dependent on the presence of free Ca2+ in the reaction buffer (Fig. S3).
To quantify the degree of CPK6-mediated SLAC1 phosphorylation activity, P81-grade filter-based kinetic in vitro phosphorylation assays were performed (Fig. 2 C and D). The Km and Vmax values of the kinase substrate pairs reveal that CPK6 very efficiently and, to a lower extent, CPK23 and OST1, phosphorylates the SLAC1-NT in vitro (Fig. 2 C and D).
Identification and Characterization of Sites Phosphorylated by CPK6.
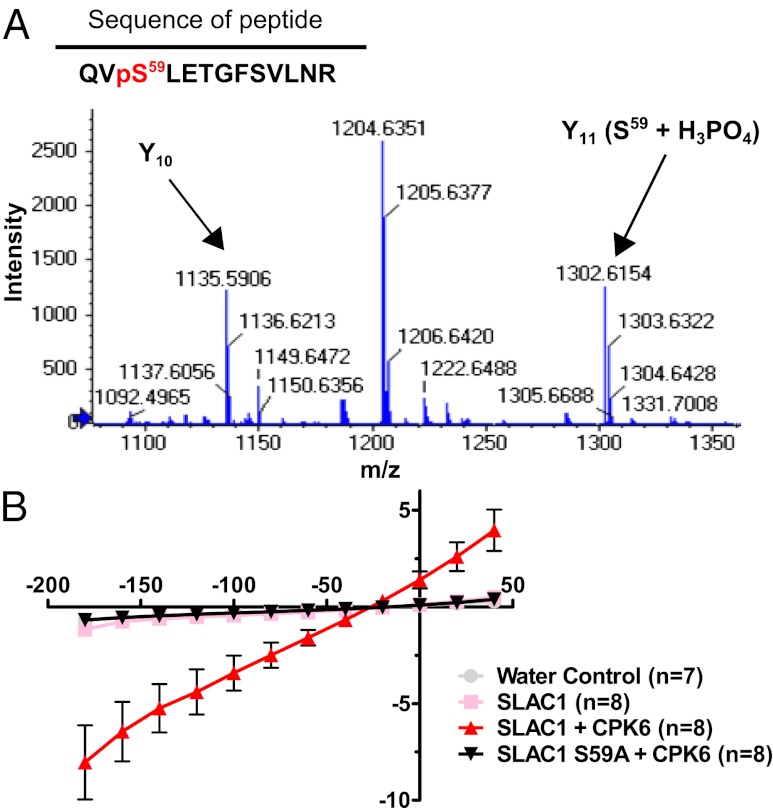
Due to activation of SLAC1-mediated ion currents by CPK6 (Figs. 1 and 3) and the in vivo function of CPK6 in guard cells (37), we focused on deciphering the properties of this regulation. To date, the only known amino acid that is crucial for SLAC1 activation by a protein kinase is serine 120 (33, 34, 41). To determine which residues may be relevant, trypsin-digested SLAC1-NT, phosphorylated by CPK6 in the presence of nonradioactive ATP, was analyzed by high-pressure liquid chromatography (HPLC) coupled to tandem mass spectroscopy (LC-MS/MS) using nanospray ionization. A SLAC1-NT phosphorylation site was identified at the amino acid serine 59 (Fig. 3A). Consequently, this amino acid was replaced by either alanine (which cannot be phosphorylated; SLAC1 S59A) or aspartate (putative phosphomimetic; SLAC1 S59D), and CPK6 activation of SLAC1 was analyzed in oocytes. Voltage-clamp recordings showed that SLAC1 S59A was not activated by CPK6 (Fig. 3B). SLAC1 S59D did not exhibit constitutive activation of anion current activity compared with wild-type SLAC1 (Fig. S4B), indicating that either this mutant is not a functional phosphomimetic or that additional phosphorylation sites are required. These findings indicate that serine 59 represents a crucial amino acid for CPK6-mediated activation of SLAC1.
Fig. 3.
Serine 59 of SLAC1 is crucial for CPK6-mediated activation of the anion channel. (A) Zoomed-in spectrum section of product ion scan for the peptide QVpSLETGFSVLNR [(M + 2H)+ = 765.4]. The fragment ion (Y11) corresponding to phosphorylated serine (S59) is labeled in the figure (Y11 (S59 + H3PO4)). The Y10 fragment is labeled to demonstrate that T62 and S65 are not phosphorylated for this ion. The complete spectrum and statistical parameters are presented in Fig. S4A and Table S1. (B) Average steady-state current–voltage relationships show that the point mutation of serine 59 to alanine (black triangles) abolishes anion channel activity due to CPK6 (red triangles). Representative data from one batch of oocytes are shown (of two batches tested).
Down-Regulation of CPK6-Activated SLAC1 Channels by Group A PP2Cs.
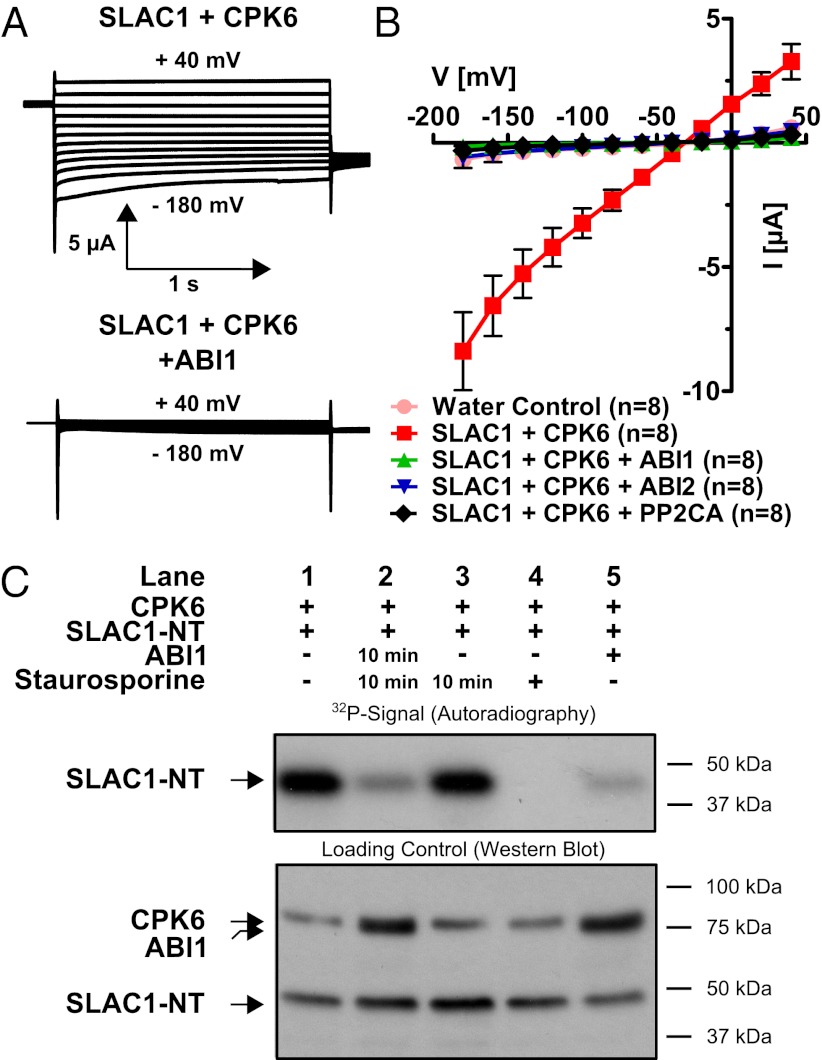
We tested several PP2Cs that function in ABA signaling for their ability to inhibit CPK6-mediated SLAC1 activation. Experiments showed that the PP2C protein phosphatases ABI1 (42, 43), ABI2, and PP2CA (44–46) were able to almost completely down-regulate CPK6-activated anion currents in oocytes (Fig. 4 A and B).
Fig. 4.
Strong inhibition of CPK6-mediated SLAC1 activation by PP2C protein phosphatases and direct SLAC1 N terminus dephosphorylation by ABI1. (A) Whole-cell current recordings for oocytes show that protein phosphatase ABI1 strongly inactivated CPK6-induced SLAC1 ion channel current. (B) Current–voltage relationships in oocytes containing SLAC1 and CPK6 demonstrate that the PP2Cs ABI1 (green triangles), ABI2 (blue triangles), and PP2CA (black diamonds) are capable of strongly inhibiting CPK6-mediated SLAC1 activity (red squares). Data from one representative batch of oocytes (of >3 batches) are shown. (C) (Upper) 32P phosphorylation of the N terminus of SLAC1. (Lower) Loading controls of the individual proteins CPK6, ABI1, and SLAC1. After initial CPK6 exposure, the strong phosphorylation state of SLAC1-NT phosphorylated by CPK6 (Upper, lane 1) decreases if subsequently coincubated with ABI1 and staurosporine 10 min after CPK6 exposure (lane 2). However, phosphorylation of SLAC1-NT by preexposure to CPK6 is not reduced if the protein kinase inhibitor staurosporine alone is added 10 min after CPK6 exposure (lane 3). If staurosporine is added simultaneously with CPK6, SLAC1-NT transphosphorylation is strongly inhibited (lane 4). The ABI1 protein phosphatase added simultaneously with CPK6 inhibits phosphorylation of SLAC1-NT (lane 5).
In in vitro kinase assays, the phosphorylation of the SLAC1-NT by CPK6 (Fig. 1C, Upper, lane 2) was decreased when recombinant ABI1 protein was included in the reaction (Fig. 1C, Upper, lane 6 and Fig. 4C, Upper, lane 5). Data further indicate that ABI1 might also be phosphorylated by CPK6 (Fig. 1C, Upper, lane 6). ABI1 alone did not produce any signal on the autoradiogram (Fig. 1C, lane 7).
Experiments were pursued to address whether ABI1 is able to dephosphorylate amino acid residues in the N terminus of SLAC1 after phosphorylation by CPK6. The kinase inhibitor staurosporine strongly inhibited CPK6 transphosphorylation activity (Fig. 4C, Upper, lane 4). To probe the ability of the PP2C ABI1 to dephosphorylate SLAC1 directly, the SLAC1-NT was first phosphorylated by CPK6 (Fig. 4C, Upper, lane 1). Ten minutes later, either staurosporine alone (Fig. 4C, lane 3, 10 min) or staurosporine and recombinant ABI1 protein (Fig. 4C, lane 2, 10 min) were added, and the reaction was incubated for an additional 35 min. Ten-minute delayed addition of staurosporine alone did not change the phosphorylation state of the SLAC1-NT (Fig. 4C, Upper, lane 3), but coincubation with ABI1 strongly decreased the prior CPK6-mediated phosphorylation (Fig. 4C, Upper, lane 2). Thus, CPK6 phosphorylates the N terminus of SLAC1. Unexpectedly, ABI1 reduces this phosphorylation by directly dephosphorylating the SLAC1-NT, which differs from previously reported OST1–ABI1 interactions in which ABI1 did not directly dephosphorylate SLAC1 after OST1-mediated phosphorylation (33). This points to a mechanism for ABA-induced SLAC1 regulation (Discussion).
Can ABA Regulate CPK6- or OST1-Dependent SLAC1 Anion Channel Activity in Oocytes?
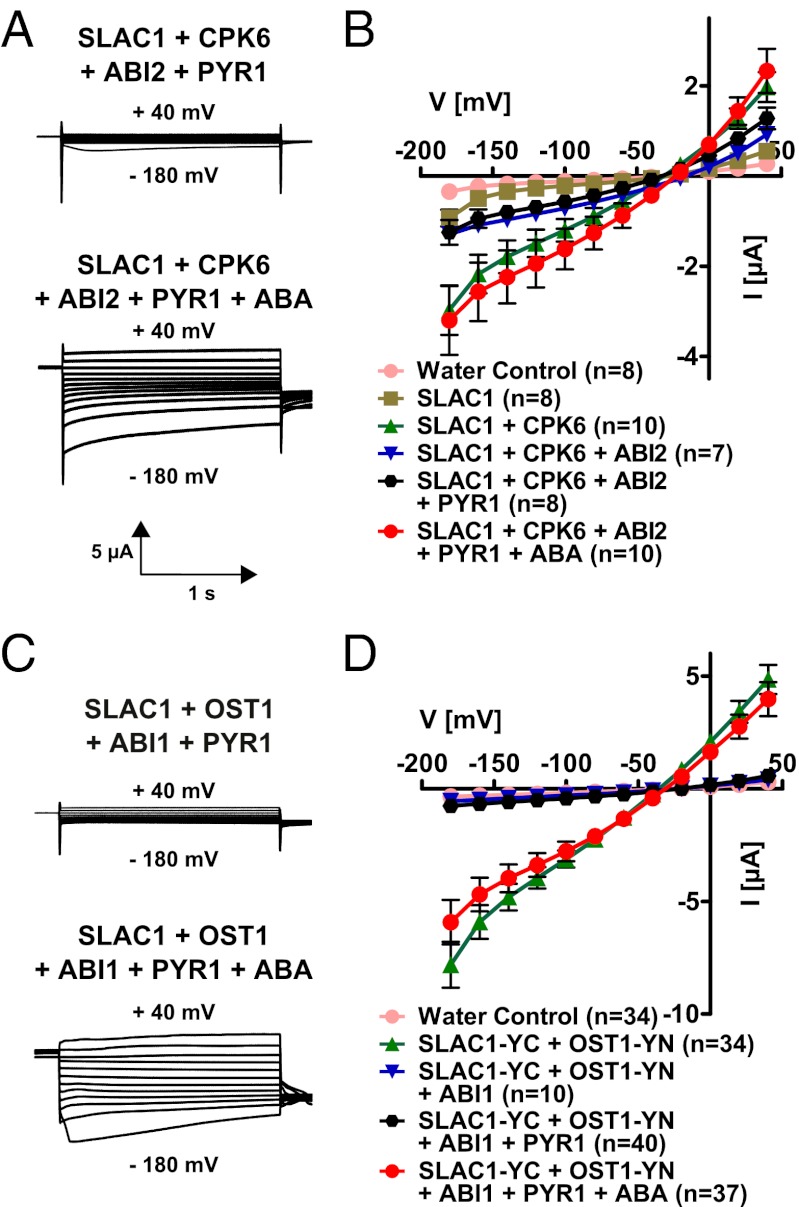
In previous studies, reconstitution of ABA activation of SLAC1 channels has not been studied (33–36). To assess whether ABA activation of SLAC1 can be reconstituted, we coinjected cRNAs of SLAC1, ABI1/ABI2, the PYR1 ABA receptor, and either CPK6 or OST1 into oocytes. In the absence of ABA, PYR1 did not affect the small magnitude of anion currents in oocytes expressing the PP2Cs ABI2 or ABI1 with SLAC1 and CPK6 (Fig. 5B) or OST1 (Fig. 5D). Extracellular ABA application did not result in SLAC1 activation (n > 6). When ABA was injected into the cytoplasm of oocytes 15 min before the recording of oocyte currents, anion channel activity was dramatically enhanced in oocytes containing SLAC1, ABI1/ABI2, PYR1, and either CPK6 or OST1 (Fig. 5). Note that to enhance the activation of SLAC1 via OST1 (Fig. 5D), split YFP (BiFC) constructs were attached to the anion channel and kinase (33) to irreversibly link these proteins. In contrast, ABA activation of SLAC1 channels via CPK6 did not require imposed linkage of the expressed signaling proteins (Fig. 5 A and B). Therefore, the analyzed ABA signaling pathway components were sufficient to reconstitute ABA activation of SLAC1 currents with either the CPK6 or OST1-Yn protein kinases. Thus, a functional ABA signaling core was reconstituted with CPK6, enabling ABA activation of SLAC1 without a SnRK2 kinase (Fig. 5 A and B).
Fig. 5.
Functional reconstitution of ABA activation of SLAC1 channels in (A and B) CPK6- and (C and D) OST1-containing signaling pathways. Current traces (A and C) show examples of the ABA activation of SLAC1 channel currents in oocytes. An ABA-dependent signaling pathway was reconstituted in oocytes using the protein kinases CPK6 (A and B) and OST1 (C and D). Split YFP (BiFC) was used in the case of SLAC1 and OST1 coexpression (C and D). The PP2C phosphatase ABI2 (A and B) or ABI1 (C and D) is able to inhibit SLAC1 currents (blue triangles in B and D). In the absence of ABA, the ABA receptor PYR1 does not activate SLAC1 currents (black hexagons in B and D). However, in the presence of injected ABA, SLAC1 currents are strongly activated (red circles in B and D). Data from one representative batch of oocytes (of >3 batches) are shown in D. Data from two to five independent batches were averaged in B.
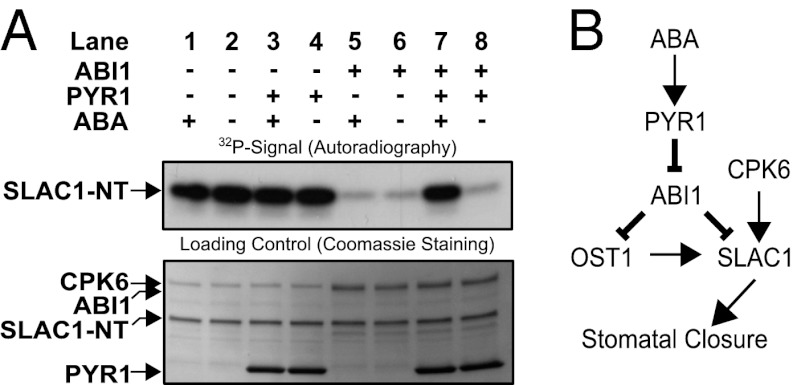
We attempted to reconstitute the ABA signaling core including PYR1, ABI1, CPK6, and SLAC1-NT in vitro. Inhibition of CPK6-mediated SLAC1-NT phosphorylation by ABI1 (Fig. 6A, Upper, lanes 5, 6, and 8) is decreased if the ABA receptor PYR1 and ABA are present (Fig. 6A, Upper, lane 7). Addition of PYR1 or ABA alone did not alter CPK6- and ABI1-dependent SLAC1-NT phosphorylation (Fig. 6A, Upper, lanes 1, 4, 5, and 8).
Fig. 6.
(A) In vitro reconstitution of an ABA-dependent signal transduction pathway including PYR1, ABI1, CPK6, and SLAC1-NT protein. Independent of ABA, CPK6 phosphorylates SLAC1-NT (lanes 1 and 2). The presence of ABI1, but not PYR1, inhibits SLAC1-NT phosphorylation, with or without ABA (lanes 3–6). If CPK6, ABI1, and PYR1 are present, addition of ABA leads to the release of ABI1-mediated inhibition of SLAC1 phosphorylation (lane 7). (B) Model for ABA activation of the SLAC1 channel via the OST1 and CPK6 protein kinases and down-regulation by the ABI1 protein phosphatase. ABA binds to PYR1, which causes inhibition of PP2C protein phosphatases, including ABI1. This leads to ABA-induced activation of SLAC1 channels in Xenopus oocytes by CPK6, which functions in native guard cells in ABA activation of S-type anion channels (37). Note that the ABI1 protein phosphatase can directly dephosphorylate the SLAC1 N terminus, which represents a previously unknown target for ABI1 and a mechanism for tight negative SLAC1 regulation, in addition to the known down-regulation of OST1.
The tryptophan 300 residue of ABI1 was identified as being crucial for stabilizing the PYL1–ABA–ABI1 complex due to interaction of this residue with the narrow hydrophobic ABA-binding pocket (7, 10). When the ABA-dependent signaling pathway containing the ABI1 W300L mutant was expressed in oocytes, ABI1 W300L was able to inhibit SLAC1 ion currents similarly to wild-type ABI1 (Fig. S5). However, SLAC1 anion currents could not be activated upon injection of ABA when coexpressing PYR1, OST1, and ABI1 W300L (Fig. S5).
Discussion
In the present study, we show that the calcium-dependent protein kinase CPK6 activates SLAC1-dependent anion currents in Xenopus oocytes (Figs. 1, 3, and 4). In vitro kinase assays demonstrate that CPK6 phosphorylates the SLAC1 N terminus (Fig. 1C), as previously observed for CPK21, CPK23, and OST1 (33–35, 41). Here, LC-MS/MS experiments revealed a phosphorylation site in SLAC1 (S59) that is crucial for SLAC1 channel activation by CPK6 in Xenopus oocytes (Fig. 3). This SLAC1 phosphorylation may lead to conformational changes in the channel, resulting in rearrangement of the phenylalanine residue 450, which is crucial for the gating of SLAC1 (47). Kinetic protein kinase quantification analyses reveal a high catalytic efficiency of CPK6 toward SLAC1-NT phosphorylation (Fig. 2 C and D), which is comparable to other plant kinases (48, 49).
A 1:10 cRNA ratio of channel versus activating kinase injection led to only very weak SLAC1 activation (Fig. S2 A and B), which appears to be puzzling, as protein kinases are potentially able to phosphorylate and thereby activate multiple channels. Potential reasons could include (i) direct CPK6-protein linkage to SLAC1, (ii) a lower expression level of the kinase compared with SLAC1, or (iii) that endogenous oocyte phosphatases might be able to remove the phosphate groups added by the kinases and thereby inhibit kinase-mediated SLAC1 activation until a certain threshold is reached. The effect of CPK3 expression on SLAC1 activity in the oocytes was statistically insignificant (Fig. S1A). Recent studies have shown differential effects of CPK3 and CPK6 on stomatal closing (50, 51). Consistent with these findings, CPK3 and CPK6 are not in the same CPK subfamily (37, 40). The CPK6 activation of SLAC1 in oocytes reported in this work and reduced ABA- and Ca2+-induced S-type anion channel activation in guard cell protoplasts (37) are in line with the recently reported drought-resistant phenotype of CPK6-overexpressing Arabidopsis plants (52).
We have further observed strong inhibition of CPK6-activated SLAC1 channel activity in the presence of the three group A PP2Cs ABI1, ABI2, and PP2CA (Fig. 4 A and B). SnRK2 kinases have been shown to be directly regulated by PP2C phosphatases through dephosphorylation of the kinase-activation loop (28–30). Phosphorylation experiments at the N terminus of a SLAC1 homolog (SLAH3) by CPK21 suggested that ABI1 inhibits kinase activity, but does not dephosphorylate the N terminus of SLAH3 (36). Interestingly, the present findings provide biochemical evidence suggesting that the ABI1 protein phosphatase can directly dephosphorylate SLAC1 (Figs. 4C and 6B). Consequently, our results with CPK6 indicate an alternate ABA signaling pathway that is distinct from the linear PYR/RCAR–PP2C–SnRK2 (OST1)–SLAC1 such that, in addition to SnRK2 down-regulation, the PP2C ABI1 directly competes with CPK6 for (de)phosphorylation of the SLAC1 N terminus, which can add a mechanism for the tight regulation of S-type anion channels found in guard cells (20, 21) (Figs. 4C and 6B). The question of whether ABI1 additionally inhibits CPK6 activity will require further investigation.
We further demonstrate functional reconstitution of ABA activation of SLAC1 ion channel activity using either the CPK6 or OST1 protein kinase coexpressed with ABI1, ABI2, and PYR1 in Xenopus oocytes (Fig. 5). We show that ABA activation requires intracellular injection of ABA. Also, CPK6 mediates SLAC1-NT phosphorylation in vitro in response to ABA in the presence of PYR1 and ABI1 (Fig. 6A). An earlier study reported reconstitution of an ABA-dependent signaling pathway consisting of PYR/RCARs, group A PP2Cs, SnRK2 kinases, and the transcription factor ABF2 using a protoplast system with a luciferase reporter (53). Interestingly, the reconstituted ABA signal transduction pathway found here expands the present model for ABA signaling cores, by showing that the calcium-dependent protein kinase CPK6 can mediate an ABA-activated ion channel response in the absence of a SnRK2 protein kinase.
Conclusions
Our findings demonstrate the complete reconstitution of ABA activation of a plant ion channel, SLAC1, in a heterologous system including CPK6. Whereas strong activation of SLAC1 via the OST1 protein kinase required induced interaction of the OST1–SLAC1 pair via split YFP fusion, CPK6 strongly activates SLAC1 channels without using additional components that enhance protein–protein interactions. A regulatory phosphorylation site in CPK6 activation of SLAC1, S59, is identified (Fig. 3). The present study expands the current model for early ABA signaling mechanisms in which ABA binds to ABA receptors, including PYR1, leading to inhibition of PP2Cs and subsequent SnRK2 kinase activation. We show here that CPKs can replace SnRK2 kinases in ABA regulation of SLAC1 channels, suggesting that both of these protein kinase families function in parallel in vivo (Fig. 6B). These data further correlate with findings showing calcium dependence of ABA-induced stomatal closure (12–15, 17, 20) and parallel Ca2+-independent mechanisms (20, 54). Moreover, data show that ABI1 directly dephosphorylates the N terminus of SLAC1, indicating a branched configuration: ABI1 deactivates SLAC1 by directly interacting with and down-regulating SnRK2s (28–30) and dephosphorylating the SLAC1 N terminus (Figs. 4C and 6B).
Materials and Methods
Construct Preparation, Electrophysiology, and Histology in X. laevis Oocytes.
All constructs were cloned into the pNB1 oocyte expression vector using the USER method (55). cRNA was prepared using the mMessage mMachine Transcription Kit (Ambion). Approximately 25 ng of each tested cRNA, in a total volume of 50 nL, was injected into each oocyte for voltage-clamp recordings, if not otherwise stated. mTq fusion proteins were visualized in fixed sections using confocal laser microscopy. For information on instrument setup, solutions used, and analyses, see SI Materials and Methods.
Site-Directed Mutagenesis, Protein Expression, Isolation, in Vitro Kinase Assays, and Western Blot Analyses.
CPK6, OST1, ABI1, PYR1, and SLAC1 N and C terminus coding sequences were cloned into modified pGEX-6P1 (GE Healthcare) vectors using the USER method (55), and the proteins were overexpressed in Escherichia coli Rosetta (DE3) pLysS (Novagen) and isolated using Strep-Tactin MacroPrep (IBA) (Fig. S6). In vitro kinase assays were performed as previously described (34). To assess phosphorylation kinetics, P81-grade filter paper (Whatman) and a scintillation counter were used. More detailed information for all methods is given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Rainer Waadt and Cawas Engineer for critical reading of the manuscript and Dr. Malik Keshwani for instruction on kinase kinetics assays. This research was supported by National Institutes of Health R01GM060396 and National Science Foundation MCB0918220; in part by grants from the Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences at the US Department of Energy (DOE; DE-FG02-03ER15449, to J.I.S.), a German Academic Exchange Service doctoral fellowship (to B.B.), and in part by Grant-in-Aid for Scientific Research on Innovative Areas 21114002 from the Ministry of Education, Science and Culture of Japan and by the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry (K.I.). A.B.S. is a Department of Energy-Biosciences Fellow of the Life Sciences Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116590109/-/DCSupplemental.
References
- 1.Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 2.Park S-Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura N, et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago J, et al. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009;60:575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- 5.Szostkiewicz I, et al. Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J. 2010;61(1):25–35. doi: 10.1111/j.1365-313X.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 6.Melcher K, et al. A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature. 2009;462:602–608. doi: 10.1038/nature08613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazono K-i, et al. Structural basis of abscisic acid signalling. Nature. 2009;462:609–614. doi: 10.1038/nature08583. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura N, et al. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science. 2009;326:1373–1379. doi: 10.1126/science.1181829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santiago J, et al. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature. 2009;462:665–668. doi: 10.1038/nature08591. [DOI] [PubMed] [Google Scholar]
- 10.Yin P, et al. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat Struct Mol Biol. 2009;16:1230–1236. doi: 10.1038/nsmb.1730. [DOI] [PubMed] [Google Scholar]
- 11.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 12.Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSilva DLR, Cox RC, Hetherington AM, Mansfield TA. Suggested involvement of calcium and calmodulin in the responses of stomata to abscisic acid. New Phytol. 1985;101:555–563. [Google Scholar]
- 14.Grabov A, Blatt MR. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacRobbie EAC. ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+(Rb+) release. Proc Natl Acad Sci USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature. 1990;343(6254):186–188. [Google Scholar]
- 17.Schwartz A. Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiol. 1985;79:1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- 19.Hedrich R, Busch H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RS, et al. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. Plant J. 2009;59:207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder JI, Schmidt C, Sheaffer J. Identification of high-affinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard cells. Plant Cell. 1993;5:1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahisalu T, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negi J, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 25.Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue S, et al. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011;30:1645–1658. doi: 10.1038/emboj.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida R, et al. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- 28.Umezawa T, et al. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlad F, et al. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell. 2009;21:3170–3184. doi: 10.1105/tpc.109.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belin C, et al. Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol. 2006;141:1316–1327. doi: 10.1104/pp.106.079327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JX, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Schelle I, Liao YJ, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiger D, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger D, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA. 2009;106:21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger D, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal. 2011;4:ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- 37.Mori IC, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franz S, et al. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant. 2011;4(1):83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- 39.Ma S-Y, Wu W-H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol. 2007;65:511–518. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- 40.Hrabak EM, et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003;132:666–680. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahisalu T, et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010;62:442–453. doi: 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- 42.Leung J, et al. Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- 43.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol. 2006;140(1):127–139. doi: 10.1104/pp.105.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheen J. Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA. 1998;95:975–980. doi: 10.1073/pnas.95.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida T, et al. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006;140(1):115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YH, et al. Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature. 2010;467:1074–1080. doi: 10.1038/nature09487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curran A, et al. Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci. 2011;2:36. doi: 10.3389/fpls.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto K, et al. Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J Biol Chem. 2012;287:7956–7968. doi: 10.1074/jbc.M111.279331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cousson A. Arabidopsis Ca2+-dependent protein kinase CPK3 mediates relationship of putative inositol triphosphate receptor with slow-type anion channel. Biol Plant. 2011;55:507–521. [Google Scholar]
- 51.Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J, et al. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta. 2010;231:1251–1260. doi: 10.1007/s00425-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 53.Fujii H, et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nour-Eldin HH, Hansen BG, Nørholm MHH, Jensen JK, Halkier BA. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 2006;34:e122. doi: 10.1093/nar/gkl635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.