Abstract
Patched (Ptc), the main receptor for Sonic Hedghog, is a tumor suppressor. Ptc has been shown to be a dependence receptor, and as such triggers apoptosis in the absence of its ligand. This apoptosis induction occurs through the recruitment by the Ptc intracellular domain of a caspase-activating complex, which includes the adaptor proteins DRAL and TUCAN, and the apical caspase-9. We show here that this caspase-activating complex also includes the E3 ubiquitin ligase NEDD4. We demonstrate that Ptc-mediated apoptosis and Ptc-induced caspase-9 activation require NEDD4. We show that Ptc, but not Bax, the prototypical inducer of the intrinsic cell-death pathway, triggers polyubiquitination of caspase-9. Moreover, a caspase-9 mutant that could not be ubiquitinated failed to mediate Ptc-induced apoptosis. Taken together, these data support the view that the Ptc dependence receptor specifically allows the activation of caspase-9 via its ubiquitination, which occurs via the recruitment by Ptc of NEDD4.
Keywords: posttranslational modification, protease, signalisation
Apoptosis is a central mechanism during embryonic development and adult tissue maintenance. Its dysregulation is causally implicated in numerous pathologies, such as cancer, neurodegenerative disorders, and autoimmune diseases (1). In higher organisms, apoptosis typically occurs through the activation of caspases, cysteinyl aspartate proteases that are the central executioners of the cell-death program (2, 3). Because caspases display the property to cleave and activate other caspases in an amplification loop, apoptosis induction is often seen as the result of the initial activation of the so-called apical caspases: for example, caspase-8, -10, or -9 (4).
Such apical caspase activation appears to occur in specific and dedicated caspase-activating complexes (5). Although the death-inducing signaling complex (DISC) allows the recruitment and activation of caspase-8 by death receptors, the apoptosome complex allows the activation of caspase-9 (5–7). Caspase-9 activation typically occurs via the apoptosome, the composition and mechanism of action of which have been elucidated (6, 7). However, caspase-9 has also been shown to be activated directly by a specific type of receptor, called “dependence receptors” (8, 9).
Dependence receptors display dual signaling that is dependent on ligand availability: in the presence of their trophic ligands, they transduce various signals, whereas in the absence of their ligands, they are not inactive but rather actively trigger apoptosis (10–12). We recently showed that the dependence-receptor Patched (Ptc) triggers apoptosis in the absence of its ligand Sonic Hedgehog (SHH) through the formation of a caspase-activating complex; that is, the dependosome, which includes down-regulated in rhadmyosarcoma LIM domain protein (DRAL), tumor up-regulated CARD-containing antagonist of caspase-9 (TUCAN), and caspase-9 (9). We investigated here the mechanism by which this Ptc-complex triggers caspase-9 activation and we observed the recruitment of Nedd4 in this complex.
Nedd4 plays a major role in protein ubiquitination, which usually requires coordinated action of a ubiquitin (Ub)-activating enzyme (E1), a Ub-conjugating enzyme (E2), and a Ub ligase (E3), such as Nedd4. Ubiquitination was initially described to play a major role in protein degradation by providing a signal for proteasome-mediated degradation. However, it has been shown recently that ubiquitination is important not only for protein degradation but also for the regulation of both prosurvival and proapoptotic signals (13–15). Considering recent data describing the ubiquitination of caspase-8 as a mechanism for caspase-8 activation (16), we investigated whether caspase-9 activation in the Ptc-complex is associated with caspase-9 ubiquitination. We show here that although caspase-9 is not ubiquitinated upon activation of the intrinsic pathway for apoptosis, caspase-9 is ubiquitinated in the Ptc complex upon SHH withdrawal and we present evidence that caspase-9 ubiquitination in the Ptc complex is required for Ptc-mediated apoptosis.
Results and Discussion
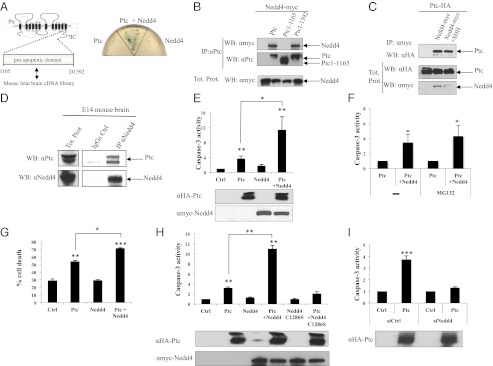
Following a two-hybrid screen, using as bait the proapoptotic domain of Ptc [Ptc 1165–1392, shown to be required for apoptosis and to interact with caspase-9 (9, 17)], Nedd4, an E3 ubiquitin ligase, was identified as a putative partner of Ptc (Fig. 1A). Nedd4 was previously shown to interact with Ptc in Drosophila (18). Nedd4 was further demonstrated to interact with Ptc by coimmunoprecipitation in HEK293T cells (Fig. 1B). This interaction appeared specific because, whereas full-length Ptc and Ptc 1–1392 clearly interacted with Nedd4, Ptc deletion of the seventh intracellular domain (Ptc 1–1165) failed to pull down Nedd4 (Fig. 1B). The Ptc/Nedd4 interaction was not affected by SHH presence, suggesting that Ptc constitutively interacts with Nedd4 (Fig. 1C). Nedd4/Ptc interaction was not only seen with ectopically expressed protein, as immunoprecipitation with an antibody raised against Nedd4 successfully pulls down Ptc in MiaPaca cells where endogenous Ptc and Nedd4 can be detected (Fig. S1A) and in brain extract from E14 mouse embryos (Fig. 1D).
Fig. 1.
Nedd4 is required for Ptc-induced apoptosis. (A) Schematic representation of a two-hybrid screen with the proapoptotic domain of Ptc-1. The Ptc-Nedd4 interaction was confirmed by direct two-hybrid: AH109 yeast transformed either with a Gal4AD plasmid fused with four WW domains of Nedd4 and a Gal4BD plasmid fused with the Ptc proapoptotic domain (Nedd4+Ptc), with a mock Gal4BD plasmid and a Gal4AD plasmid fused with four WW domains of Nedd4 (Nedd4), or with a mock Gal4AD plasmid and a Gal4BD plasmid fused with the Ptc proapoptotic domain (Ptc). (B and C) Coimmunoprecipitations were performed on HEK293T cells transiently expressing (B) Nedd4-myc alone or with Ptc (Ptc), Ptc-1–1165 (Ptc deleted of the seventh intracellular domain) or Ptc1-1392 (P1-1392) (C) Ptc-HA alone or with Nedd4-myc in the absence (Nedd4-myc) or in the presence of SHH (Nedd4-myc+SHH). Pull-down with anti-Ptc (B) or anti-myc (C) antibodies was used to immunoprecipitate Ptc and Nedd4, respectively. Nedd4 and Ptc were then revealed by Western blot by using anti-myc and anti-HA antibodies, respectively. Western blot on lysates before pull-down are shown (Tot. prot.). (D) Coimmunoprecipitation experiments were performed on embryonic mouse brain at E14. Pull-down with anti-Nedd4 antibody was used to immunoprecipitate Nedd4 followed by Western blot using anti-Ptc (αPtc) or anti-Nedd4 (αNedd4), as a control of immunoprecipitation efficiency. (E, F, H, and I) Caspase-3 activity assay was performed on HEK293T cells 24 h after transfection (E) with empty vector (Ctrl), Ptc-encoding vector (Ptc), Nedd4-encoding vector (Nedd4) or Ptc- and Nedd4-encoding vectors (Ptc+Nedd4) (F) with Ptc-encoding vector (Ptc) or Ptc and Nedd4-encoding vectors (Ptc+Nedd4) in the absence (−) or in the presence (+) of MG132 (H) with empty vector (Ctrl), Ptc-encoding vector (Ptc), Nedd4-encoding vector (Nedd4), Ptc- and Nedd4-encoding vectors (Ptc+Nedd4), Nedd4C1286S-encoding vector (Nedd4 C1286S), or Ptc- and Nedd4C1286S-encoding vectors (Ptc+Nedd4C1286S) (I) with empty vector (Ctrl) or Ptc-encoding vector (Ptc), in the presence of siRNA control (siCtrl) or siRNA Nedd4 (siNedd4). (E, H, and I) Anti-HA (αHA-Ptc) and anti-myc (αmyc-Nedd4) immunoblots are shown as controls of specificity and loading. (G) Cell death was analyzed by Trypan blue exclusion assay in HEK293T cells 24 h after transfection of an empty vector (Ctrl), a Ptc-encoding vector (Ptc), a Nedd4-encoding vector (Nedd4) or Ptc- and Nedd4-encoding vectors (Ptc+Nedd4). For all caspase-3 activity, folds over control of each experiment is represented. For all caspase-3 activity and cell death assays, error bars are SEMs. *P < 0.05, **P < 0.01, and ***P < 0.001.
Nedd4 as Ub ligase (E3) plays a major role in protein ubiquitination. Because ubiquitination was initially described to play a major role in protein degradation by providing a signal for proteasome-mediated degradation, the Nedd4 interaction with Ptc might at first appear to be a mechanism to increase proteasome-dependent degradation of Ptc, and thus to negatively regulate Ptc-induced apoptosis. However, forced expression of Nedd4 in HEK293T failed to reduce the level of Ptc expression (Fig. 1E), and more importantly, dramatically potentiated Ptc-induced apoptosis as measured by caspase-3 activation (Fig. 1E). This effect was not related to protein degradation, because treatment with the proteasome inhibitor MG132, although blocking degradation of proteins such as P21, had no effect on the Nedd4-mediated potentiation of Ptc-induced caspase-3 activation (Fig. 1F and Fig. S1B). A similar potentiating effect was observed when cell death per se, rather than caspase activation, was analyzed (Fig. 1G). This effect appeared to be specific for Nedd4 because another E3 ubiquitin ligase—mdm2—did not potentiate Ptc-induced apoptosis (Fig. S1C). Potentiation of Ptc-induced apoptosis was caused by the ligase activity of Nedd4, because forced expression of Nedd4 C1286S, a Nedd4 catalytic dead mutant, failed to promote Ptc-induced caspase-3 activation (Fig. 1H). We next performed a loss-of-function experiment to determine whether Nedd4 is required for Ptc-mediated apoptosis. As shown in Fig. 1I, Ptc-induced apoptosis was completely inhibited when Nedd4 was silenced by a siRNA approach (Fig. S1D). Thus, Nedd4 promotes Ptc-induced apoptosis via its E3 ligase activity and is required for Ptc proapoptotic signaling.
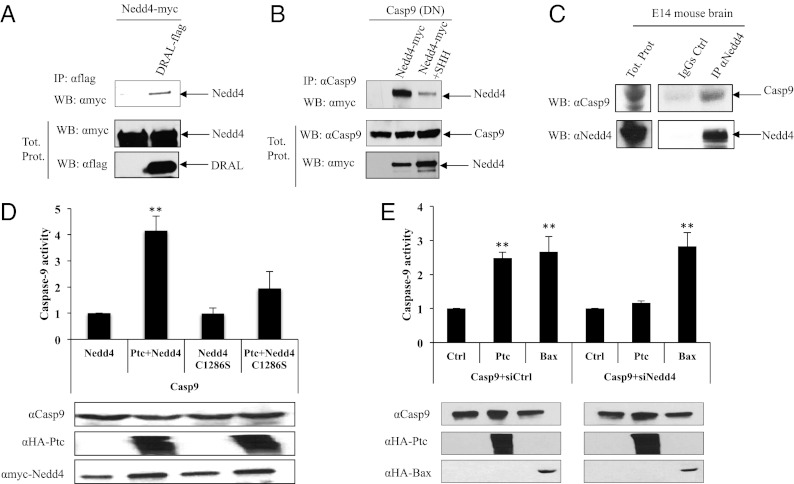
Ptc triggers apoptosis through the recruitment of a caspase-activating complex—the dependosome—that includes DRAL and caspase-9, and leads to direct caspase-9 activation (9). Nedd4 was shown to be a component of this caspase-activating complex because, in HEK293T coimmunoprecipitation, Nedd4 was pulled down with both DRAL and caspase-9 (Fig. 2 A and B) [i.e. a caspase-9 mutated at its catalytic cysteine, casp9(DN) was used here instead of wild-type caspase-9 to avoid cell death induction observed upon wild-type caspase-9 expression]. The interaction of Nedd4 with caspase-9 occurs in vivo with endogenous proteins because immunoprecipitation of Nedd4 pulls down caspase-9 in brain extract from embryonic day E14 mouse embryos (Fig. 2C). The presence of SHH, which has been shown to disrupt this caspase-activating complex (9), inhibits the interaction of Nedd4 with caspase-9 (Fig. 2B). Moreover, although Ptc triggers an increased caspase-9 activation when Nedd4 is overexpressed, such Ptc-mediated caspase-9 activation was not detected upon forced expression of the Nedd4 catalytic dead mutant (Fig. 2D). Conversely, Nedd4-silencing by siRNA transfection inhibited caspase-9 activation (Fig. 2E). Of interest, Nedd4 silencing had no effect on activation of caspase-9 mediated by Bax, the prototypical inducer of the intrinsic pathway for apoptosis. Thus, although the intrinsic pathway for apoptosis and the apoptosome activate caspase-9 independently of Nedd4, these data support the view that Nedd4 is recruited to the dependosome in the absence of SHH and participates in Ptc-mediated caspase-9 activation.
Fig. 2.
Nedd4 is part of the dependosome and regulates caspase-9 activation. (A) DRAL immunoprecipitation (IP: αflag) was performed on HEK293T cells transfected with either Nedd4 alone or Nedd4 and DRAL together (DRAL-flag). DRAL interaction with Nedd4 was revealed by Western blot using an anti-myc (Nedd4-myc) antibody. Western blot on lysates before pull-down are shown (Tot. prot.). (B) Coimmunoprecipitations were performed on HEK293T cells transiently expressing Casp-9(DN) alone or with Nedd4-myc in the absence (Nedd4-myc) or in the presence of SHH (Nedd4-myc+SHH). Western blot on lysates before pull-down are shown (Tot. prot.). (C) Coimmunoprecipitation experiments were performed on embryonic mouse brain at E14. Pull-down with anti-Nedd4 antibody was used to immunoprecipitate Nedd4 followed by Western blot using anti–caspase-9 (αCasp9) or anti-Nedd4 (αNedd4) as a control of immunoprecipitation efficiency. (D and E) Caspase-9 activity assay was performed using a proluminogenic caspase-9 substrate 18 h after transfection of HEK293T cells with wild-type caspase-9 together (D) with Nedd4-expressing construct or Nedd4C1286S-encoding vector or (E) with siRNA control (Casp9+siCtrl) or siRNA Nedd4 (Casp9+siNedd4), in the absence (Ctrl) or in the presence of Ptc (Ptc). Anti-HA (αHA-Ptc and αHA-Bax), anti–caspase-9 (αCasp9) and anti-myc (αmyc-Nedd4) immunoblots are shown as a control of specificity and loading. Folds over control are represented and error bars are SEM. **P < 0.01.
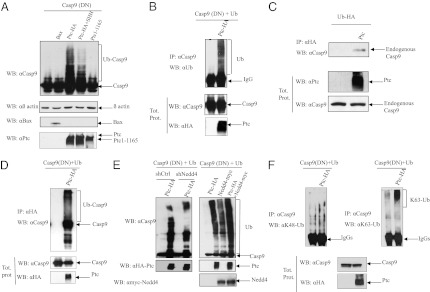
We therefore investigated the mechanism by which Nedd4 E3 ligase activity within the dependosome complex promotes caspase-9 activation, and consequently cell death. Considering recent data describing the ubiquitination of caspase-8 as a mechanism for caspase-8 activation (16), we investigated whether caspase-9 activation in the dependosome is associated with caspase-9 ubiquitination. In HEK293T cells forced to express a catalytically-dead caspase-9 [casp9(DN)], a caspase-9 immunoblot performed after cell extraction with SDS (but not the milder detergent Triton X-100) revealed putative ubiquitination of caspase-9 upon Ptc expression, but Bax had no effect (Fig. 3A). As expected from the dependence-receptor paradigm, the addition of SHH inhibited Ptc-associated caspase-9 ubiquitination. Similarly, enforced expression of Ptc deleted of the proapoptotic domain (Ptc 1–1165) was not associated with caspase-9 ubiquitination (Fig. 3A). Caspase-9 immunoprecipitation, performed under denaturing conditions to disrupt binding to other proteins, followed by Ub immunoblot, confirmed the ubiquitination of caspase-9 (Fig. 3B). Ubiquitination of endogenous caspase-9 was also observed upon Ptc expression after immunoprecipitation of HA-tagged Ub (Fig. 3C). To further analyze whether the caspase-9 recruited to the dependosome is ubiquitinated, we performed Ptc immunoprecipitation and assayed for caspase-9 ubiquitination. As shown in Fig. 3D, the Ptc-associated caspase-9 is ubiquitinated.
Fig. 3.
Caspase-9 is polyubiquitinated during Ptc-induced apoptosis. (A) Western blot using caspase-9 antibody was performed on HEK293T cells lysed in lysis buffer containing 1% SDS, 48 h after transient transfection of Casp9(DN) together with an empty vector, with Bax-encoding vector (Bax), with Ptc-encoding vector in the absence (Ptc-HA) or in the presence (Ptc-HA+SHH) of SHH, or with Ptc-1–1165 encoding vector (Ptc-1-1165). Anti–β-actin (αβ actin), anti-Ptc (αPtc), and anti-Bax (αBax) immunoblots are shown as controls of loading. (B and F) Immunoprecipitation of caspase-9 (IP: αCasp9) was performed on SDS-lysed HEK293T cells expressing Casp9(DN) and human Ub in the absence or in the presence of Ptc (Ptc-HA). Ubiquitinated caspase-9 (Ub) was revealed by Western blot using anti-ubiquitin (B) or anti–K48-ubiquitin and anti–K63-ubiquitin (F) antibodies. Western blots on lysates before pull-down are shown (Tot. prot.). (C) Immunoprecipitation of HA-ubiquitinated proteins (IP αHA-Ub) was performed on HEK293T cells lysed in the presence of SDS and expressing HA-ubiquitin encoding vectors in the absence or in the presence (Ptc) of Ptc. Endogenous caspase-9 was revealed by Western blot using an anti–caspase-9 antibody. Western blot on lysates before pull-down are shown (Tot. prot.). (D) Ptc immunoprecipitation (IP α-HA-Ptc) was performed on HEK293T cells expressing Casp9(DN) and human Ub in the absence or in the presence of Ptc (Ptc-HA). Ubiquitinated caspase-9 was revealed by Western blot using an anti–caspase-9 antibody. Western blot on lysates before pull-down are shown (Tot. prot.). (E) Western blot using caspase-9 antibody was performed on HEK293T cells lysed in 8 M urea lysis buffer, 48 h after transient transfection of Casp9(DN) and Ub together with shRNA control (shCtrl) or a shRNA against Nedd4 (shNedd4) in the presence of an empty vector or Ptc-encoding vector (Ptc-HA) or together with an empty vector, with Ptc-encoding vector (Ptc-HA), with Nedd4-encoding vector (Nedd4-myc) or with Nedd4- and Ptc-encoding vectors (Ptc-HA+ Nedd4-myc). Anti-HA (α HA-Ptc) and anti-myc (αmyc-Nedd4) immunoblots are shown as controls of expression.
To link the fact that Nedd4 is required for Ptc-induced apoptosis with the fact that Ptc triggers caspase-9 ubiquitination, we investigated, using both gain-of-function and loss-of-function experiments, whether Nedd4 is required for Ptc-mediated caspase-9 ubiquitination. As shown in Fig. 3E, silencing of Nedd4 by a shRNA approach (Fig. S2A) strongly reduced Ptc-induced caspase-9 ubiquitination but overexpression of Nedd4 strengthened Ptc-induced caspase-9 ubiquitination. Thus, taken together, these data support the view that, in the absence of SHH, Ptc directly interacts with Nedd4, which is required for ubiquitination of caspase-9 within the dependosome.
Ubiquitination involves covalent attachment of Ub to proteins, and this occurs either through addition of monoubiquitin or polyubiquitin chains linked via internal lysines. Although K48-linked poly-Ub frequently provides a signal for proteasome-mediated degradation, K63-linked chains are more frequently associated with a functional effect on the targeted protein (19). The pattern of ubiquination of Ptc-mediated caspase-9 ubiquitination supports a poly-ubiquitination of caspase-9 (Fig. 3 A, B, and D), and we therefore investigated the nature of this polyubiquitination. Using specific antibodies recognizing either the K48- or K63-linked chain, we observed that Ptc does not trigger any K48-Ub modification of caspase-9, but caspase-9 is clearly covalently linked to K63-Ub (Fig. 3F).
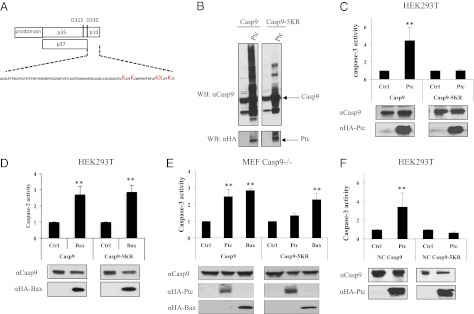
To determine whether this caspase-9 K63-ubiquitination could affect caspase-9 activity, we attempted to silence caspase-9 ubiquitination. Caspase-9 contains in its P10 fragment five putative lysines that represent potential ubiquitination sites (Fig. 4A). We therefore mutated these five lysines either individually (K394R, K398R, K409R, K410R, K414R) or all together (K394/398/409/410/414R). By investigating caspase-9 ubiquitination via a caspase-9 immunoblot performed after cell extraction with SDS, we did not detect any effect of the single mutation on Ptc-induced-induced caspase-9 ubiquitination. However, enforced expression of Ptc was associated with a strong reduction in caspase-9 ubiquitination when the caspase-9 K394/398/409/410/414R mutant (i.e., caspase-9 5KR) was used instead of wild-type caspase-9 (Fig. 4B). Similar results were obtained when caspase-9 ubiquitination was assessed by caspase-9 immunoprecipitation, performed under denaturing conditions followed by Ub immunoblot (Fig. S2B). We therefore assessed caspase activation in response to Bax or Ptc expression in the presence of either wild-type caspase-9 or caspase-9 mutants. A first set of experiments was performed in HEK293T cells. None of the single mutations in caspase-9 abrogate Ptc-induced caspase-3 activation (Fig. S2C). As shown in Fig. 4C, although Ptc triggered caspase-3 activation in wild-type caspase-9 settings, Ptc-induced caspase-3 activation was markedly reduced when caspase-9 5KR was used. The suggested ubiquitination-dependent activation of caspase is specific for Ptc, because Bax triggered caspase activation whether caspase-9 wild-type or 5KR was used (Fig. 4D). As expected, according the view that Nedd4 is required for Ptc-mediated caspase-9 ubiquitination and activation, both silencing of Nedd4 and overexpression of Nedd4 failed to have any effect on caspase-9 activity and ubiquitination when the caspase-9 5KR mutant was used instead of wild-type caspase-9 (Fig. S3).
Fig. 4.
Ubiquitination of caspase-9 is required for Ptc-induced caspase activation. (A) Schematic representation of caspase-9. The position of five lysines mutated in C9-5KR constructs is indicated. (B) Anti–caspase-9 immunoblot was performed on HEK293T cells transfected with wild-type caspase-9 (Casp9) or caspase-9 mutated on five lysines (Casp9-5KR) in the absence or in the presence of Ptc (Ptc). Anti-HA (αHA-Ptc) immunoblot is shown as a control of loading. (C–F) Caspase-3 activity assay was performed in HEK293T cells (C, D, and F) or in MEF deficient for caspase-9 (MEF Casp9−/−) (E), 24 h after transfection with wild-type caspase-9 (Casp9), caspase-9 mutated on five lysines (Casp9-5KR), or noncleavable caspase-9 (NC Casp9) together with either an empty vector (Ctrl), a Ptc-encoding vector (Ptc), or a Bax-encoding vector (Bax). Anti-HA (αHA-Ptc and αHA-Bax) and anti–caspase-9 (αCasp9) immunoblots are shown as controls of specificity and loading. Folds over control are represented and error bars are SEM. **P < 0.01.
Because, in the HEK293T cells, endogenous caspase-9 is expressed and may interfere with the ectopically expressed caspase-9, we performed the same type of experiment in mouse embryonic fibroblast (MEF) cells deficient for caspase-9 (Fig. S4A) (20). As a control, Ptc or Bax expression failed to trigger caspase activation in caspase-9–deficient MEF cells (Fig. S4B). However, as shown in Fig. 4E, although expression of wild-type caspase-9 allows Ptc- and Bax-induced caspase activation, expression of caspase-9 5KR was associated with apoptosis induction via Bax but not with Ptc. Thus, Ptc triggers caspase activation via ubiquination of caspase-9.
Because caspase-9 cleavage has been shown to play a role in caspase-9 activation and amplification (6), HEK293T cells were forced to express either a noncleavable form of caspase-9 or a noncleavable caspase-9 mutated at the five lysines that represent potential ubiquitination sites. As shown in Fig. 4F, Ptc triggered caspase-3 activation in uncleavable caspase-9 settings, but it did not when uncleavable caspase-9 5KR was used. Thus, caspase-9 activation mediated by Ptc requires ubiquitination of caspase-9 by Nedd4, but no necessarily the cleavage of caspase-9.
Taken together, these data support the view that Ptc triggers direct activation of caspase-9 within the dependosome by a mechanism that requires Nedd4 recruitment and caspase-9 ubiquitination. Interestingly, there is a strong analogy with the elegant observation made by Ashkenazi and colleagues, who first described the contribution of cullin3-based polyubiquitination of caspase-8 in caspase-8 activation (16). Interestingly, both types of receptors—the death receptors DR4 and DR5, and the dependence receptor Ptc—recruit at the membrane caspase-activating complexes: the DISC in the former case and the dependosome in the latter. These receptors then trigger initiator caspase activation, caspase-8 by the death receptors DR4 and DR5, and caspase-9 by the dependence receptor Ptc. It is even more intriguing to note that, although caspase-9 ubiquitination appears to be a prerequisite for caspase-9 activation in the Ptc dependosome, we failed to detect any caspase-9 ubiquitination upon apoptosome activation, and we failed to observe, upon Bax overexpression, any loss of caspase-9 activity using a Ub-dead mutant of caspase-9. Thus, depending on whether an extrinsic or intrinsic pathway is used, two different mechanisms of initiator caspase activation are used: one requires initiator caspase ubiquitination, be it caspase-8 or caspase-9, and occurs at the membrane; the other is independent of initiator caspase ubiquitination and occurs in the cytosol. One may wonder, what is the role of adding K63 polyubiquitination to promote caspase-9 activation, specifically for the dependosome and not the apoptosome? In the case of caspase-8, ubiquitination was shown to allow caspase-8 translocation from receptor-associated DISC to Ub-rich foci, although to date there has been no evidence that these Ub-rich foci actively participate in caspase-8 activity. In the case of caspase-9 described here, it is of interest to see that the proteolytic cleavage of caspase-9 is not required for Ptc-induced caspase activation. It is then intriguing to compare this observation with caspase-9 processing in the apoptosome. Although, in the initial steps of apoptosome-dependent caspase-9 activation, processing of caspase-9 is not required, this processing appears important for prolonged and efficient apoptosis induction (21). Future biochemical work will need to be performed to define the role of polyubiquitination in caspase-9 catalytic activation.
Experimental Procedures
A complete discussion of materials and methods is presented in SI Experimental Procedures.
Site-Directed Mutagenesis and Plasmid Constructs.
A list of the constructs used and a description of the cloning strategies are described in SI Experimental Procedures.
Cell Cultures, Transfection Procedures, Reagents.
Transient transfection of HEK293T cells was performed with calcium phosphate for coimmunoprecipitation or with Jetprime (Polyplus) for cell death assay and immunoblot according to the manufacturer’s instructions. MEF cells were cultured in DMEM media supplemented with 10% (vol/vol) calf fetal serum and 2 μL of β-mercaptoethanol. MEF cells were transfected with Jetprime (Polyplus) for cell death assay. Recombinant SHH-N was from R&D Systems and was added at the time of transfection at 600 ng/mL. MG132 was used at 0.5 μg/mL for 2 h, and was purchased from Sigma. For siRNA experiments, cells were transfected with 60 pmols siRNA using Jetprime reagent. Nedd4 and control siRNAs were from Sigma.
Two-Hybrid Analysis.
Matchmaker two-hybrid system III (Clontech) was used according to the manufacturer’s instructions using AH109 yeast cotransformed with pGBKT7-DNA binding domain GAL4 fused to Ptc 1165–1392 (pGBKT7-Ptc7IC) and the pGADT7-GAL4 transcriptional activation domain AD fused to four WW domains of Nedd4 (pGADT7-WWNedd4) (Clontech).
Coimmunoprecipitation and Immunoblotting Analysis.
Coimmunoprecipitation were carried out on endogenous proteins in E14 mouse brain or in MiaPaca-2 human pancreatic cell line and on over-expressed proteins in HEK293T cells, as described previously (9) and as described in SI Experimental Procedures. Immunoblots were performed as previously described (9) and as described in SI Experimental Procedures.
Cell Death Analysis and Caspase Assays.
Cell death was analyzed 24 h after transfection using Trypan blue staining procedures. Caspase-3 activity assay was performed 24 h after transfection using the caspase-3 fluorometric assay kit (BioVision), as described previously (9). Caspase-9 activity was measured 18 h after transfection using the luminescent Caspase-Glo 9 Assay according to the manufacturer’s instructions (Promega).
Quantitative RT-PCR.
Real-time quantitative RT-PCR was performed as described in SI Experimental Procedures. Primers and probes were given by Universal Probe Library Assay Design Center Web site (Roche Applied Science). Sequences are shown in Table S1.
Statistics.
The statistical significance of differences between groups was evaluated by the Mann–Whitney U test. Mean values for all outcome variables are presented with SEMs. Data presented are representative of at least four independent experiments. All statistical tests were two-sided, and P values less than 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank D. E. Bredesen for critical reading of the manuscript; A. P. Arrigo, D. Green, and G. Melino for sharing reagents; O. Ayrault for key recommendation on ubiquitination detection; and N. Zala for technical help. This work was supported by institutional grants from the Ligue Contre le Cancer, Institut National du Cancer, Agence Nationale de la Recherche, and European Community Seventh Framework Programme Apo-Sys.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200094109/-/DCSupplemental.
References
- 1.Zörnig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–F37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 2.Green D, Kroemer G. The central executioners of apoptosis: Caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 3.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Boatright KM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 5.Mace PD, Riedl SJ. Molecular cell death platforms and assemblies. Curr Opin Cell Biol. 2010;22:828–836. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pop C, Timmer J, Sperandio S, Salvesen GS. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Riedl SJ, Salvesen GS. The apoptosome: Signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 8.Forcet C, et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci USA. 2001;98:3416–3421. doi: 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mille F, et al. The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex. Nat Cell Biol. 2009;11:739–746. doi: 10.1038/ncb1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell Death Differ. 2005;12:1031–1043. doi: 10.1038/sj.cdd.4401680. [DOI] [PubMed] [Google Scholar]
- 11.Goldschneider D, Mehlen P. Dependence receptors: A new paradigm in cell signaling and cancer therapy. Oncogene. 2010;29:1865–1882. doi: 10.1038/onc.2010.13. [DOI] [PubMed] [Google Scholar]
- 12.Mehlen P, Bredesen DE. Dependence receptors: From basic research to drug development. Sci Signal. 2011;4:mr2. doi: 10.1126/scisignal.2001521. [DOI] [PubMed] [Google Scholar]
- 13.Wertz IE, Dixit VM. Signaling to NF-kappaB: Regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, et al. Bcr-Abl ubiquitination and Usp9x inhibition block kinase signaling and promote CML cell apoptosis. Blood. 2011;117:3151–3162. doi: 10.1182/blood-2010-03-276477. [DOI] [PubMed] [Google Scholar]
- 15.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 16.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Thibert C, et al. Inhibition of neuroepithelial patched-induced apoptosis by Sonic Hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Liu S, Kornberg TB. The C-terminal tail of the Hedgehog receptor Patched regulates both localization and turnover. Genes Dev. 2006;20:2539–2551. doi: 10.1101/gad.1461306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Forero I, Rouzaut A, Palazon A, Dubrot J, Melero I. Lysine 63 polyubiquitination in immunotherapy and in cancer-promoting inflammation. Clin Cancer Res. 2009;15:6751–6757. doi: 10.1158/1078-0432.CCR-09-1225. [DOI] [PubMed] [Google Scholar]
- 20.Marsden VS, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 21.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.