Abstract
Elevating Akt activation is an obvious clinical strategy to prevent progressive neuronal death in neurological diseases. However, this endeavor has been hindered because of the lack of specific Akt activators. Here, from a cell-based high-throughput chemical genetic screening, we identified a small molecule SC79 that inhibits Akt membrane translocation, but paradoxically activates Akt in the cytosol. SC79 specifically binds to the PH domain of Akt. SC79-bound Akt adopts a conformation favorable for phosphorylation by upstream protein kinases. In a hippocampal neuronal culture system and a mouse model for ischemic stroke, the cytosolic activation of Akt by SC79 is sufficient to recapitulate the primary cellular function of Akt signaling, resulting in augmented neuronal survival. Thus, SC79 is a unique specific Akt activator that may be used to enhance Akt activity in various physiological and pathological conditions.
Keywords: drug discovery, cell signaling
Akt/PKB, a serine/threonine protein kinase with antiapoptotic activity, is one of the major downstream targets of PtdIns(3,4,5)P3 signaling pathway. It contains a pleckstrin homology domain (PH domain) that specifically binds PtdIns(3,4,5)P3 on the plasma membrane. Under physiological condition, the PtdIns(3,4,5)P3-mediated membrane translocation of Akt is essential for its phosphorylation at Thr308 and Ser473 and the subsequent activation. Activated Akt, in turn, phosphorylates a variety of proteins, including several associated with cell survival/death pathways such as BAD and FoxOs (1, 2). Akt is a crucial mediator of cell survival and its deactivation is implicated in various stress-induced pathological cell death and degenerative diseases. For example, Akt was shown to be important in mediating the survival of a range of neuronal cell types. Deactivation of Akt was implicated in pathogenesis of numerous neurological diseases (3–7). In addition, Akt signaling is critically important for myelination of axons (8, 9). Activation of Akt in myelin-forming cells will be beneficial for various neuropathies, such as multiple sclerosis, caused by demyelination of axons. The acute activation of Akt is also beneficial to prevent apoptosis of cardiomyocytes upon ischemic injury (6).
Elevating Akt signaling can be achieved by activating upstream components by using certain growth factors or growth factor receptor agonists. Akt phosphorylation and activation are directly determined by the level of PtdIns(3,4,5)P3 on the plasma membrane, which is regulated by phosphatidylinositol 3′-kinases (PI3-kinase or PI3K), the tumor suppressor PTEN, SHIP, and 5ptase IV (a phosphoinositide-specific inositol polyphosphate 5-phosphatase IV). It was reported that two inositol phosphates, InsP7 and Ins(1,3,4,5)P4, compete for Akt-PH domain binding with PtdIns(3,4,5)P3 both in vitro and in vivo, providing another level of regulation for Akt membrane translocation and activation (10–13). Thus, activation of Akt can also be achieved by manipulating these related cellular factors. However, none of these approaches are specific because multiple signaling pathways are activated downstream of the receptors or PtdIns(3,4,5)P3.
Despite the great need of Akt activator for a variety of therapeutic applications, the effort of identifying genuine activator of Akt was ultimately unsuccessful. In this study, we setup a cell-based high-throughput chemical genetic screening system aiming to identifying novel Akt inhibitors that specifically target PtdIns(3,4,5)P3-mediated Akt membrane translocation. However, paradoxically, from this screening, we discovered a genuine activator of Akt that suppressed PHAKT-GFP plasma membrane translocation but enhanced Akt phosphorylation. It enables cytosolic activation of Akt independent of PtdIns(3,4,5)P3-mediated Akt membrane translocation. In addition, this activator-induced Akt activation recapitulates the primary function of Akt signaling because it efficiently prevented excitotoxicity-induced neuronal death.
Results
High-Throughput Screening for Inhibitors of Akt Plasma Membrane Translocation.
To visualize Akt translocation, we used the PH domain of Akt (PHAkt) fused with green fluorescent protein (PHAkt-GFP) as a marker. When serum-starved cells were stimulated with insulin-like growth factor (IGF), the membrane translocation of PHAkt-GFP occurred within 5 min. As a control, IGF-elicited membrane translocation was significantly suppressed in cells treated with LY294002, which inhibits PI3K activity and, therefore, reduces the level of PtdIns(3,4,5)P3 on the plasma membrane (SI Appendix, Fig. S1). Using a stable HeLa cell line expressing PHAkt-GFP fusion protein (SI Appendix, Fig. S2), we performed a cell-based chemical genetic screening for compounds that suppress IGF-induced PHAkt-GFP membrane translocation (SI Appendix, Fig. S3). The pilot screening identified 21 positive hits from a library containing 480 bioactive compounds. Several chemicals known to be able to inhibit PtdIns(3,4,5)P3 signaling, including Wortmannin, Celastrol, Quercetin, and LY294002, were among the identified compounds (SI Appendix, Fig. S4 and Table S1). The subsequent high-throughput screening (HTS) of more than 60,000 synthetic chemical compounds (SI Appendix, Table S2) identified 125 positive hits (Fig. 1A and SI Appendix, Table S3 and Fig. S5).
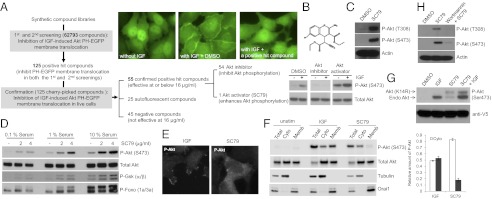
Fig. 1.
High-throughput screening identifies a synthetic compound SC79 that suppresses PHAKT-GFP plasma membrane translocation but enhances Akt phosphorylation and activation in the cytosol. (A) High-throughput screening identified SC79 as an inhibitor of Akt-PH domain translocation and an enhancer of Akt phosphorylation. (B) Chemical structure of SC79. (C) SC79 augmented Akt phosphorylation at both the Thr308 and S473 sites. (D) SC79 enhanced Akt phosphorylation and its kinase activity. Phosphorylation of downstream targets, GSK-3β and Foxo, was detected by using specific Phospho-GSK3β and Phospho-Foxo antibodies. (E) The representative images of SC79-induced cytosolic phosphorylation of Akt. Cells were serum-starved for overnight and then treated with IGF or SC79 for 15 min. The fixed cells were immunostained with the phospho-Akt (S473) antibody. (F) SC79-induced cytosolic phosphorylation of Akt analyzed by Western blotting. HeLa cells were serum starved for 1 h and treated with IGF (100 ng/mL) or SC79 (4 μg/mL) for 30 min. Total cell lysate, cytosolic, and membrane fractions were resolved by SDS/PAGE and analyzed for phospho-Akt (S473), Total Akt, Tubulin (cytosolic marker), and Orai1 (membrane marker) by Western blotting. (G) SC79 treatment led to phosphorylation of Akt (K14R) mutant, a PH domain mutant incapable of binding to PtdIns(3,4,5)P3. Serum-starved cells stably expressing the V5-tagged Akt (K14R) were treated with IGF, SC79, or in combination before analysis for Akt phosphorylation. The phosphorylated forms of endogenous and mutant Akt (K14R) were indicated by arrows. A mouse monoclonal V5 antibody was used to detect Akt (K14R) mutant and to serve as the loading control. (H) Pretreatment with PI3K inhibitor wortmannin abolished SC79-induced Akt activation.
To confirm the inhibitory effect of the positive hit compounds on PHAkt-GFP membrane translocation, we examined PH-Akt translocation by using time-lapse fluorescent imaging. We found that 25 of the positive compounds generated autofluorescence and their effect on PH-Akt membrane translocation was, in fact, the result of greatly enhanced background fluorescence (SI Appendix, Table S3). Fifty-four of the initial 125 positive compounds could inhibit IGF-induced Akt membrane translocation at the concentration of 8 μg/mL (SI Appendix, Figs. S6 and S7). However, these compounds may have a general translocation inhibitory effect and, thus, prevent any protein from translocating to the plasma membrane. In addition, some “positive hits” may affect PtdIns(3,4,5)P3 level via modulating cellular levels of phosphoinositides. To eliminate these possibilities, we examined PtdIns(4,5)P2-mediated protein translocation. We used a cell line stably expressing GFP-PLC-delta1-PH domain, which specifically binds to PtdIns(4,5)P2, but not PtdIns(3,4,5)P3 (14, 15). It appeared that none of the positive hit compounds suppressed the membrane localization of GFP-PLC-delta1-PH (SI Appendix, Fig. S8). Another general issue in cell-based HTS is the indirect effects of compounds on the assay readout (e.g., via inducing cell death or affecting transcription or translation). None of the positive hit compounds caused morphological changes in this short period, suggesting that the inhibited PHAKT-GFP plasma membrane translocation was not the result of cell death (SI Appendix, Fig. S9). However, many positive compounds turned out to be toxic when cells were incubated with them for longer time (SI Appendix, Fig. S9 and Table S4), indicating that these chemicals may target cellular components other than Akt.
Synthetic Compound SC79 Suppresses PHAKT-GFP Plasma Membrane Translocation but Enhances Akt Phosphorylation and Activation in the Cytosol.
To investigate the effect of each positive hit compound on Akt activity, we next assessed IGF-elicited Akt phosphorylation, a commonly used reporter for Akt activation. To our surprise, we unexpectedly found that one of the positive hits, SC79, could suppress PHAKT-GFP plasma membrane translocation but enhance Akt phosphorylation (Fig. 1B). Upon PtdIns(3,4,5)P3-mediated recruitment to the plasma membrane, Akt is phosphorylated at two different regulatory sites, T308 and S473, respectively. Both phosphorylations were augmented by SC79 treatment (Fig. 1C). In addition, SC79 not only enhanced IGF1-induced Akt phosphorylation in serum-starved cells, but also elevated the level of Akt phosphorylation in cells grown in serum-rich medium (Fig. 1D). The kinetic studies revealed that Akt phosphorylation could be increased within a minute of SC79 treatment (SI Appendix, Fig. S10).
Various chemicals targeting the ATP binding pocket of Akt and inhibiting its kinase activity paradoxically lead to hyperphosphorylation of Akt (16, 17) (SI Appendix, Fig. S11). Importantly, once the drug is removed, such phosphorylated Akt was shown to be fully active (18). One explanation for SC79-induced Akt phosphorylation might be that SC79 transiently binds to the ATP binding pocket, leading to Akt phosphorylation, and then readily dissociates from the activated Akt. To rule out this possibility, we first determined whether SC79 could inhibit the Akt kinase activity in intact cells. At a comparable concentration, SC79 failed to demonstrate any inhibition toward Akt kinase activity. Instead, phosphorylation of several downstream effectors of Akt, including and FOXO, was much enhanced in SC79-treated cells, suggesting that SC79-induced augmentation of Akt phosphorylation led to enhanced Akt kinase activity (Fig. 1D). A compound that shares structure similarity with SC79, HA14-1, showed the same effect (SI Appendix, Fig. S11).
SC79 enhanced Akt phosphorylation but inhibited PtdIns(3,4,5)P3-mediated Akt membrane translocation, indicating that the binding between Akt and PtdIns(3,4,5)P3 may not be essential for SC79-induced Akt hyperactivation. Supporting this notion, both immunostaining and biochemical analyses indicate that IGF-induced Akt phosphorylation occurred near the plasma membrane where the PtdIns(3,4,5)P3 was generated, whereas SC79-induced Akt phosphorylation mainly occurred in the cytosol (Fig. 1 E and F). In addition, SC79 also enhanced the phosphorylation of an Akt mutant (K14R) that is deficient in PtdIns(3,4,5)P3 binding function and, thus, cannot be activated by IGF (Fig. 1G). Nevertheless, it is noteworthy that even in the presence of SC79, Akt phosphorylation relied on production of a certain amount of PtdIns(3,4,5)P3, because PDK1 still required PtdIns(3,4,5)P3 for activation. Therefore, treatment with PI3K inhibitor wortmannin significantly suppressed Akt phosphorylation in both SC79-treated and -untreated cells (Fig. 1H).
SC79 Enhances Phosphorylation of all Three Akt Isoforms and Elevates Akt Activation in Multiple Cell Types.
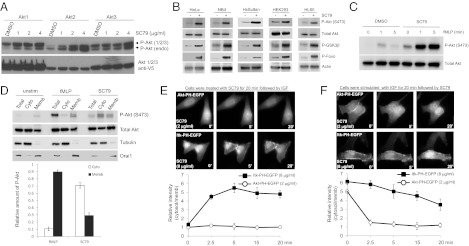
There are three Akt isoforms in mammalian cells with an almost identical PtdIns(3,4,5)P3-binding PH domain. The original screening was conducted by using Akt1-PH domain. In some contexts, the differential activation and functional specificity of Akt isoforms have been observed. For instance, in neutrophils, Akt2 but not Akt1 was shown to be recruited to the plasma membrane upon chemoattractant stimulation (19). We, therefore, wondered whether SC79 could show any isoform specific activity. We tested this possibility in cells stably expressing individual Akt isoform. SC79 was able to potently activate all isoforms, indicating that it may act as a pan-Akt activator (Fig. 2A). In addition, the effect of SC79 on Akt activation was not limited to HeLa cells. SC79-induced Akt hyperphosphorylation was detected in all cell types examined in this study, including HEK293, HeLa, HL60, NB4, and HsSulton (B cells) cells (Fig. 2B).
Fig. 2.
SC79 is a pan-Akt activator that specifically enhances Akt phosphorylation and activation in multiple cell types and in both receptor tyrosine kinase- and GPCR-mediated signaling. (A) HEK293 cells stably expressing the individual Akt isoforms were treated with indicated amounts of SC79. (B) Indicated cell lines was treated with SC79 (8 μg/mL) for 30 min and phosphorylation of Akt (Ser473) and its downstream targets, GSK-3β and Foxo1, was analyzed. Total Akt and actin were used as loading control. (C) Differentiated HL-60 cells was pretreated with SC79 (4 μg/mL) or DMSO for 5 min and then stimulated with fMLP for indicated time. Phosphorylation of Akt (Ser473) was analyzed as described above. (D) dHL60 were stimulated as described above. Total cell lysate, cytosolic, and membrane fractions were resolved by SDS/PAGE and analyzed for phospho-Akt (S473), Total Akt, Tubulin (cytosolic marker), and Orai1 (membrane marker) by Western blotting as described in Fig. 1F. (E) Pretreatment of SC79 prevented PtdIns(3,4,5)P3-mediated membrane translocation of Akt PH but not Itk PH domain. (F) SC79 led to dissociation of membrane-bound Akt PH but not Itk PH domain.
SC79 Specifically Enhances Akt Phosphorylation and Activation in both Receptor Tyrosine Kinase- and GPCR-Mediated Signaling.
It is known that PH-domain membrane translocation can also be induced by activation of G protein coupled receptors (GPCRs) (8, 11). The general Akt activator should also enhance GPCR-mediated Akt activation. To test this hypothesis, we examined the effect of SC79 on Akt phosphorylation in neutrophil-like differentiated HL60 cells (dHL60). Chemoattractants, such as methionyl-leucyl-phenylalanine (fMLP), bind receptors on cell membrane, leading to activation of GPCR and PtdIns(3,4,5)P3/Akt signaling. Treatment with SC79-elevated Akt phosphorylation in both unstimulated- and fMLP-stimulated dHL60 cells, demonstrating that SC79 also acts as an Akt activator in GPCR signaling (Fig. 2C). Similar to what was observed in HeLa cells, SC79-elicited Akt activation in dHL60 cells also mainly occurred in cytosol (Fig. 2D).
Effect of SC79 on Akt appeared to be specific. A recent systematic study revealed a great diversity among various PtdIns(3,4,5)P3-binding PH domains (20). For example, the PH domain of IL2-inducible T-cell Kinase (ITK), a Tec family protein tyrosine kinase, is diverged from that of Akt (SI Appendix, Fig. S12). Although both Akt and ITK were translocated to the plasma membrane upon IGF stimulation, pretreatment with SC79 significantly inhibited Akt-PH domain translocation, but not Itk-PH domain translocation, even at a higher concentration (Fig. 2E). We also carried out a converse experiment in which cells were prestimulated with IGF followed by SC79 treatment. Consistent with its inhibition of membrane translocation, SC79 led to an efficient dissociation of Akt-PH domain from the membrane while it showed a relative inefficacy toward Itk PH domain (Fig. 2F).
SC79 Directly Binds to Akt and Converts It to an Active Conformation More Amenable to Be Phosphorylated by Upstream Kinases.
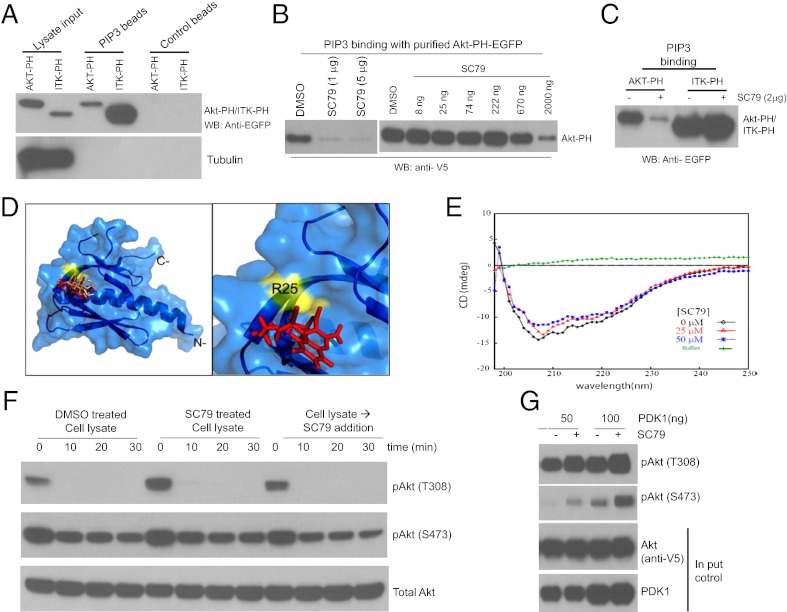
We hypothesize that SC79 directly binds to Akt-PH domain and converts Akt from an inactive conformation to an active conformation, leading to hyperactivation of Akt. To determine whether SC79 directly interacts with the Akt-PH domain, we performed an in vitro binding assay with PtdIns(3,4,5)P3-coated beads. In the presence of increasing amounts of SC79, Akt-PH-EGFP brought down by PtdIns(3,4,5)P3 beads was found to be reduced, indicating that SC79 could directly bind to PH domain and compete with PtdIns(3,4,5)P3 for such binding (Fig. 3 A and B). In contrast to Akt PH domain, the in vitro PtdIns(3,4,5)P3 binding capacity of Itk PH domain was unaffected by SC79, although Itk PH domain manifested a relatively higher affinity toward PtdIns(3,4,5)P3 compared with Akt-PH domain (Fig. 3C).
Fig. 3.
SC79 directly binds to Akt and converts it from an inactive conformation to an active conformation, leading to hyperactivation of Akt. (A) Both Akt-PH domain and Itk-PH domain could bind to PtdIns(3,4,5)P3. (B) SC79 inhibited PtdIns(3,4,5)P3 binding function of Akt PH domain. (C) SC79 did not affect the PtdIns(3,4,5)P3 binding function of Itk PH domain. (D) In silico docking of SC79 ligand onto the Akt PH domain structure. (Left) Docked SC79 ligand (rendered red) onto crystal structure of Akt PH domain (PDB ID code: 1UNR, rendered marine). Shown in “wheat” color is the IP4 ligand that docks at the site almost similar to the SC79 binding site. Highlighted in yellow is the residue surface located adjacent to the SC79 binding site that exhibits significant interaction with ligand SC79. (Right) Zoomed region, as in Left, shows docked SC79 ligand and interaction residue R25. (E) CD spectra of human Akt1 and its complex with SC79. An overlay of CD spectra from 5 μM human Akt1 (shown in black) alone and in the presence of SC79 of 25 μM (shown in red) or 50 μM (shown in blue) collected at 25 °C is shown. Baseline was corrected for buffer contribution. (F) SC79 did not affect Akt dephosphorylation in vitro. HEK293 cells grown in serum-rich medium were treated with DMSO or SC79 (4 μg/mL) for 20 min. Cells were lysed on ice in a control buffer devoid of phosphatase inhibitors or supplemented with SC79 (4 μg/mL). After centrifugation at 4 °C, the lysates were kept on ice (time 0 min) or incubated at 37 °C for indicated time points. The kinetics of Akt dephosphorylation at T308 and S473 was determined by Western blot. (G) SC79 directly enhanced Akt phosphorylation by purified recombinant PDK1 in vitro.
The structure of Akt PH domain has been reported (21). PtdIns(3,4,5)P3 binds to a shallow pocket in the Akt PH domain largely mediated through several salt bridges between the phosphate groups and basic residues in the protein. Molecular docking of Akt-PH domain with SC79 revealed that SC79 binds to the same PtdIns(3,4,5)P3 binding pocket (Fig. 3D). By performing circular dichroism (CD) spectroscopy, we further examined whether the binding of SC79 to the PH domain can alter the overall structure of Akt. Far-UV CD spectra were recorded for full-length human Akt1 in the presence or absence of SC79 (Fig. 3E). The overall secondary structural content of Akt1 was decreased by 4.3% because of ligand binding at both 25 μM and 50 μM concentrations (Fig. 3E and SI Appendix, Table S5). At 25 μM, SC79 binding resulted in a decrease in α-helical content by 17% and an increase in β-strand content by 19% compared with Akt1 alone (Fig. 3E and SI Appendix, Table S5). These results indicate that SC79 can physically interact with and modulate the structure of Akt.
SC79 significantly increased the level of Akt phosphorylation. One possibility is that, in the presence of SC79, the phosphorylated Akt could be more resistant to dephosphorylation by cellular phosphatases. To test this possibility, we examined whether SC79 affects Akt dephosphorylation by using an in vitro assay. When the cytosolic extract of HEK293 cells was incubated at 37 °C, a dramatic dephosphorylation of T308 ensued, whereas that of S473 was progressed in a relatively slower kinetics. Under this condition, neither the cytosolic extract from SC79 pretreated cells nor addition of SC79 to the cell lysate attenuated dephosphorylation of either T308 or S473 site (Fig. 3F).
To provide direct evidence that SC79-bound Akt adopts a conformation more amenable to be phosphorylated by upstream kinases, we explored the effect of SC79 on Akt phosphorylation by using a cell-free assay. Unphosphorylated Akt was immunoprecipitated from the lysate of serum-starved cells. In vitro phosphorylation by purified PDK1 was conducted in the presence or absence of SC79. This assay revealed that SC79 could enhance Akt phosphorylation at T308 site by PDK1. Interestingly, when T308 site was highly phosphorylated, the phosphorylation at S473 site was also dramatically increased in the absence of any PDK2 kinases (Fig. 3G). The in vitro autophosphorylation at S473 site, which depends on T308 phosphorylation, has been reported (22). Thus, our finding is consistent with this report and supports a positive role for SC79 in T308 phosphorylation by PDK1.
SC79 Reduces Neuronal Excitotoxicity and Prevents Stroke-Induced Neuronal Death.
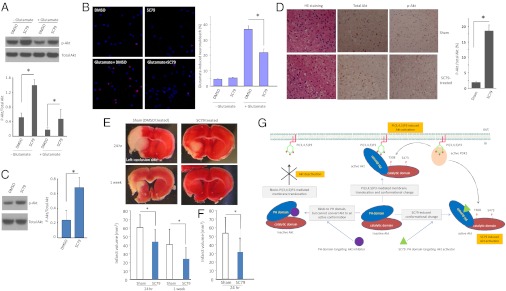
Next, we determined whether SC79-induced Akt activation could recapitulate the physiological function of Akt signaling. Because Akt deactivation is a causal mediator of neuronal death in various neurological diseases, we investigated whether SC79 can suppress such pathological neuronal death by preventing Akt deactivation. We first examined the effect of SC79 on neuronal death elicited by glutamate excitotoxicity. Excitotoxicity-induced neuronal death is a unique type of cell death that is mainly mediated by N-methyl-d-aspartate (NMDA) receptors (3, 23–25). Akt deactivation is a causal mediator of excitotoxicity-induced neuronal death (26). Treatment of cultured cortical neurons with Akt activator SC79 markedly enhanced Akt phosphorylation without altering total Akt levels (Fig. 4A). Similarly, SC79 treatment substantially reduced the death of a glutamate-challenged hippocampal neurons as monitored by cellular morphology and nuclear staining (26) (Fig. 4B).
Fig. 4.
SC79 suppresses excitotoxicity and alleviates stroke-induced neuronal death. (A) SC79 elevated Akt activation in cultured cortical neurons. All samples were normalized to the amount of total Akt. Data presented are the means (±SD) of three independent experiments. (B) SC79 suppresses excitotoxicity-induced neuronal death. Primary neuronal cultures (14 d) were treated with 50 μM glutamate for 40 min, and toxicity was assayed 4 h after glutamate exposure. Cell viability was assessed by fluorescence microscopy after Hoechst nuclear staining and propidium iodide (PI) staining. At least three separate experiments were performed with a minimum of 1,000 neurons counted per data point. The results are the means of three independent experiments. Bars indicate means ± SD. (C) SC79 treatment led to Akt hyperactivation in the brain of live animals. Protein extracts collected from the brain of untreated and SC79-treated mice were resolved on SDS/PAGE and immunoblotted with indicated antibodies. SC79 was applied via i.p. injection at a concentration of 0.04 mg/g of body weight. Data are from five independent experiments (mean ± SD). *P < 0.05 (Student t test). (D) SC79 enhanced Akt activity during neuronal cell death in an in vivo mouse ischemia model. Mice (C57 Black/6) were subjected to permanent focal cerebral ischemia by middle cerebral artery occlusion (MCAO) for 24 h. Brain slices around ischemic penumbra were prepared, and total and phosphorylated Akt/PKB were analyzed. Data are representative of five experiments. (E) SC79 alleviated stroke-induced neuronal death. Infarct volume was assessed in SC79 treated and Sham-treated control mice at each indicated time points. Data are from five independent experiments (mean ± SD). *P < 0.05 (Student t test). SC79 was injected i.p. once (0.04 mg/g of mouse body weight) 5 min before permanent MCAO. (F) The experiment was conducted as described above except that extra SC79 was injected (0.04 mg/g of mouse body weight, once per hour for 6 h). (G) Schematic model of SC79-induced Akt hyperactivation.
To ascertain whether SC79 can lead to Akt hyperactivation and prevent glutamate-mediated neurotoxicity in intact organisms, an ischemic stroke model was used. We subjected mice to middle cerebral artery occlusion (MCAO), which elicits substantial cell death in the area of the occlusion. Most clinical symptoms of stroke, such as paralysis, aphasia, visual disturbance, and memory loss, are caused by neuronal death elicited by oxygen-glucose deprivation (OGD), which is a result of lack of blood flow. Stroke-initiated OGD in affected brain regions induces a dramatic elevation of glutamate in the synaptic clefts that, in turn, causes massive excitotoxicity-elicited neuronal cell death (27, 28). Akt was identified as a potential target for treating stroke-induced neuronal death (29–32). Consistently, i.p. pretreatment with SC79 in mice effectively prevented stroke-induced Akt deactivation (Fig. 4 C and D). Consequently, it provided protection from excitotoxicity-induced brain damage in both the cortical area and striatum. The effect of SC79 was potent, with a single dose of SC79, 0.04 mg/g of body weight (equivalent to 0.5 μM), reducing the neocortical lesion size by 35% 24 h after MCAO and more than 40% 1 wk after MCAO (Fig. 4E). More drastic effect was observed when SC79 was injected multiple times (Fig. 4F).
Discussion
PtdIns(3,4,5)P3-mediated plasma membrane translocation is a prerequisite for Akt activation by regulatory phosphorylation. It is reported that PtdIns(3,4,5)P3–PH domain interaction may not only bring Akt and PDK1, the kinase involved in Thr308 phosphorylation, to the membrane, but also result in conformational changes of Akt, exposing two residues Thr308 and Ser473 for phosphorylation (33–35). Nevertheless, whether such conformational changes alone could contribute to Akt phosphorylation and activation is not clear. Here, we demonstrate that the necessity of Akt membrane recruitment can be bypassed via chemical-induced allosteric activation of cytosolic Akt. From a cell-based high-throughput chemical genetic screening, we identified an Akt activator, SC79, that specifically targets Akt PH domain. SC79 may act similarly to PtdIns(3,4,5)P3 to induce Akt conformation favorable for phosphorylation. It enabled the cytosolic activation of Akt, bypassing the requirement of PtdIns(3,4,5)P3-mediated Akt membrane translocation (Fig. 4G). It has been shown that Akt and PDK1 interact in the cytosol (35). In the absence of PtdIns(3,4,5)P3, the Akt phosphotylation by PDK1 in this complex is inhibited by the intramolecular interaction between Akt PH and kinase domain (i.e., unfavorable conformation for phosphorylation). The binding of PtdIns(3,4,5)P3 to PH domain was shown to release this intramolecular constraint, thus making it a more favorable conformation. Given that SC79 acts as a PtdIns(3,4,5)P3 mimetic and induces an Akt conformational change (Fig. 1 D and E), our results also support such possibility. We further demonstrated that SC79-induced Akt activation could recapitulate the primary function of Akt signaling because it efficiently prevented the excitotoxicity-induced neuronal death both in vitro and in vivo. Although the activation of Akt signaling plays significant roles in protecting the ischemic-injury induced neuronal death, other cellular pathways and components are also important. Nevertheless, the SC79-induced Akt activation alone manifested a significant protection. Identifying other cellular pathways leading to synergistic protection in combination with SC79 would be important.
Deactivation of Akt contributes to pathogenesis of numerous neurological diseases (3–5) and, thus, elevating Akt activity becomes an obvious clinical strategy to suppress progressive neuronal death under these pathological conditions. However, this endeavor has been hindered because of the lack of specific Akt activators. Our results suggest that chemical mimetics of PtdIns(3,4,5)P3, such as SC79, could be explored to develop legitimate Akt activators. SC79 is relatively unstable in aqueous environment (SI Appendix, Fig. S13A). Intriguingly, however, after the removal of SC79, the sustained level of phosphorylated Akt was observed both in cell culture and in vivo (SI Appendix, Fig. S13 B and C), indicating that SC79 may act irreversibly. SC79 contains the chemical moieties (i.e., nitrile group) that could be modified and/or reacts with amino acids. Nevertheless, SC79 appeared to be a relatively safe drug. SC79 treatment, even at much high dose (0.4 mg/g of body weight), did not induce any detectable changes in body weight, survival rate, appearance, and behavior in mice (SI Appendix, Fig. S14). The fact that neuronal protective effect was achieved by i.p. injection suggests that SC79 also has a good penetration of blood–brain barrier. Therefore, SC79 can be used as a chemical platform to develop novel drugs for neurological and other complications (SI Appendix).
Materials and Methods
In this study, infarct volume was used to evaluate MCAO-induced brain damage. For histological examination of infracted area, mice were deeply anesthetized either 24 h or 1 wk after permanent MCAO by excess pentobarbital sodium (100 mg/kg). Upon removal, brains were sectioned coronally into five slices of 1 mm thickness starting from the frontal pole by using a mouse brain matrix (ASI Instruments). Slices were stained with 2% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC; Sigma) for 30 min at room temperature. Areas ipsilateral to the occlusion, which were not stained, were recorded as infarcted. After fixation with 4% paraformaldehyde/PBS, the unstained area of infarction was measured on the posterior surface of each coronal section by using an Image J system (Wayne Rasband, National Institutes of Health). Because of substantial hemispheric swelling after ischemia, the corrected infarct volume was calculated by using an indirect method to compensate for the effect of brain edema. The infarcted area of the ipsilateral (ischemic) hemisphere (II) was determined by subtracting the noninfarcted area of the ipsilateral hemisphere (IN) from the total area of the contralateral (uninfarcted) hemisphere (CT): II = CT − IN. Total infarct volume was then determined by multiplying the area of infarct for each slice by the slice thickness (1 mm) and summing for the seven brain slices.
Other materials and methods are presented in SI Appendix. Analysis of statistical significance for indicated datasets was performed by using the Student t test capability on Microsoft Excel.
Supplementary Material
Acknowledgments
The authors thank Solomon H. Snyder, Sangwon Kim, and Lewis C. Cantley for very helpful comments and suggestions on the manuscript; Leslie Silberstein, John Manis, and Li Chai for helpful discussions; Natsuko Fujii, Yusuke Moriya, Yoko Takahari for help with middle cerebral artery occlusion model; and Bo Chen and Kai Yao for intraocular injections. H.L. is supported by National Institutes of Health Grants HL085100, AI076471, HL092020, and GM076084.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202810109/-/DCSupplemental.
References
- 1.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: A hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 5.Dudek H, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 6.Harvey PA, Leinwand LA. The cell biology of disease: Cellular mechanisms of cardiomyopathy. J Cell Biol. 2011;194:355–365. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo R, et al. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int Rev Neurobiol. 2009;85:407–423. doi: 10.1016/S0074-7742(09)85028-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotter L, et al. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 2010;328:1415–1418. doi: 10.1126/science.1187735. [DOI] [PubMed] [Google Scholar]
- 10.Luo HR, et al. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates PtdIns(3,4,5)P3 signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad A, et al. Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)-trisphosphate signaling. Nat Immunol. 2011;12:752–760. doi: 10.1038/ni.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty A, et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razzini G, Brancaccio A, Lemmon MA, Guarnieri S, Falasca M. The role of the pleckstrin homology domain in membrane targeting and activation of phospholipase Cbeta(1) J Biol Chem. 2000;275:14873–14881. doi: 10.1074/jbc.275.20.14873. [DOI] [PubMed] [Google Scholar]
- 15.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 16.Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2009;113:1723–1729. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- 17.Han EK, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 18.Okuzumi T, et al. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5:484–493. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Tang H, Hay N, Xu J, Ye RD. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115:4237–4246. doi: 10.1182/blood-2009-11-255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park WS, et al. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell. 2008;30:381–392. doi: 10.1016/j.molcel.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 22.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 23.Yu SW, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 24.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 25.Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr Opin Pharmacol. 2003;3:101–109. [PubMed] [Google Scholar]
- 26.Luo HR, et al. Akt as a mediator of cell death. Proc Natl Acad Sci USA. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 28.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 30.Hillion JA, et al. Involvement of Akt in preconditioning-induced tolerance to ischemia in PC12 cells. J Cereb Blood Flow Metab. 2006;26:1323–1331. doi: 10.1038/sj.jcbfm.9600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyawaki T, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano S, et al. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:351–360. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Andjelković M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 34.Stokoe D, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 35.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: Structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.