Abstract
The lectin pathway of complement activation is an important component of the innate immune defense. The initiation complexes of the lectin pathway consist of a recognition molecule and associated serine proteases. Until now the autoactivating mannose-binding lectin-associated serine protease (MASP)-2 has been considered the autonomous initiator of the proteolytic cascade. The role of the much more abundant MASP-1 protease was controversial. Using unique, monospecific inhibitors against MASP-1 and MASP-2, we corrected the mechanism of lectin-pathway activation. In normal human serum, MASP-2 activation strictly depends on MASP-1. MASP-1 activates MASP-2 and, moreover, inhibition of MASP-1 prevents autoactivation of MASP-2. Furthermore we demonstrated that MASP-1 produces 60% of C2a responsible for C3 convertase formation.
Keywords: innate immunity, complement system, directed evolution, phage display, canonical inhibitor
The lectin pathway of the complement system serves as a first line of defense against microbial intruders. The innate immune system recognizes danger signals presented by the pathogens (pathogen-associated molecular patterns) or altered host cells (damage-associated molecular patterns) by means of germline-encoded cell-surface bound or soluble pattern recognition molecules (1, 2). These pattern recognition molecules have evolved against evolutionarily conserved structures of microorganisms, such as carbohydrates and acetylated compounds. The prompt action of the innate immune system provides sufficient time for the adaptive immune system to react with less conservative antigens (e.g., proteins) to build up a more specific response. In humans, five different humoral pattern recognition molecules have been identified that are able to initiate the lectin pathway: mannose-binding lectin (MBL) (3), three ficolins (M-, L-, and H-ficolin; also called ficolin-1, -2, and -3) (4), and collectin 11 (CL11 or CL-K1) (5). The pattern recognition molecules do not act alone; they are associated with other proteins, mainly serine proteases (6). These serine proteases [MBL-associated serine proteases (MASPs)] are present as proenzymes (zymogens) in the complexes and become activated to initiate the complement cascade when the recognition molecules bind to their target. Activation of the complement cascade culminates in the destruction and elimination of pathogens via opsonization or direct cell lysis. Although the lectin pathway was discovered some 20 years ago (7), the mechanism of the activation is still enigmatic. One of the most controversial issues is the role of the serine proteases. Up to now, three serine proteases have been discovered and designated as MASP-1, MASP-2, and MASP-3. In addition to the proteases, two nonenzymatic fragments of the MASPs, MAp44 (8, 9) and MAp19 (10), have also been found in the recognition complexes. MASP-1, MASP-3, and MAp44 are the alternative splice products of the MASP-1/3 gene, and MASP-2 and MAp19 are encoded by the MASP-2 gene. The only consensus point in the literature is that MASP-2 can autonomously initiate the complement cascade (11, 12). Zymogen MASP-2 can autoactivate and active MASP-2 cleaves complement proteins C4 and C2, the components of the C4b2a C3–convertase complex (13, 14). Although the concentration of MASP-2 in human plasma is relatively low (0.5 μg/mL), especially compared with the concentration of C1r and C1s, the initiator proteases of the analogous classical pathway (50 μg/mL), it can efficiently trigger the complement cascade (15). In the absence of MASP-2, the lectin pathway is not functional as was shown in the case of MASP-2–depleted human serum (16) and, in the case of MASP-2 knockout mouse (17). The role of the most abundant lectin-pathway protease, MASP-1, is rather controversial. MASP-1 can autoactivate, but it cannot initiate the complement cascade alone, because it can cleave C2 but not C4 (13, 18). Because the absence of MASP-1 does not prevent lectin-pathway activation, but rather causes a delay in the onset of the activation process, it was suggested that MASP-1 has only a supportive role in the lectin pathway (19).
Recently we have developed specific small (35 amino acid) protein inhibitors against MASP-1 and MASP-2 by using phage display. Starting with a canonical serine protease-inhibitor scaffold, in vitro evolution yielded SGMI-1 and SGMI-2, which are tight binding (having nanomolar Ki) monospecific inhibitors of MASP-1 and MASP-2, respectively. We have also solved the structure of the protease-inhibitor complexes (20).
In the present study we used the specific inhibitors to test their effect on the three activation pathways of complement in human serum. As expected, inhibition of MASP-2 by SGMI-2 selectively blocked the lectin pathway, leaving the classical and the alternative pathways intact and fully functional. However, to our great surprise, inhibition of MASP-1 by SGMI-1 also completely abolished the lectin-pathway activation. This result was unexpected and suggested that the current picture of lectin-pathway activation is not complete. To reveal the correct mechanisms of the lectin-pathway activation we further studied the role of MASP-1 and MASP-2 using the MASP-specific inhibitors. Our results show that MASP-1 is the professional activator of MASP-2 in normal human serum. We also demonstrated that MASP-1 produces 60% of the C2a component of the C3–convertases generated upon lectin-pathway activation.
Results
Selective Inhibition of MASP-1 Permanently Blocks Lectin-Pathway Activity.
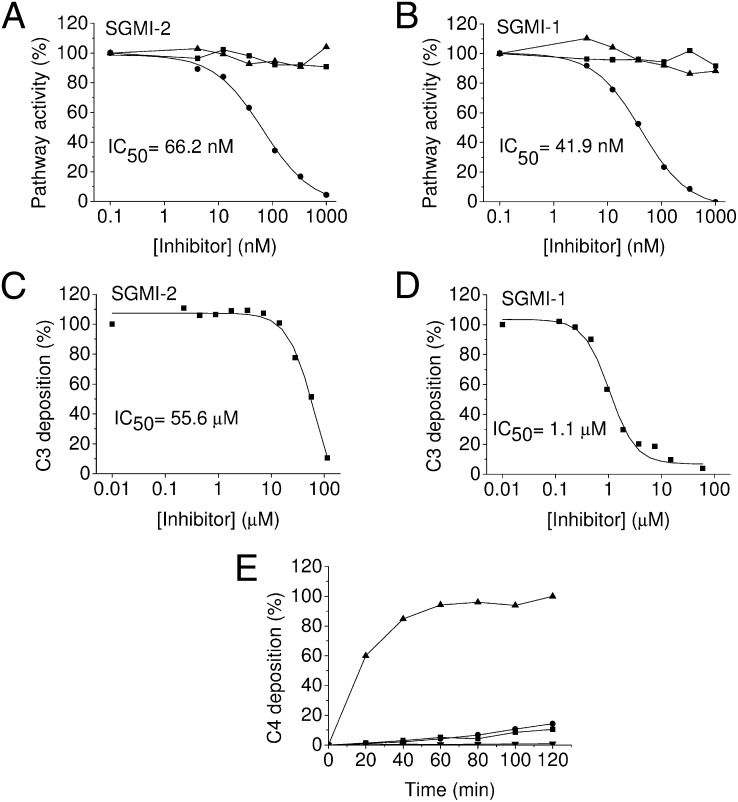
SGMI-1 and SGMI-2 specifically inhibit MASP-1 and MASP-2, respectively (20). To test the effect of these inhibitors on the three activation pathways of the complement system in pooled human serum, we applied the Wieslab complement assay. As was expected, inhibition of MASP-2 by SGMI-2 selectively blocked the lectin pathway (IC50 ≅ 66 nM), leaving the classical and the alternative pathways intact and fully functional (Fig. 1A). However, to our surprise, inhibition of MASP-1 by SGMI-1 also completely abolished the lectin-pathway activation (IC50 ≅ 42 nM) (Fig. 1B). We also demonstrated the inhibitory capacity of SGMI-1 and -2 on individual sera (Fig. S1). To confirm these results under near-physiological conditions, we tested the effect of our inhibitors in twofold diluted serum (Fig. S2) and in lepirudin (recombinant hirudin) treated whole blood (Fig. 1 C and D). Both SGMI-2 and SGMI-1 blocked the lectin-pathway-mediated C3 deposition efficiently even in these conditions. These results clearly suggest that the current picture of the activation mechanism of the lectin pathway needs to be revised as previously MASP-2 had been regarded as the autonomous activator of the lectin pathway.
Fig. 1.
Inhibitory effect of SGMI-1 and SGMI-2 on the complement pathways. (A), (B) The formation of the membrane attack complex was detected by using the Wieslab kit. The activity of the classical (■), lectin (●), and alternative (▲) pathways were measured by separate experiments. (A) Inhibition by SGMI-2. (B) Inhibition by SGMI-1. (C), (D) Inhibition of the lectin pathway mediated C3 deposition in lepirudin-treated whole blood. (C) Inhibition by SGMI-2. (D) Inhibition by SGMI-1. (E) The time course of the inhibitory effect of the MASP-1–specific SGMI-1 (●) and the MASP-2–specific SGMI-2 (■) on the MBL–lectin pathway was monitored by measuring C4 deposition. As control experiments, lectin-pathway activity was monitored in the absence of inhibitors (▲) and in the presence of a strong broad-spectrum serine protease inhibitor FUT-175 (▼).
In MASP-1 knockout mice there is lectin-pathway activity but the activation process is slower, which results in a delay in the onset. It was therefore of utmost importance to study the time course of the inhibitory effect of SGMI-1. We incubated normal human serum with a high concentration (5 μM) of SGMI-1 on a mannan-coated surface for two hours at 37 °C. At this concentration, SGMI-1 completely blocks MASP-1 but does not inhibit MASP-2. We measured the lectin-pathway activation by detecting C4 deposition. Because MASP-1 does not cleave C4, the assay measures the activity of MASP-2. As a control experiment, we incubated the serum with same concentration of the MASP-2–specific inhibitor (SGMI-2). Our results show that inhibition of MASP-1 permanently blocks lectin-pathway activation (Fig. 1E). Even after two hours of incubation both SGMI-1 and SGMI-2 prevented C4 deposition almost completely. These findings clearly demonstrate that the activation of the lectin pathway can be fully and permanently prevented by inhibiting either MASP-1 or MASP-2 in normal human serum.
SGMI-1 Inhibits MASP-1 in both MBL–MASP and Ficolin–MASP Complexes.
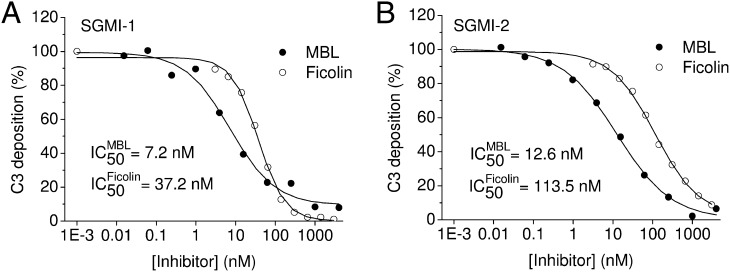
The pattern recognition molecules of the lectin pathway are MBL and ficolins. To compare the ability of SGMI-1 to prevent lectin-pathway activation through MBL–MASP and ficolin–MASP complexes, we used mannan and acetylated BSA (acBSA) as solid phase ligands for MBL and ficolin, respectively. The lectin-pathway activation was followed by measuring C3 deposition. The results of the C3 deposition assays show that SGMI-1 effectively inhibits the activation of the lectin pathway irrespective of whether the recognition complexes contain MBL or ficolins (Fig. 2A). Mannan binds MBL, and acBSA interacts predominantly with H-ficolin (ficolin-3) (21). The efficient inhibition of C3 deposition in both cases indicates that the mechanism of complement activation and its inhibition are similar in the case of MBL–MASP and ficolin–MASP complexes. We also confirmed that both MBL–MASP and ficolin–MASP complexes contain a sufficient amount of MASP-1, which plays a central role in triggering the lectin pathway. Inhibition of MASP-2 by the specific inhibitor SGMI-2 either in the MBL–MASP or in the ficolin–MASP complexes resulted in complete abolition of C3 deposition, as expected (Fig. 2B).
Fig. 2.
C3 deposition assay. (A) The MASP-1–specific SGMI-1 inhibits the lectin-pathway-mediated C3 deposition both on mannan-coated plates (●), as well as on acBSA-coated plates (○). (B) The MASP-2–specific SGMI-2 also inhibits the lectin pathway both on mannan-coated plates (●) and on acBSA-coated plates (○).
Inhibition of MASP-1 Prevents the Activation of MASP-2.
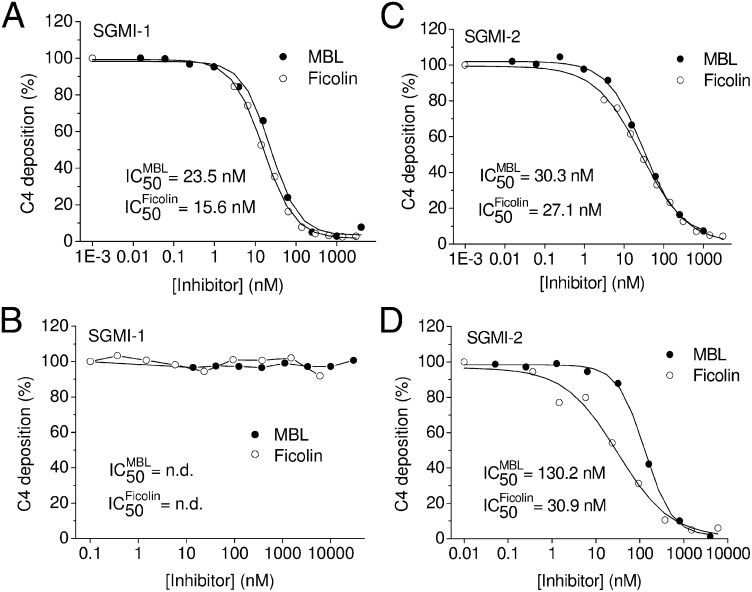
To further analyze the mechanism of the lectin-pathway activation and to reveal the role of MASP-1 in this process, we applied two types of C4 deposition experiments. In one type of assay, we measured the deposition of C4b directly from human serum where the MASPs were present initially as inactive proenzymes (zymogens). This setting was similar to the C3 deposition measurement. In the other type of C4 deposition assay, the MBL–MASP or ficolin–MASP complexes were first immobilized on surfaces coated with mannan or acBSA, respectively, where the proenzyme MASP-1 and MASP-2 were allowed to activate into their proteolytically active forms. Then we added purified C4 together with various concentrations of the inhibitor to the activated complexes and followed the deposition of C4b (22). In the first type of assay when we applied nonactivated serum, SGMI-1 prevented C4 deposition very efficiently in a dose-dependent manner on either mannan- or acBSA-coated surface (Fig. 3A). In the assay where the MASP-1 and MASP-2 were activated before adding purified C4 and inhibitor, the SGMI-1 inhibitor was completely ineffective (Fig. 3B). In other words, inhibition of MASP-1 in this case could not prevent the MASP-2–mediated cleavage and deposition of C4. On the other hand, C4 cleavage was readily blocked in both assays by applying the MASP-2–specific inhibitor (SGMI-2) (Fig. 3C and D).
Fig. 3.
C4 deposition assays. (A) MASP-1–selective SGMI-1 prevented deposition of C4 efficiently when the MBL–MASP (●) or H-ficolin–MASP (○) complexes from the serum were immobilized in the presence of the inhibitor and the direct C4 cleavage was detected. (B) SGMI-1 was totally ineffective when the immobilized MBL–MASP (●) or H-ficolin–MASP (○) complexes were let to activate before purified C4 and SGMI-1 were added. The MASP-2–specific inhibitor SGMI-2 efficiently prevents C4 deposition from both normal (C) and preactivated (D) serum in the case of both MBL–MASP (●) or H-ficolin–MASP (○) complexes.
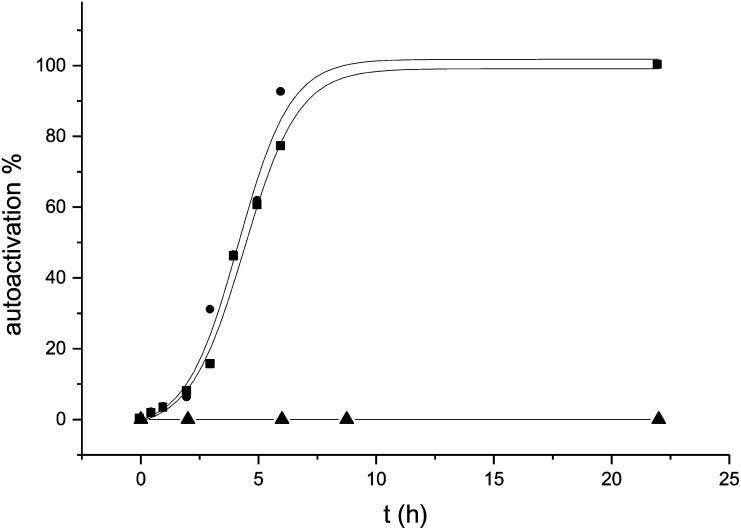
These results suggested that SGMI-1 precludes activation of MASP-2 by inhibiting MASP-1; therefore, active MASP-1 is needed for MASP-2 activation. To exclude the theoretically sound but unlikely possibility that SGMI-1 directly interacts with zymogen MASP-2, we tested the effect of SGMI-1 on the autoactivation of isolated zymogen MASP-2. The recombinant catalytic fragment of MASP-2, which contains the serine protease domain and the two complement control protein modules (CCP1–CCP2–SP), was prepared in zymogen form and the rate of autoactivation was followed at 37 °C. As Fig. 4 shows, SGMI-1 did not affect the autoactivation of zymogen MASP-2, even at a high concentration (1 μM). Similar concentration of the MASP-2–specific inhibitor (SGMI-2), however, completely impeded the autoactivation process.
Fig. 4.
Effect of SGMI-1 and SGMI-2 on the autoactivation of isolated MASP-2. The time course of autoactivation of isolated zymogen catalytic fragment of MASP-2 was studied in the presence or absence of inhibitors. At 1-μM inhibitor concentration, the MASP-2–specific SGMI-2 (▲) completely inhibited the autoactivation process, and the MASP-1–specific SGMI-1 (■) had no effect at all compared with the noninhibited sample (●).
The finding that MASP-1 is essential for activating MASP-2 is further supported by our kinetic measurements showing that MASP-1 activates zymogen MASP-2 about 20 times more efficiently than MASP-2 activates its own zymogen form (Fig. S3).
SGMI-1 and SGMI-2 Do Not Inhibit MASP-3.
Because MASP-3 is the second most abundant protease in the lectin pathway, and its physiological role is unknown, it was important to test whether the SGMIs inhibit MASP-3. Our results show that SGMI-1 does not inhibit MASP-3 even at high concentration, and SGMI-2 is a very poor inhibitor of this enzyme (Ki = 5.2 ± 0.3 μM). We can therefore state that MASP-3 inhibition did not bias the effects that our inhibitors exerted on the lectin pathway.
MASP-1 Generates Most of C2a Responsible for C3–Convertase Formation.
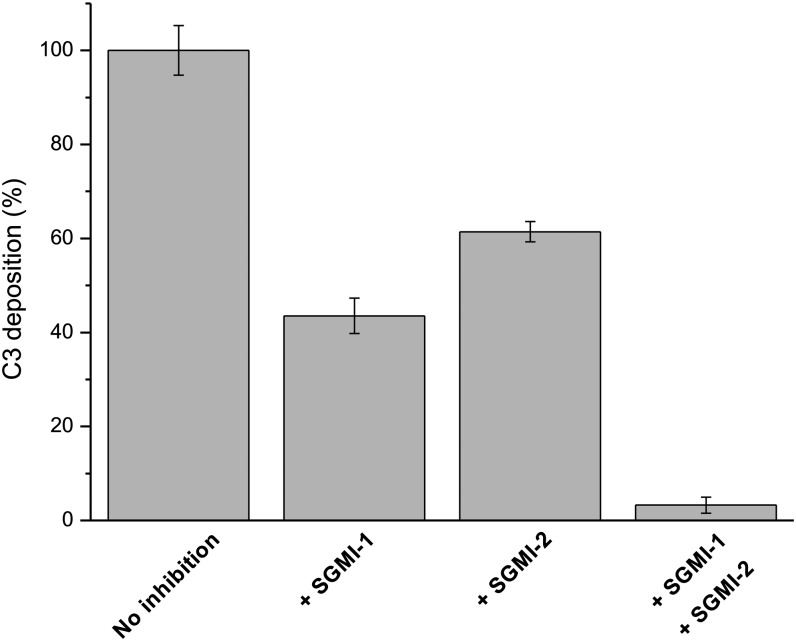
Because MASP-1 cleaves C2, it was proposed earlier that MASP-1 augments the C3–convertase (C4b2a) forming ability of MASP-2 (12, 16). Using our MASP-1–specific inhibitor we could test this hypothesis and, importantly, we could quantitatively determine the contribution of MASP-1. For this purpose we combined the C4 and C3 deposition assays. In the first step, we immobilized the MBL–MASP complexes from human serum on a mannan-coated surface and, after several washing steps, we added purified C4 and incubated it at 37 °C for one hour to make a C4b-saturated plate. Due to the C4b saturation of the plate, the limiting step for C3–convertase formation is the subsequent C2 cleavage. In the second step we added human serum again as a C2 and C3 source, with or without inhibitors, and measured the C3 deposition. The positive control was the serum without any inhibitor. As a negative control, we applied FUT-175, which is a strong broad-spectrum inhibitor of serum serine proteases.
By using the MASP-1–specific inhibitor in the second step of the assay, we measured the contribution of MASP-2 to the generation of the C2a component of C4b2a. This contribution was expressed in terms of percentage of the total (uninhibited) C3 deposition. In this case, the only C2 cleaving agent was MASP-2 and its contribution was 43.5% (Fig. 5). In the complementary experiment we used the MASP-2–specific inhibitor to determine the contribution of MASP-1 to generating the C2a component of the C3–convertase, and it proved to be 61%. When we used the MASP-1 and MASP-2–specific inhibitors together, the level of C3 deposition dropped to 3.3%, which equals the level of the negative control (FUT-175). Our results clearly indicate that MASP-1 contributes significantly to the C3–convertase formation in human serum as it generates about 60% of C2a responsible for C3 cleavage in the C4b2a enzyme complex.
Fig. 5.
Contribution of MASP-1 and MASP-2 to C3–convertase formation through C2 cleavage. The C2 cleaving ability of MASP-1 and MASP-2 was determined through measuring C3 deposition on C4b-saturated plate using the MASP-1– and MASP-2–specific inhibitors. When MASP-1 was inhibited by SGMI-1, the level of C3 deposition dropped to 43.5% of the noninhibited experiment. In accordance with this result, when MASP-2 was inhibited by SGMI-2, the level of C3 deposition dropped to 61%. The two independent measurements unequivocally show that MASP-1 is the major contributor of the C2 cleavage. When applying both inhibitors at the same time the level of C3 deposition is reduced to 3.3%.
SGMI-1 and SGMI-2 Have No Effect on Blood Coagulation.
Similarly to the complement, blood coagulation is also a complex system involving a large number of delicately regulated trypsin-like proteases. To obtain additional information on the level of selectivity of our inhibitors, we tested whether SGMI-1 or SGMI-2 interfere with blood clotting. Their inhibitory potential to slow down the coagulation process was tested by measuring their effects in three standard coagulation assays, the thrombin time (TT), prothrombin time (PT), and the activated partial thromboplastin time (APTT). SGMI-1 and SGMI-2 were applied at 5 μM, a concentration that is over 700-fold higher than the Ki values of SGMI-1 and SGMI-2 on MASP-1 and MASP-2, respectively, and about 100-fold higher than their lectin-pathway-inhibiting IC50 values (in Wieslab assay). However, even at this high concentration, the two SGMI variants did not slow down the blood clotting process. The corresponding data in terms of seconds are as follows: (TT)—control 23.2; SGMI-1 22.5; SGMI-2 22.9; (PT)—control 11.2; SGMI-1 11.0; SGMI-2 11.1; (APTT)—control 25.6; SGMI-1 26.0; SGMI-2 26.2. The three assays together imply that SGMI-1 and SGMI-2 do not inhibit any of the six blood coagulation proteases thrombin, fVIIa, fIXa, fXa, fXIa, and fXIIa.
Discussion
The first report about the MBL-initiated complement activation appeared more than twenty years ago (7). The discovery of the lectin pathway, the third route of complement activation, gave new impetus to the research and placed the complement system into its rightful position in the innate immunity. Although the overall scheme of complement activation in the lectin pathway seems to be very similar to that of the classical pathway, the role of the individual components is still enigmatic. We decided to clarify the role of the serine proteases by using phage display selected inhibitors. Previously we developed two peptide inhibitors against MASP-1 and MASP-2 using the sunflower trypsin inhibitor scaffold (23). The MASP-2–selective inhibitor (SFMI-2) blocked completely the lectin-pathway activation. This result was in concordance with the widely accepted view that MASP-2 is the key enzyme of the lectin pathway. The other peptide inhibitor (SFMI-1) was mainly MASP-1–specific, but it also inhibited MASP-2, although to a much lesser extent. This inhibitor was an even more efficient lectin-pathway inhibitor than the MASP-2–specific SFMI-2, which indicates that MASP-1 has some role in the activation process. Although the concentration of MASP-1 far exceeds that of MASP-2, only a supportive role was assigned to MASP-1 in the literature. To clarify the role of MASP-1, we developed a highly specific inhibitor, SGMI-1, using the SGPI protease-inhibitor scaffold.
SGMI-1 efficiently inhibits MASP-1, but it is totally ineffective on isolated active (20) or zymogen MASP-2. In consequence, it does not prevent autoactivation of isolated zymogen MASP-2. However, to our great surprise, SGMI-1 completely inhibited the lectin pathway, suggesting that it somehow prevented autoactivation of MASP-2 in normal human serum. This unequivocally showed that MASP-1 fully controls lectin-pathway activation. The question remained of how it works.
MASP-1 inhibition was ineffective in regards to blocking C4 deposition in complexes already containing activated MASP-2. We also showed that isolated MASP-1 activates zymogen MASP-2 about 20 times more efficiently than MASP-2 activates itself. Taking all these together led us to two fundamental conclusions. One is that MASP-1 directly and exclusively activates zymogen MASP-2 in normal human serum. The other is that, in normal human serum, the inherent autoactivating capacity of zymogen MASP-2 does not manifest. As explained below, we believe that the above conclusions are the two sides of the same coin.
Activation of zymogen MASP-2 by MASP-1 in normal human serum requires close proximity of these components (e.g., inside the recognition complex or on the surface of the pathogen). As the concentration of MASP-1 (11 μg/mL) (24) is about 20 times higher than that of MASP-2 (0.5 μg/mL), every zymogen MASP-2 molecule should be surrounded by multiple copies of MASP-1 molecules on the activator surface. This could explain our first conclusion, the efficient MASP-1–mediated MASP-2 activation in human serum.
The same setup could also explain our second conclusion. If the more abundant MASP-1 molecules topologically separate MASP-2 zymogens from one another, inhibition of MASP-1 should prevent autoactivation of MASP-2. Although this simple model powerfully explains our two major findings, only a detailed structural understanding of the surface-driven activation process could yield a more precise mechanistic explanation.
Nevertheless, let us consider what kind of functional consequences our simple model would predict for the entire removal of MASP-1 from the system as opposed to its in situ inhibition. A complete removal of MASP-1 would override the naturally isolated state of the MASP-2 zymogens allowing their autoactivation. This way the lectin pathway could activate, and the central role of MASP-1 would remain hidden. Note, however, that because MASP-2 is 20-fold less abundant than MASP-1 and because MASP-2 is a 20-fold less efficient MASP-2 activator than MASP-1, a significantly diminished pathway activation would be expected.
In fact, as listed below, exactly these phenomena were observed previously when MASP-1 was entirely removed from the serum. It was already suggested that MASP-1 facilitates the activation of MASP-2 but it was also stated that MASP-1 is not essential because, in the absence of MASP-1, MASP-2 can initiate the lectin pathway (19). To this end, all previous studies defined MASP-2 as the single key enzyme claiming that the lectin pathway is functional without MASP-1 (17, 19).
It was previously shown in vitro that isolated MASP-2 is capable of autoactivation and the initiation complex reconstituted from recombinant MBL and recombinant MASP-2 can initiate the complement cascade through C3–convertase generation (11, 14). As a consequence, there is lectin-pathway activity in the MASP-1–depleted human serum and in the serum of MASP-1 knockout mouse, although it is diminished compared with the activity of the intact serum and there is a significant delay in activation of MASP-2 (16, 19). On the other hand, no C3 deposition was observed on the mannan-coated surface using serum from MASP-2 knockout mouse (17). These observations, indeed, logically pointed to the key role of MASP-2 and the inferior role of MASP-1. However, as we already noted, our simple model is fully coherent with all of the above findings despite their virtual contradiction with our present results.
Our MASP-1 selective inhibitor proved to be a unique research tool. It allowed for the studying of the role of MASP-1 in intact human serum and ex vivo in whole-blood, experimental settings much closer to normal human physiology than the knockout mouse or the manipulated (depleted) human sera. As already explained, we argue that this important difference in experimental setup is fully responsible for the substantial discrepancy between the previous findings and conclusion and those we present here.
Another important achievement of the current study is that we quantified the contribution of MASP-1 to the C3–convertase formation via its C2 cleaving ability. Because MASP-1 cleaves C2, it is not surprising that it enhances the ability of MASP-2 to generate C4b2a. The unexpected finding is that MASP-1 is the main contributor to this process, as it is responsible for the generation of about 60% of the total C4b2a complexes. Although MASP-1 cleaves C2 less efficiently than MASP-2 (13), its concentration far exceeds that of MASP-2 (24). Our results are in accord with that of Møller-Kristensen et al. (16), who showed that MASP-1 and MASP-2 act synergistically in C3b deposition in human serum. The cooperation between MASP-1 and MASP-2 can explain the observation that although every C4b deposited by MBL–MASPs complex can form C3–convertase, only one out of four C4b deposited by the classical pathway C1 complex can do the same (25). Based on our model, MASP-2–generated C4b should land on a nearby surface abundant in MASP-1 molecules, which could then very efficiently provide the C2a component for the C4b2a C3–convertase.
Our results also demonstrated that both the MBL–MASP and the ficolin–MASP complexes contain MASP-1, and the inhibition of MASP-1 results in complete arrest of the lectin-pathway activation in both cases. We conclude that the structure of the MBL–MASP and the ficolin-MASP complexes are very similar and their activation mechanism is the same. Note that we measured the complement fixing ability of the H-ficolin complexes (21). The L- and M-ficolins can also activate complement on appropriate targets (26). We can assume that the same activation mechanism is also valid at these complexes; however, more specific assays are needed to prove this.
Besides MASP-1 and -2, other MASP-related proteins (MASP-3, MAp44, and MAp19) are also present on the recognition complexes. It might be possible that these components could decrease the efficiency of the activation process, but at least in the case of MBL and H-ficolin, they do not liberate MASP-2 from the control of MASP-1.
In fact, our findings show that MASP-1 entirely controls the activation of MASP-2 and, through this, the lectin pathway (Fig. 6). This demonstrates that MASP-1 is an essential central component of the innate immune system. We showed that both MASP-1 and MASP-2 are indispensable for a quick and strong response against invading pathogens or altered host structures via the initiation of the lectin pathway in humans.
Fig. 6.
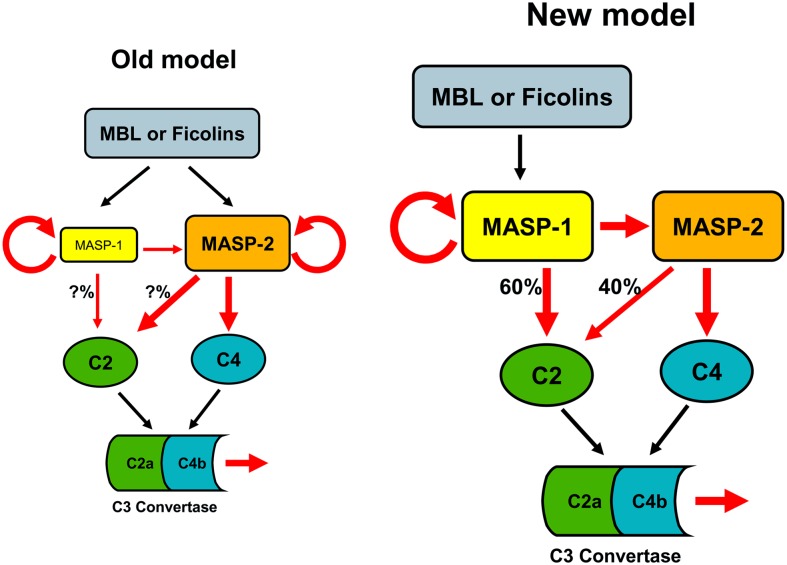
Comparison of the old model and our corrected (new) model of the lectin-pathway activation. According to the old model, MASP-2 is the autonomous activator of the lectin pathway, because it can autoactivate and cleave C4 and C2, while MASP-1 has only supportive role. In contrast to this, our model states that MASP-1 controls the entire activation process. MASP-1 autoactivates and cleaves zymogen MASP-2. The active MASP-2 then cleaves C4 and the deposited C4b binds C2, which is then cleaved primarily (60%) by MASP-1 and to a lesser extent (40%) by MASP-2. This results in the formation of the C4b2a complex. Red arrows indicate proteolytic cleavage.
Uncontrolled activation of the complement system results in serious self-tissue damage in numerous diseases, such as in the case of ischemia–reperfusion (IR) injury (e.g., myocardial infarction, stroke) (27, 28). Complement proteins have recently become the targets of drug development (29). A very recent study shows that in MASP-2 knockout mice, MASP-2 deficiency lessens myocardial IR injury and in wild-type mice anti-MASP-2 antibody reduces gastrointestinal IR injury (17). MASP-2 therefore is a relevant target in drug development against IR injury.
A major practical significance of our results is that MASP-1 is an equally relevant target, because it tightly controls MASP-2 activation. Transient inhibition of MASP-1 may protect from the harmful consequences of IR injury, which should be an attractive therapeutic approach.
Finally, a general conclusion is that complete removal of a protein (via KO animals or depletion) versus in situ inhibition of the same protein (in in vitro assays or in wild-type animals) can lead to contradicting effects. Whenever this happens, a new functional model has to be established that is coherent with the observations of both experimental arrangements. Without that, the real function of the protein remains controversial.
Materials and Methods
Complement activity was measured using the Wieslab kit (30), ELISA-type C3 and C4 deposition assays on diluted serum (21–23), and also on twofold diluted serum. The autoactivation of isolated MASP-2 catalytic fragment was measured by SDS/PAGE densitometry. Standard blood coagulation assays were applied. The activity of MASP-3 was measured on synthetic substrate. The details of these procedures are described in SI Text.
Ex Vivo Whole-Blood Experiments.
Whole blood was drawn from a healthy human donor into plastic syringes containing the anticoagulant lepirudin (Refludan) at a final concentration of 50 μg/mL To selectively measure the lectin-pathway activation we applied the compound sodium polyanethole sulfonate (SPS) which, at an appropriate concentration, inhibits the classical and the alternative pathway but does not influence the lectin pathway (21, 31). Our preliminary experiments demonstrated that SPS in a final concentration of 120 μg/mL inhibited complement activation in 1:1 diluted whole blood on IgG-coated (classical pathway) and on LPS-coated (alternative pathway) plates, while the lectin-pathway activation on a mannan-coated surface was efficient. To measure the inhibitory effects of SGMI-1 and -2 the collected whole blood was diluted (1:1) with a solution of SPS and the SGMI inhibitors in barbital buffer (4 mM barbiturate, 145 mM NaCl, 0.5 mM MgCl2, 2 mM CaCl2, and 0.1% Tween 20, pH 7.4). Formerly, high binding microtiter plates were coated with 5 μg/mL mannan and then blocked with 1% BSA in TBS. The lepirudin-treated whole-blood samples containing the inhibitors were immediately applied to the plates and complement activation was allowed for 20 min at 37 °C under continuous shaking (400 rpm). After extensive washing, antihuman C3c antibody (1:30,000) (Dako A0062) was added to the wells and incubated for 40 min at 37 °C. The plates were developed as described earlier (23).
Quantitative Determination of the Contribution of MASP-1 and -2 to the C3–Convertase Formation Through C2 Cleavage.
The assay combines the C4 and the C3 deposition measurements. First, C4-saturated wells are produced so that the limiting step for C3–convertase formation is the C2 cleavage. Then human serum is added to the C4-saturated wells as C2 and C3 source with or without the inhibitors. Finally, the extent of C3 deposition, which depends on the amount of cleaved C2, is detected in the wells. Mannan-coated wells were incubated with 100 μl of pooled human serum diluted 1:1 in 40 mM Hepes, 2 M NaCl, and 10 mM CaCl2, pH 7.4 (high salt serum dilution buffer) for 1 h at 4 °C. The wells were then washed three times with 300 μL of high salt wash buffer (20 mM Hepes, 1 M NaCl, 5 mM CaCl2, and 0.1% Tween-20, pH 7.4) and three times with 300 μL of normal wash buffer (20 mM Hepes, 5 mM CaCl2, and 0.1% Tween-20, pH 7.4) at 37 °C. Purified C4 (0.4 μg/well) in 100 μL wash buffer was added to the wells and incubated for 1 h at 37 °C. Serum samples were diluted 100 times and SGMI inhibitors were added at a concentration of 3 μM. Following three further washes with wash buffer, serum samples (100 μL) with or without the inhibitors were applied to the wells and incubated for 30 min at 37 °C. After three washes, the deposited C3 was detected as described in the C3 deposition assay.
Supplementary Material
Acknowledgments
The authors thank Dr. Miklós Sahin-Tóth for critical reading of the manuscripts and for his valuable suggestions. This work was supported by Ányos Jedlik Grant NKFP_07_1-MASPOK07; Hungarian Scientific Research Fund (OTKA) Grants NK77978, NK100769, NK100834, K68408, and NK81950; National Development Agency Grant KMOP-1.1.2-07/1-2008-0003; as well as by European Union and the European Social Fund (TÁMOP) Grant 4.2.1./B-09/KMR-2010-0003. We thank Ms. Júlia Balczer and Mr. Dániel Datz for protein purification.
Footnotes
Conflict of interest statement: D.H., P.G., G.P., and P.Z. filed patent application for the SGMI inhibitors.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202588109/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: Past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo Y, Matsushita M, Fujita T. The role of ficolins in the lectin pathway of innate immunity. Int J Biochem Cell Biol. 2011;43:705–712. doi: 10.1016/j.biocel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Hansen S, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol. 2010;185:6096–6104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 6.Gál P, Dobó J, Závodszky P, Sim RB. Early complement proteases: C1r, C1s and MASPs. A structural insight into activation and functions. Mol Immunol. 2009;46:2745–2752. doi: 10.1016/j.molimm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987;262:7451–7454. [PubMed] [Google Scholar]
- 8.Degn SE, et al. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J Immunol. 2009;183:7371–7378. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 9.Skjoedt MO, et al. A novel mannose-binding lectin/ficolin-associated protein is highly expressed in heart and skeletal muscle tissues and inhibits complement activation. J Biol Chem. 2010;285:8234–8243. doi: 10.1074/jbc.M109.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover CM, et al. Two constituents of the initiation complex of the mannan-binding lectin activation pathway of complement are encoded by a single structural gene. J Immunol. 1999;162:3481–3490. [PubMed] [Google Scholar]
- 11.Vorup-Jensen T, et al. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–2100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 12.Chen CB, Wallis R. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J Biol Chem. 2004;279:26058–26065. doi: 10.1074/jbc.M401318200. [DOI] [PubMed] [Google Scholar]
- 13.Ambrus G, et al. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: A study on recombinant catalytic fragments. J Immunol. 2003;170:1374–1382. doi: 10.4049/jimmunol.170.3.1374. [DOI] [PubMed] [Google Scholar]
- 14.Gál P, et al. A true autoactivating enzyme. Structural insight into mannose-binding lectin-associated serine protease-2 activations. J Biol Chem. 2005;280:33435–33444. doi: 10.1074/jbc.M506051200. [DOI] [PubMed] [Google Scholar]
- 15.Møller-Kristensen M, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–167. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Møller-Kristensen M, Thiel S, Sjöholm A, Matsushita M, Jensenius JC. Cooperation between MASP-1 and MASP-2 in the generation of C3 convertase through the MBL pathway. Int Immunol. 2007;19:141–149. doi: 10.1093/intimm/dxl131. [DOI] [PubMed] [Google Scholar]
- 17.Schwaeble WJ, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi V, et al. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J Biol Chem. 2001;276:40880–40887. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, et al. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J Immunol. 2008;180:6132–6138. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 20.Héja D, et al. Monospecific inhibitors show that both mannan-binding lectin-associated serine protease (MASP)-1 and -2 are essential for lectin pathway activation and reveal structural plasticity of MASP-2. J Biol Chem. 2012;287:20290–20300. doi: 10.1074/jbc.M112.354332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hein E, et al. Functional analysis of Ficolin-3 mediated complement activation. PLoS ONE. 2010;5:e15443. doi: 10.1371/journal.pone.0015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 23.Kocsis A, et al. Selective inhibition of the lectin pathway of complement with phage display selected peptides against mannose-binding lectin-associated serine protease (MASP)-1 and -2: significant contribution of MASP-1 to lectin pathway activation. J Immunol. 2010;185:4169–4178. doi: 10.4049/jimmunol.1001819. [DOI] [PubMed] [Google Scholar]
- 24.Thiel S, et al. MASP-1, a serine protease associated with humoral pattern-recognition molecules: normal and acute-phase levels in serum and stoichiometry of lectin pathway components. Clin Exp Immunol. 2012;169:38–48. doi: 10.1111/j.1365-2249.2012.04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawal N, Rajagopalan R, Salvi VP. Activation of complement component C5: Comparison of C5 convertases of the lectin pathway and the classical pathway of complement. J Biol Chem. 2008;283:7853–7863. doi: 10.1074/jbc.M707591200. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun. 2010;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 29.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seelen MA, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Palarasah Y, et al. Sodium polyanethole sulfonate as an inhibitor of activation of complement function in blood culture systems. J Clin Microbiol. 2010;48:908–914. doi: 10.1128/JCM.01985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.