Abstract
Infarction occurs when myocardial perfusion is interrupted for prolonged periods of time. Short episodes of ischemia and reperfusion protect against tissue injury when the heart is subjected to a subsequent prolonged ischemic episode, a phenomenon known as ischemic preconditioning (IPC). Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that mediates adaptive responses to hypoxia/ischemia and is required for IPC. In this study, we performed a cellular and molecular characterization of the role of HIF-1 in IPC. We analyzed mice with knockout of HIF-1α or HIF-1β in Tie2+ lineage cells, which include bone marrow (BM) and vascular endothelial cells, compared with control littermates. Hearts were subjected to 30 min of ischemia and 120 min of reperfusion, either as ex vivo Langendorff preparations or by in situ occlusion of the left anterior descending artery. The IPC stimulus consisted of two cycles of 5-min ischemia and 5-min reperfusion. Mice lacking HIF-1α or HIF-1β in Tie2+ lineage cells showed complete absence of protection induced by IPC, whereas significant protection was induced by adenosine infusion. Treatment of mice with a HIF-1 inhibitor (digoxin or acriflavine) 4 h before Langendorff perfusion resulted in loss of IPC, as did administration of acriflavine directly into the perfusate immediately before IPC. We conclude that HIF-1 activity in endothelial cells is required for acute IPC. Expression and dimerization of the HIF-1α and HIF-1β subunits is required, suggesting that the heterodimer is functioning as a transcriptional activator, despite the acute nature of the response.
Keywords: CD39, CD73, coronary artery disease, myocardial infarction, oxygen
The heart requires a constant supply of O2 for generation of ATP, 95% of which is derived from oxidative phosphorylation (1). Coronary artery stenosis due to an atherosclerotic plaque results in reduced perfusion and myocardial ischemia, especially under conditions of increased myocardial O2 demand, as occurs when heart work is increased in response to physical exertion or emotional stress. Plaque rupture is a catastrophic event that results in complete arterial occlusion and, within ∼20 min, the onset of progressive death of cardiac cells due to O2 deprivation (2). Rapid reperfusion after ischemia limits infarct size, while at the same time reperfusion contributes to tissue injury (3).
Exposure of the heart to short (5-min) episodes of ischemia and reperfusion protects the heart against injury caused by a subsequent prolonged episode of ischemia and reperfusion (IR), a phenomenon known as ischemic preconditioning (IPC) (4). The protection against myocardial injury following IR that is afforded by IPC (“cardioprotection”) consists of an acute/early phase, with onset immediately following the IPC stimulus and a duration of protection lasting several hours (4), followed by a delayed/late phase, with onset ∼24 h after the IPC stimulus and a duration of protection lasting several days (5, 6). According to the prevailing paradigm, molecular events during the early phase consist of posttranslational modifications of preexisting proteins, whereas the late phase involves de novo gene expression and protein synthesis (7–9). A corollary of the prevailing paradigm is that many of these molecular events are thought to occur within cardiomyocytes, although the role of endothelial cells (ECs) in IPC has been investigated to a much more limited extent (10).
Hypoxia-inducible factor 1 (HIF-1) is a transcription factor, which functions as a master regulator of adaptive responses to reduced O2 availability (11). HIF-1 regulates both O2 delivery, through effects on vascular growth and function, and O2 utilization, by determining the balance between oxidative and glycolytic metabolism (8, 12, 13). HIF-1 is a heterodimer consisting of HIF-1α and HIF-1β subunits (14, 15). HIF-1α is the O2-regulated subunit that is specific to HIF-1, whereas HIF-1β is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) because it can also dimerize with the aryl hydrocarbon receptor (16). HIF-1α is subjected to O2-dependent modification by the prolyl hydroxylase PHD2, which targets the protein for ubiquitination and proteasomal degradation under normoxic conditions, whereas these events are inhibited under conditions of continuous hypoxia (11, 12, 17). Cycles of hypoxia and reoxygenation also potently increase HIF-1α protein levels and HIF-1 transcriptional activity (18–21). HIF-1α activation has been demonstrated in human hearts under conditions of myocardial ischemia and infarction (22) and patients with coronary artery disease who carry genetic polymorphisms at the human HIF1A locus are more likely to present to medical attention with stable angina rather than with myocardial infarction (23) and are less likely to have coronary collaterals (24).
Mice that are homozygous for a knockout allele at the Hif1a locus die at midgestation with major cardiac malformations (25–27). Hif1a+/− mice, which are heterozygous for the knockout allele, develop normally but the acute protective effects of IPC are completely absent in the hearts of these mice (28). Infusion of small interfering RNA (siRNA) targeting HIF-1α mRNA into the left ventricle of wild-type mice also abolished the acute cardioprotective effects of IPC, whereas siRNA targeting PHD2 mRNA induced cardioprotection in the absence of IPC (29). HIF-1 is likely to activate the expression of multiple pathways that contribute to cardioprotection (9, 13). Among these, HIF-1–dependent adenosine signaling was implicated as an important mechanism by which HIF-1 may mediate the protective effects of IPC (3, 29). Consistent with this hypothesis, infusion of adenosine into Hif1a+/− hearts induced significant protection against IR injury (28). Mice with reduced expression of PHD2 in the heart are protected against myocardial injury after IR in the absence of IPC (29, 30), as are wild-type mice treated with the prolyl hydroxylase inhibitor dimethyloxalylglycine (29).
The finding that the O2-dependent subunit of HIF-1 was required for the acute/early protective effects of IPC was unexpected on the basis of the prevailing paradigm of early- vs. late-phase cardioprotection. Several mechanisms could be invoked to explain these surprising data. First, basal HIF-1 activity under normoxic conditions might be required for the transcription of genes encoding proteins that are subject to posttranslational modification during IPC. According to this model, the induction of HIF-1 transcriptional activity would not be required during IPC. Second, HIF-1α induced by IPC might bind to one or more proteins and regulate their activity. Under this model, the effects of HIF-1α would be independent of its dimerization with HIF-1β and its known role as a transcription factor. Third, HIF-1 transcriptional activity induced by IPC might lead to the expression of target genes that are critical for cardioprotection. In addition to uncertainty regarding the molecular mechanism of action, there are no data regarding the cardiac cell type(s) in which HIF-1α expression is required for cardioprotection. In this study we have performed experiments to further delineate the molecular and cellular mechanisms by which HIF-1α contributes to cardioprotection induced by IPC.
Results
Effects of IPC on Hif1a+/− Hearts Subjected to Global Ischemia ex Vivo or Coronary Artery Occlusion in Situ.
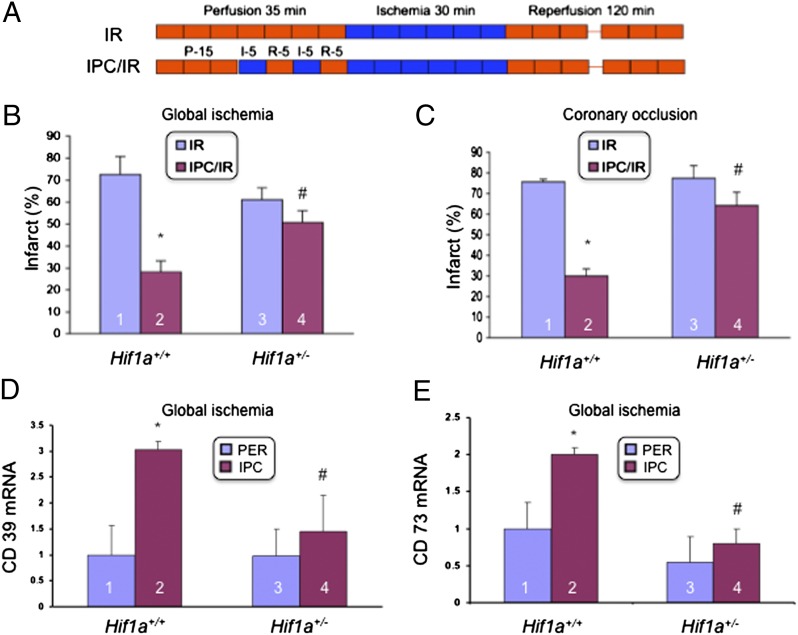
Our previous study involved the analysis of isolated Langendorff-perfused mouse hearts (28). In this system, when perfusion of the heart with buffer containing O2 and glucose is stopped, leading to global ischemia, the heart stops beating. Using littermate mice, we directly compared the results obtained with this model to those obtained with an in situ model of coronary artery occlusion, in which the heart continues to beat during the ischemia, which is restricted to the myocardium perfused by the left anterior descending coronary artery. In both models, hearts were subjected to 30 min of ischemia followed by 120 min of reperfusion, either alone (IR; Fig. 1A) or preceded by two cycles of 5-min ischemia/5-min reperfusion (IPC/IR; Fig. 1A). In the Langendorff perfusion model, we found that IPC significantly reduced myocardial infarct size in Hif1a+/+ (wild type) but not in Hif1a+/− hearts (Fig. 1B). These data replicate results that we have previously reported (28). In the coronary artery occlusion model, we obtained remarkably similar results (Fig. 1C), demonstrating that the dependence on HIF-1α was not an artifact of ex vivo perfusion. Next, we analyzed the expression of HIF-1 target genes encoding CD39 and CD73, which catalyze the conversion of ATP to adenosine and have been implicated in the cardioprotection induced by IPC (30). Both CD39 (Fig. 1D) and CD73 (Fig. 1E) mRNA levels were significantly increased after IPC in wild-type hearts but not in Hif1a+/− hearts.
Fig. 1.
Loss of the cardioprotective effect of ischemic preconditioning in Hif1a+/− mice. (A) Hearts were subjected to 30 min of ischemia and 120 min of reperfusion, either alone (IR) or preceded by ischemic preconditioning (IPC), consisting of two cycles of 5 min of ischemia and 5 min of reperfusion (IPC/IR). (B and C) Effect of IPC on infarct size (infarct area/total area at risk × 100%; mean ± SD, n = 3 hearts in each group), as determined by staining with 1% triphenyltetrazolium chloride (TTC), was analyzed in ex vivo Langendorff-perfused hearts (B) or in hearts subjected to in situ coronary artery occlusion (C). *P < 0.01 vs. column 1; #P < 0.01 vs. column 2 (Student’s t test). (D and E) Hearts isolated from Hif1a+/+ and Hif1a+/− mice were either subjected to IPC or perfused continuously (PER) for 40 min and total RNA was isolated and analyzed for the expression of CD39 (D) and CD73 (E) mRNA by quantitative real-time RT-PCR (mean ± SEM; n = 5 hearts in each group). *P < 0.05 vs. column 1; #P < 0.05 vs. column 2 (Student’s t test).
Generation and Analysis of Mice Lacking HIF-1α or HIF-1β in Tie2+ Lineage Cells.
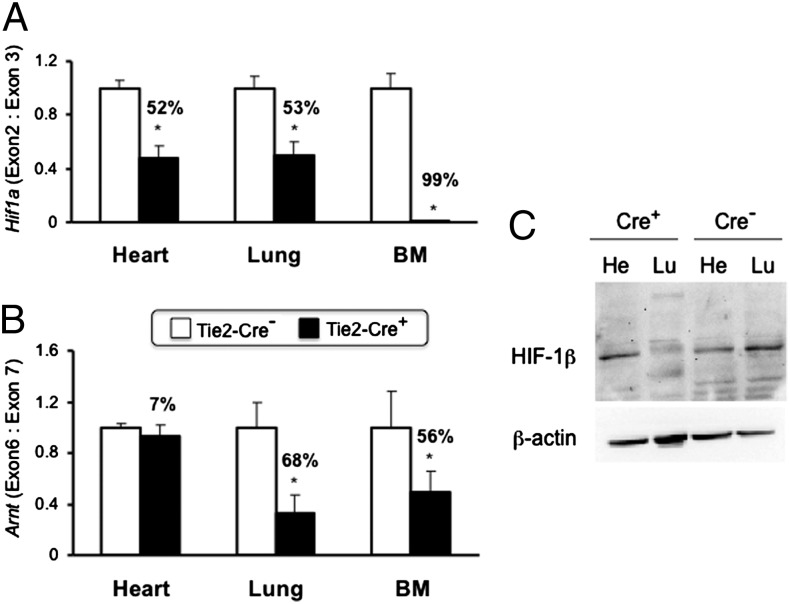
We next used mice that were homozygous for a floxed allele at the locus encoding HIF-1α (Hif1af/f) and also carried (Tie2-Cre+) or lacked (Tie2-Cre−) a transgene in which expression of Cre recombinase was driven by the Tie2 gene promoter (31). We performed matings of Hif1af/f; Tie2-Cre− female mice with Hif1af/f; Tie2-Cre+ male mice. Expression of Cre in Tie2+ lineage cells [ECs and bone marrow (BM) cells] results in deletion of the floxed Hif1a exon 2, thereby disrupting the HIF-1α coding sequence (32, 33). Deletion of Hif1a exon 2 was extremely efficient in BM cells and in lung tissue, in which ECs comprise approximately half of the total cell population (Fig. 2A). Surprisingly, almost half of all cells in the heart also showed deletion of Hif1a exon 2. We also analyzed Arntf/f; Tie2-Cre− and Arntf/f; Tie2-Cre+ littermate mice (34) and found that the floxed Arnt exon 6 was deleted with high efficiency in the lung, at reduced efficiency in BM, and at very low efficiency in the heart (Fig. 2B). The data from heart and lung tissue were confirmed at the level of HIF-1β protein expression (Fig. 2C) and were consistent with the extent to which ECs contribute to the total cell population in these organs. Specifically, ECs represent a minor cell population in the heart, which accounts for the low efficiency of Arnt gene deletion in the heart (7%) and the failure to detect any reduction in HIF-1β protein levels in the hearts of Arntf/f; Tie2-Cre+ compared with Arntf/f; Tie2-Cre− mice.
Fig. 2.
Analysis of gene deletion in conditional knockout mice. (A and B) DNA was isolated from heart, lung, and bone marrow of Hif1af/f (A) and Arntf/f (B) mice that were either Tie2-Cre− (open bars) or Tie2-Cre+ (closed bars). Efficiency of gene deletion was determined by quantitative real-time PCR as the ratio of the targeted exon to the next downstream exon × 100%. *P < 0.05 vs. Tie2-Cre− (n = 4–6; Student’s t test on log-converted ratios). (C) Immunoblot assay of HIF-1β and β-actin protein levels in heart (He) and lung (Lu) tissue lysates from Arntf/f; Tie2-Cre+ and Arntf/f; Tie2-Cre− mice.
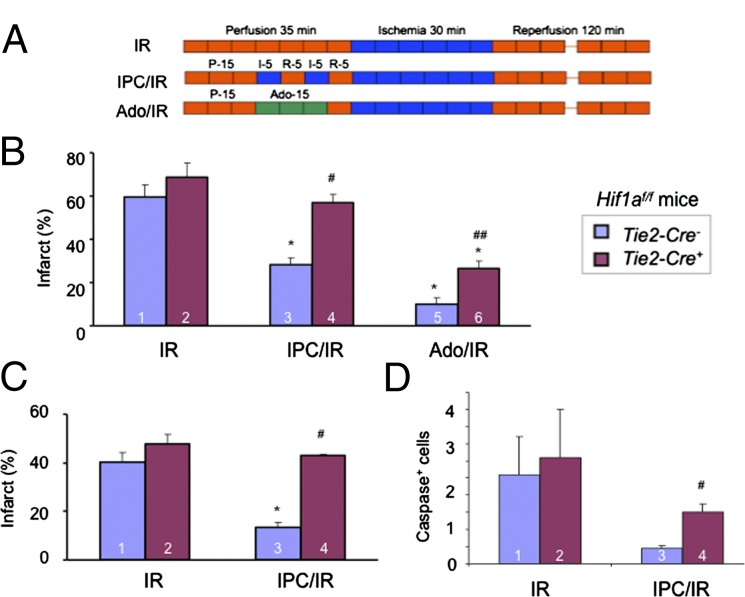
Analysis of infarct size in Langendorff-perfused hearts from Hif1af/f; Tie2-Cre− mice subjected to IR or IPC/IR (Fig. 3A) revealed significant cardioprotection afforded by IPC, an effect that was absent in hearts from Hif1af/f; Tie2-Cre+ mice (Fig. 3B and Fig. S1A). In contrast, adenosine perfusion immediately before IR (Fig. 3A) protected hearts from both Tie2-Cre− and Tie2-Cre+ mice, although infarct size was significantly smaller in Tie2-Cre− hearts (Fig. 3B and Fig. S1A). The observed effects of IPC and adenosine on Tie2-Cre− vs. Tie2-Cre+ mice are remarkably similar to those reported for Hif1a+/+ vs. Hif1a+/− mice (Fig. 1B and ref. 28). The loss of IPC-induced acute-phase cardioprotection was also observed in the hearts of Tie2-Cre+ mice subjected to in situ coronary artery occlusion (Fig. 3C and Fig. S1B), which was again similar to Hif1a+/− mice (Fig. 1C). The number of apoptotic cells identified by the presence of activated caspase 3 was significantly greater in Hif1af/f; Tie2-Cre+ compared with Hif1af/f; Tie2-Cre− hearts after IPC/IR (Fig. 3D), as previously reported for Hif1a+/− vs. Hif1a+/+ hearts (28).
Fig. 3.
Effect of IPC or adenosine perfusion in hearts of Hif1af/f;Tie2-Cre mice. (A–C) Hearts were subjected to 30 min of ischemia and 120 min of reperfusion (IR), ischemic preconditioning consisting of two cycles of 5-min ischemia + 5-min reperfusion followed by IR (IPC/IR), or 200-μM adenosine infusion for 15 min followed by IR (Ado/IR) in either the ex vivo Langendorff perfusion (B) or in situ coronary artery occlusion (C) model. Infarct size (mean ± SEM) was determined by TTC staining. *P < 0.05 vs. column 1; #P < 0.05 vs. column 3; ##P < 0.05 vs. column 5 (n = 3–4, Student’s t test). (D) Hearts were subjected to IR and IPC/IR as described except that reperfusion was for 45 min rather than 120 min and heart sections were analyzed by immunohistochemistry using an antibody specific for cleaved (activated) caspase 3. #P < 0.05 vs. column 3 (n = 3; Student’s t test).
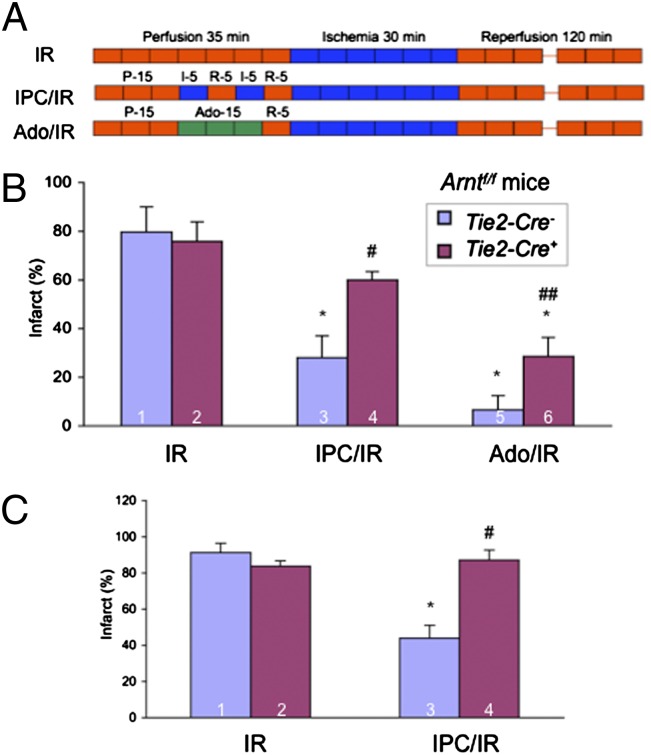
The Langendorff preparations were perfused with buffer rather than blood, suggesting that loss of HIF-1α expression in BM-derived cells was unlikely to play an important role in the loss of acute cardioprotection in Hif1af/f; Tie2-Cre+ hearts. However, because of the unexpected Hif1a deletion in ∼50% of cardiomyocytes in Hif1af/f; Tie2-Cre+ hearts (35), we could not distinguish between a role for HIF-1α in ECs vs. cardiomyocytes. To resolve this uncertainty and to determine whether HIF-1β was also required for cardioprotection, we analyzed Arntf/f; Tie2-Cre+ mice. Remarkably, Arntf/f; Tie2-Cre+ hearts (in which HIF-1β was expressed at normal levels in cardiomyocytes as shown in Fig. 2 B and C) also showed a complete lack of cardioprotection after IPC in both Langendorff perfusion and coronary artery occlusion models (Fig. 4). Thus, the inactivation of the Arnt gene specifically in ECs, representing ∼7% of the cells in the heart, resulted in a loss of IPC-mediated cardioprotection. Both Arntf/f; Tie2-Cre+ and Arntf/f; Tie2-Cre− hearts showed a significant reduction in infarct size after adenosine perfusion, although the protection was significantly greater in Arntf/f; Tie2-Cre− hearts.
Fig. 4.
Effect of IPC or adenosine perfusion in hearts of Arntf/f; Tie2-Cre mice. (A–C) Hearts were subjected to 30 min of ischemia and 120 min of reperfusion (IR), ischemic preconditioning consisting of two cycles of 5-min ischemia + 5-min reperfusion followed by IR (IPC/IR), or 200-μM adenosine infusion for 15 min followed by IR (Ado/IR) in either the ex vivo Langendorff perfusion (B) or in situ coronary artery occlusion (C) model and infarct size (mean ± SEM; n = 3 hearts in each group) was determined by TTC staining. *P < 0.05 vs. column 1; #P < 0.05 vs. column 3; ##P < 0.05 vs. column 5 (Student’s t test).
Effect of HIF-1 Inhibitors on Cardioprotection Induced by IPC.
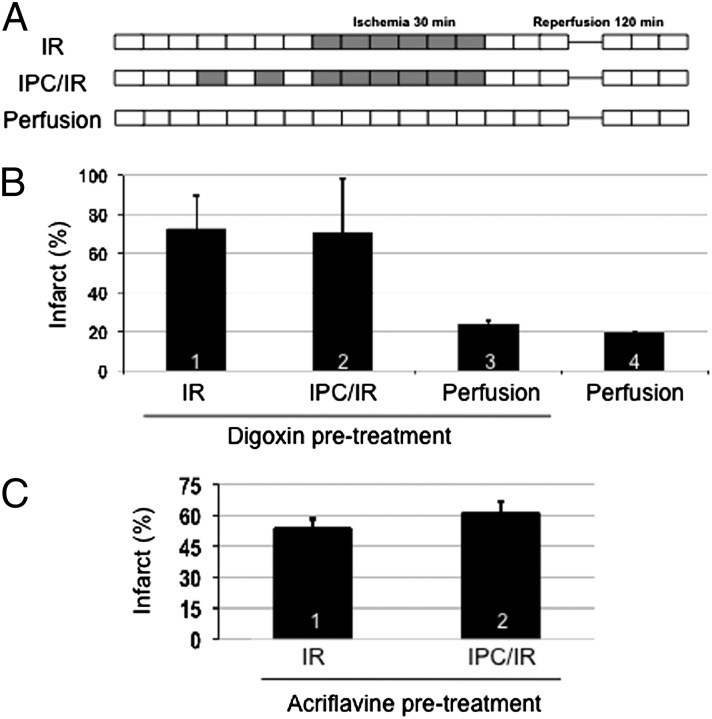
The analysis of Hif1af/f; Tie2-Cre and Arntf/f; Tie2-Cre conditional knockout mice described above provides evidence that both HIF-1α and HIF-1β expression in ECs is required for acute phase cardioprotection induced by IPC. To determine whether acute induction of HIF-1 activity during IPC is required for cardioprotection, we treated mice with drugs that inhibit HIF-1. Digoxin inhibits the translation of HIF-1α mRNA into protein (36), whereas acriflavine binds to HIF-1α and inhibits dimerization with HIF-1β (37). These drugs have been shown to inhibit HIF-1 activity in multiple tissues of experimental animals, including retina (38), lungs (39), and tumor xenografts (36, 37, 40, 41). We first administered digoxin to wild-type mice at a dose of 2 mg/kg by i.p. injection and harvested the heart for Langendorff perfusion 4 h later (Fig. 5A). Infarct size was not significantly different in hearts subjected to IR vs. IPC/IR (Fig. 5B, compare columns 1 and 2), indicating that the protective effect of IPC was lost in hearts from mice pretreated with digoxin. Infarct size in hearts that were subjected to perfusion only (i.e., no IR) was similar regardless of whether the mice were pretreated with digoxin or not (Fig. 5B, compare columns 3 and 4), indicating that digoxin pretreatment did not increase infarct size in the absence of IR.
Fig. 5.
Effect of parenteral administration of HIF-1 inhibitors on cardioprotection mediated by IPC. (A–C) Wild-type mice were pretreated with an i.p. injection of digoxin (B) or acriflavine (C) at a dose of 2 mg/kg. Four hours later, Langendorff heart preparations were subjected to IR, IPC/IR, or perfusion (without IPC or IR) and infarct size was determined by TTC staining (mean ± SEM, n = 3–4 hearts in each group).
To confirm that the effect of digoxin was mediated by its inhibition of HIF-1, we next used acriflavine, a HIF-1 inhibitor that is structurally distinct from digoxin and that inhibits HIF-1 by a distinct mechanism of action. Mice received an i.p. injection of acriflavine at a dose of 2 mg/kg and the hearts were harvested for Langendorff perfusion 4 h later. Infarct size was not significantly different in hearts subjected to IR vs. IPC/IR (Fig. 5C, compare columns 1 and 2), indicating that the protective effect of IPC was lost in hearts from mice pretreated with acriflavine.
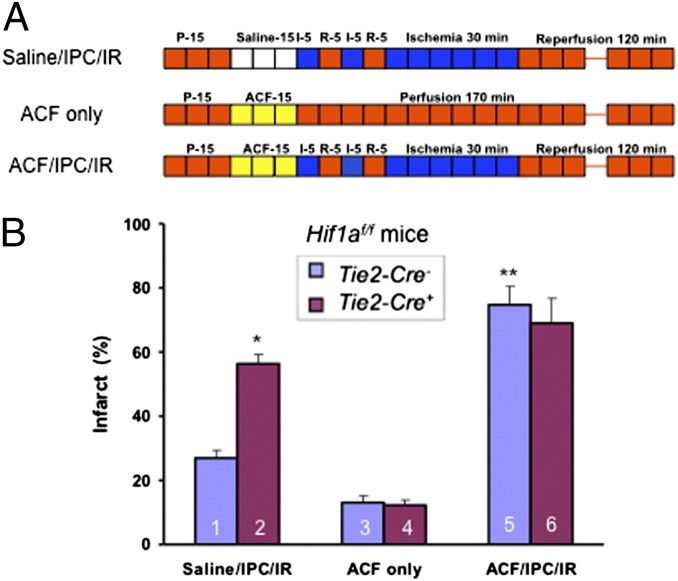
To demonstrate a direct effect of acriflavine on the heart, we next isolated hearts from Hif1af/f; Tie2-Cre+ and Hif1af/f; Tie2-Cre− mice and pretreated them with saline or acriflavine (at a concentration of 1 μM) by direct introduction of the inhibitor into the perfusate over 15 min immediately before IR or IPC/IR (Fig. 6A). IPC resulted in a significant reduction in infarct size in Hif1af/f; Tie2-Cre− compared with Hif1af/f; Tie2-Cre+ hearts that were pretreated with saline; in contrast, the protective effect of IPC on Hif1af/f; Tie2-Cre− hearts was not observed after acriflavine pretreatment (Fig. 6B). Acriflavine perfusion by itself did not increase infarct size in Hif1af/f; Tie2-Cre− or Hif1af/f; Tie2-Cre+ hearts that were not subjected to IR.
Fig. 6.
Effect of acriflavine infusion immediately before IPC/IR on cardioprotection. (A and B) Langendorff heart preparations from Hif1af/f; Tie2-Cre− and Hif1af/f; Tie2-Cre+ mice were perfused with saline or 1 μM acriflavine (ACF) for 15 min before IPC/IR or perfusion only (A) and infarct size (B) was determined by TTC staining (mean ± SEM, n = 3). *P < 0.05, **P < 0.01 vs. column 1; Student’s t test.
Discussion
In this study, we provide evidence that activity of the transcription factor HIF-1 during the IPC stimulus is required for subsequent acute phase cardioprotection and show that loss of HIF-1 in vascular ECs is sufficient to abolish acute phase cardioprotection induced by IPC. This finding shifts a paradigm in which transcriptional regulation has been viewed as a major mechanism for late, but not acute phase cardioprotection induced by IPC. The finding that a transcription factor is required for acute phase cardioprotection is surprising and suggests that less time may be required for HIF-1–dependent transcription of essential target genes involved in the response to IPC than is required for responses to continuous hypoxia. Indeed, whereas continuous hypoxia increases the stability of HIF-1α, cycles of hypoxia and reoxygenation increase both the synthesis and the stability of HIF-1α (20).
Our data also shift a paradigm in which the cardiomyocyte has been viewed as the primary locus of adaptive responses triggered by IPC to one in which ECs must also be considered as playing an essential role in cardioprotection. However, our findings do not exclude a role for HIF-1 in cardiomyocytes as well. Transgenic mice with HIF-1α expression driven by the α-myosin heavy chain gene promoter had reduced infarct size after coronary artery ligation compared with controls, although this effect may have been due to increased vascular density in the transgenic hearts (42). In contrast, there was no difference in vascular density in Hif1af/f; Tie2-Cre− compared with Hif1af/f; Tie2-Cre+ hearts (35), indicating that differences in vascularization cannot explain the loss of IPC-induced protection in Hif1af/f; Tie2-Cre+ hearts. The Tie2 promoter is also active in BM cells, resulting in Cre expression and loss of HIF-1 activity in myeloid and lymphoid cells of Tie2-Cre+ mice (Fig. 2). However, the isolated Langendorff hearts were perfused with buffer rather than blood and thus the differences observed in the acute infarction model between Tie2-Cre− and Tie2-Cre+ mice are unlikely to be due to loss of HIF-1 activity in inflammatory cells. However, these results do not exclude a role for HIF-1 expression in inflammatory cells in the process of postinfarction cardiac remodeling.
In contrast to the loss of IPC-mediated cardioprotection, adenosine perfusion resulted in protection of HIF-1–deficient hearts, although the protection was of lesser magnitude than in wild-type hearts. Adenosine levels increase in hearts subjected to IPC and pretreatment with adenosine receptor antagonists blocks the protective effect of IPC, whereas treatment with adenosine receptor agonists mimics IPC (43). Adenosine is formed from extracellular ATP through the activity of ectonucleoside trisphosphate diphosphohydrolase 1 (also known as CD39), which converts ATP to AMP, and ecto-5′-nucleotidase (NT5E, also known as CD73), which converts AMP to adenosine. CD73 expression is induced in hypoxic cells via direct binding of HIF-1 to the Nt5e gene (44). IPC-mediated cardioprotection is lost in both CD73-null (45) and CD39-null (46) hearts. We found that the expression of both CD39 and CD73 mRNA was rapidly induced in wild-type hearts, but not in Hif1a+/− hearts, in response to IPC, suggesting that CD39 expression is also regulated by HIF-1, perhaps in concert with SP1 (47).
Adenosine administration bypasses the defect in HIF-1–dependent adenosine production, triggering protection of HIF-1–deficient hearts. However, HIF-1 also regulates expression of the A2B adenosine receptor (29, 48). Adenosine administration does not bypass decreased receptor expression, which may account for the observation that compared with wild-type hearts, infarct size was significantly greater in HIF-1–deficient hearts after adenosine perfusion. Considering the well-known expression of CD73 in vascular ECs (49) and IPC-mediated induction of CD39 expression in cardiac ECs (46), it is likely that HIF-1–dependent CD39 and CD73 expression in ECs leads to the increased generation of adenosine, which plays a critical role by activating the A2B adenosine receptor, which is also required for IPC-mediated cardioprotection (29) through downstream survival signaling pathways mediated by phosphatidylinositol 3-kinase, AKT, and ERK (50). Two predictions of this model are: (i) HIF-1–dependent increases in cardiac adenosine levels in response to IPC (29) should be lost in mice with Tie2-Cre–dependent knockout at the Hif1a or Arnt locus, and (ii) Tie2-Cre–dependent knockout of CD39 or CD73 expression should result in loss of IPC-mediated cardioprotection. Thus, additional studies are needed to determine the extent to which the adenosine signaling pathway contributes to the requirement for endothelial HIF-1 activity in IPC-mediated cardioprotection.
Materials and Methods
Generation of Hif1a and Arnt Conditional Knockout Mice.
Mice with Tie2-Cre–dependent knockout at the Hif1a or Arnt locus were generated as described (32). Briefly, animals containing loxP sites flanking exon 2 of Hif1a (B6.129-Hif1atm3Rsjo/J; Jackson Laboratory) or exon 6 of Arnt (51) were crossed with Tie2-Cre transgenic mice (B6.Cg-TgTek-cre1Ywa/J; Jackson Laboratory). Genotype was determined by PCR analysis of DNA from tail biopsies. Primers used to distinguish floxed from nonfloxed alleles were: 5′-GCA GTT AAG AGC ACT AGT TG-3′ and 5′-GGA GCT ATC TCT CTA GAC C-3′ for Hif1a and 5′-ACG CAC TAC AAC ACC TGA GCT AA-3′ and 5′-GCA TGC TGG CAC ATG CCT GTC T-3′ for Arnt. Primers used to detect the Tie2-Cre transgene were: 5′-CGC ATA CCA TAC ATA GGT GGA G-3′ (in Tie2) and 5′-GCA TCG ACC GGT AAT GCA GGC A-3′ (in Cre). Primers used to analyze deletion of Hif1a in tissues by quantitative real-time PCR (qPCR) were: 5′-CGG CGA AGC AAA GAG TCT GAA G-3′ and 5′-GAT GGT GAG CCT CAT AAC AGA AGC-3′ (floxed exon 2) and 5′-GAT GAG ATG AAG GCA CAG ATG-3′ and 5′-CCC ATG TAT TTG TTC ACG TTA TC-3′ (nonfloxed Hif1a exon 3). Primers used to analyze deletion of Arnt in tissues by qPCR were: 5′-GGC GGC GAC GGA ACA AGA TG-3′ and 5′-TCA AGG ACT TCA TGT GAG AAA CGG-3′ (floxed exon 6) and 5′-GGC TTT CTG TTT ATT GTC TCC-3′ and 5′-CGA AGT TTA TCC ACA TCA TCT G-3′ (nonfloxed exon 7). All experiments were performed with littermates derived from Hif1af/f-Tie2-Cre+ × Hif1af/f-Tie2-Cre− or Arntf/f-Tie2-Cre+ × Arntf/f-Tie2-Cre− matings. At the time of cardiac studies, mice were 8–12 wk old. All procedures were approved by The Johns Hopkins University Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85–23, revised 1996).
Langendorff Perfusion System.
Mice were anesthetized by i.p. injection of pentobarbital (70 mg/kg). After the chest was opened, the heart was excised and the ascending aorta was cannulated with a blunt needle. The heart was perfused at a constant pressure with Krebs–Henseleit buffer (in mmol/L: glucose 17, NaCl 120, NaHCO3 25, CaCl2 2.5, KCl 5.9, MgSO4 1.2, and EDTA 0.5), which was maintained at 37 °C and bubbled continuously with a mixture of 95% O2 and 5% CO2 (vol/vol). Global ischemia was induced by cessation of perfusion for 5–30 min, followed by reperfusion for 5–170 min. The IPC stimulus consisted of two cycles of 5-min ischemia + 5-min reperfusion. Adenosine was infused into the perfusate for 15 min at a final concentration of 200 μmol/L. There was no change in heart rate or rhythm associated with adenosine administration. Acriflavine was perfused for 15 min at a final concentration of 1 μmol/L. At the end of the experiment, myocardial infarct size was measured as described below.
Coronary Artery Ligation Model.
Mice were anesthetized by i.p. injection of pentobarbital (70 mg/kg) and were mechanically ventilated. The depth of anesthesia was confirmed by lack of toe pinch response. The electrocardiogram (ECG) was recorded continuously. The mice were monitored with a rectal probe and body temperature was maintained at 37 °C with heating lamps. A left thoracotomy was performed, the left anterior descending coronary artery was identified visually, and a 7-0 suture was placed around the artery ∼1 mm below the left auricle. A loop was made in the suture around the coronary artery and tightened over a small piece of polyethylene tubing. Occlusion of the coronary artery resulted in changes in heart color (pallor) and ECG (ST-segment elevation and widening of the QRS complex). Reperfusion was accomplished by loosening the loop and confirmed by return of red color to the region and improved cardiac contraction. All mice were subjected to ischemia for 30 min and reperfusion for 120 min, with or without preceding IPC, which consisted of a two cycles of ischemia for 5 min and reperfusion for 5 min. Mice were euthanized by transection of the aorta and removal of the heart for determination of infarct size.
Assessment of Myocardial Infarct Size.
At the end of Langendorff perfusion, hearts were frozen at −80 °C for 10 min, sliced into five sections, incubated in 1% (wt/vol) triphenyltetrazolium chloride (TTC) for 30 min, and scanned. Viable myocardium stained brick red and infarct tissue appeared pale white (Fig. S1A). Infarct and left ventricular areas were measured by Image J1.30 software (NIH). Infarct size was calculated as infarct area divided by left ventricular area. For the in situ model, just before euthanasia, the loop around the coronary artery was retightened, 1% (wt/vol) Evans Blue solution was injected into the aorta, and the dye passed into the coronary arteries and distributed throughout the not-at-risk ventricular wall proximal to the coronary artery ligature. The heart was frozen and cut transversely into five sections, each of which was weighed and incubated in TTC for 15 min at 37 °C. The area not at risk appeared blue, viable myocardium in the at-risk area stained brick red, and infarcted tissue was pale white (Fig. S1B). The two sides of each section were photographed. The areas of blue, red, or white color were measured by computerized planimetry. Infarct size was measured as described above.
Assessment of Myocardial Apoptosis.
Following 30 min of ischemia and 45 min of reperfusion, hearts were cut transversely into five sections, fixed in 10% buffered formalin, routinely processed, paraffin embedded, and sectioned. Apoptosis was analyzed by immunohistochemistry, using an antibody that specifically recognizes cleaved caspase 3 (Cell Signaling Technology). For each section, apoptotic cells were counted in 10 random 200× fields.
Immunoblot Assays.
At the end of the experiments, hearts were collected and protein lysates were prepared in RIPA buffer containing complete protease inhibitor (Roche Diagnostics) and fractionated by SDS/PAGE. Antibodies against HIF-1β and β-actin were from Novus Biologicals and Santa Cruz Biotechnology, respectively.
Quantitative Real-time Reverse Transcription-PCR (RT-PCR).
Total RNA from mouse hearts was extracted and analyzed as previously described (35).
Statistical Analysis.
Differences between two groups were assessed with Student’s t test. Ratios and percentages were normalized with a log10 conversion before parametric test analysis. Differences were considered significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank Amanda Cardona for technical assistance with genotyping and Karen Padgett of Novus Biologicals for generously providing anti–HIF-1β antibody. G.L.S. is the C. Michael Armstrong Professor at The Johns Hopkins University School of Medicine. This work was supported by Grant P01-HL65608 from the National Institutes of Health and by funds from the Johns Hopkins Institute for Cell Engineering.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208314109/-/DCSupplemental.
References
- 1.Lopaschuk GD, Kelly DP. Signalling in cardiac metabolism. Cardiovasc Res. 2008;79:205–207. doi: 10.1093/cvr/cvn134. [DOI] [PubMed] [Google Scholar]
- 2.Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357:1631–1638. doi: 10.1056/NEJMra065985. [DOI] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 5.Kuzuya T, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 6.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 7.Bolli R. Preconditioning: A paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H19–H27. doi: 10.1152/ajpheart.00712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shohet RV, Garcia JA. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med (Berl) 2007;85:1309–1315. doi: 10.1007/s00109-007-0279-x. [DOI] [PubMed] [Google Scholar]
- 9.Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008;15:686–690. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- 10.Laude K, Beauchamp P, Thuillez C, Richard V. Endothelial protective effects of preconditioning. Cardiovasc Res. 2002;55:466–473. doi: 10.1016/s0008-6363(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 15.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 17.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Cai Z, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 19.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 20.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng YJ, et al. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 23.Hlatky MA, et al. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am Heart J. 2007;154:1035–1042. doi: 10.1016/j.ahj.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Resar JR, et al. Hypoxia-inducible factor 1α polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–791. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 25.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan HE, Lo J, Johnson RS. HIF-1 α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Compernolle V, et al. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1α. Cardiovasc Res. 2003;60:569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Cai Z, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 α. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 29.Eckle T, Köhler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 30.Hyvärinen J, et al. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285:13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar K, et al. Tie2-dependent knockout of HIF-1 impairs burn wound vascularization and homing of bone marrow-derived angiogenic cells. Cardiovasc Res. 2012;93:162–169. doi: 10.1093/cvr/cvr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang N, et al. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Yim SH, et al. Disruption of the Arnt gene in endothelial cells causes hepatic vascular defects and partial embryonic lethality in mice. Hepatology. 2006;44:550–560. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei H, et al. Endothelial HIF-1 protects the murine heart and aorta from pressure overload by suppression of TGF-β signaling. Proc Natl Acad Sci USA. 2012;109:E841–E850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Yoshida T, et al. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abud EM, et al. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci USA. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong CC, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl) 2012;2012:10. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Kido M, et al. Hypoxia-inducible factor 1-α reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 43.Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 44.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckle T, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 46.Köhler D, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 47.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 49.Koszalka P, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1α. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.