Abstract
Beneficial microbe functions range from host dietary supplementation to development and maintenance of host immune system. In mammals, newborn progeny are quickly colonized with a symbiotic fauna that is provisioned in mother’s milk and that closely resembles that of the parent. Tsetse fly (Diptera: Glossinidae) also depends on the obligate symbiont Wigglesworthia for nutritional supplementation, optimal fecundity, and immune system development. Tsetse progeny develop one at a time in an intrauterine environment and receive nourishment and symbionts in mother’s milk. We show that the host Peptidoglycan Recognition Protein (PGRP-LB) is expressed only in adults and is a major component of the milk that nourishes the developing progeny. The amidase activity associated with PGRP-LB may scavenge the symbiotic peptidoglycan and prevent the induction of tsetse's Immune Deficiency pathway that otherwise can damage the symbionts. Reduction of PGRP-LB experimentally diminishes female fecundity and damages Wigglesworthia in the milk through induction of antimicrobial peptides, including Attacin. Larvae that receive less maternal PGRP-LB give rise to adults with fewer Wigglesworthia and hyperimmune responses. Such adults also suffer dysregulated immunity, as indicated by the presence of higher trypanosome densities in parasitized adults. We show that recPGRP-LB has antimicrobial and antitrypanosomal activities that may regulate symbiosis and impact immunity. Thus, PGRP-LB plays a pivotal role in tsetse’s fitness by protecting symbiosis against host-inflicted damage during development and by controlling parasite infections in adults that can otherwise reduce host fecundity.
Keywords: maternal transfer, transgenerational immune effects, antimicrobial activity, antitrypanosomal activity
Maternal effects profoundly affect the phenotype of the offspring (1). In vertebrates, maternal transfers include immune proteins such as antibodies, which are vital in shaping the B- and T-cell repertoire of the offspring and protecting juveniles against fatal infections (2, 3). Maternal transfers also include symbiotic microbes that have coevolved with their eukaryotic hosts. Newborn progeny are quickly colonized with a microbial fauna that closely resembles that of the parent. Gut colonization with commensal bacteria during the juvenile state influences the expression and localization of pattern recognition receptors and antimicrobial peptides later in the adult state (4, 5). In germ-free animals, it has been shown that the development of immune-related organs, such as the lymphoid system as well as antibody production, is severely impaired.
Unlike vertebrates that harbor complex microbiomes with many taxa, most of which cannot be cultivated in vitro, invertebrates—particularly insects—harbor a vertically transmitted microbiome that is highly restricted in composition. The symbiotic functions in insects also range from nutritional supplementation of host diets to provisioning host physiological processes, including fecundity and immunity (6–8). The insect systems are easier to maintain with shorter generation times and less husbandry costs. Thus, they make excellent models to understand the functional aspects of host–symbiont biology and the contribution(s) of individual symbionts toward host physiology as we explore here in the tsetse system.
Tsetse flies are the sole vectors of medically and agriculturally important African trypanosomes. In addition, tsetse is an excellent model system for studying host–symbiont dynamics. Both male and female tsetse feed on only vertebrate blood, and the female has viviparous reproduction in which the offspring develop one at a time in utero. The larva is nourished by mother’s milk produced by accessory glands. The milk contains lipids and proteins, including Transferrin and Milk Gland Protein (a novel protein in the lipocalin family), as well as tsetse’s microbial symbionts (9). Females give birth to a single mature larva, which pupates and remains dormant until eclosion. All tsetse harbor the obligate mutualist Wigglesworthia glossinidia, which resides intracellularly in the midgut bacteriome organ and extracellularly in mother’s milk (7, 10). Wigglesworthia supplements tsetse’s nutritionally restricted blood diet with vitamins (11), and without Wigglesworthia, females are reproductively sterile. Juvenile progeny that develop without Wigglesworthia give rise to immune-compromised adults that are especially deficient in cellular immune responses and that exhibit unusual susceptibility for trypanosome infections (7). Some natural populations and laboratory lines of tsetse can also harbor the commensal Sodalis glossinidius and the parasitic Wolbachia pipientis. Whereas Wolbachia is vertically transmitted transovum, Sodalis is maternally transmitted in mother’s milk (9). Tsetse’s viviparous reproduction limits the microbial exposure of the immature stages developing in the uterus only to the maternally transmitted symbionts (Wigglesworthia, Sodalis, and Wolbachia).
Because hosts require the beneficial microbes for full fitness, they have likely evolved high tolerance for these microbes, while they can resist closely related pathogens (12). In tsetse, both host- and symbiont-mediated mechanisms may contribute to tolerance to microbial fauna. Modifications in the major coat protein (Outer Membrane Protein; OmpA) of Sodalis confer resistance to the symbiont against host immune peptides (Antimicrobial peptides; AMP) (13). Host–pathogen recognition processes involve a family of Peptidoglycan Recognition Proteins (PGRPs). In insects, PGRP-LC has been shown to be the receptor of the Immune Deficiency (Imd) pathway and can bind microbial peptidoglycan (PGN), which leads to the synthesis of a battery of AMPs (14). Humans PGRPs can clear pathogens directly via bactericidal activity (15). The Drosophila PGRP-LB has a catalytic amidase activity that can degrade PGN and prevent host immune activation (16, 17). The ability of PGRP-LB to control host immune activation provides protection to beneficial microbes in several insects (18–20). In tsetse, pgrp-lb is expressed in Wigglesworthia harboring tissues (bacteriome and milk gland), and there is a positive correlation between Wigglesworthia density and pgrp-lb expression levels. When PGRP-LB is reduced through RNA interference-based gene silencing, tsetse’s Imd pathway is activated, resulting in production of AMPs, which in turn damage Wigglesworthia (21). Interestingly, silencing PGRP-LB also results in higher susceptibility to trypanosome infections, suggesting a possible antiprotozoal function for PGRP-LB. Given that parasitism induces host immunity, which reduces host fecundity, PGRP-LB can be a strong driver for Wigglesworthia symbiosis and host fitness (22).
Here, we investigated the regulation of the microbiome during the maternal transmission process in tsetse pregnancy with a focus on the role of PGRP-LB and Wigglesworthia dynamics for host fitness traits. Spatial and temporal analysis of PGRP-LB reveals that it is an essential milk protein transmitted to the intrauterine progeny. We evaluated the long-term consequences of PGRP-LB reduction in pregnant females on fitness traits in emerging adult progeny. We expressed recPGRP-LB in Drosophila cells and tested its amidase, antimicrobial, and antiprotozoal activities to understand the mechanism(s) by which PGRP-LB may regulate symbiotic homeostasis and influence parasitism. We discuss the regulation of maternal processes that enable efficient transfer and colonization of progeny with the symbiotic fauna and the fine-tuned regulation of symbiotic densities that maximize host fitness traits (23, 24).
Results
Spatial and Temporal Expression of PGRP-LB.
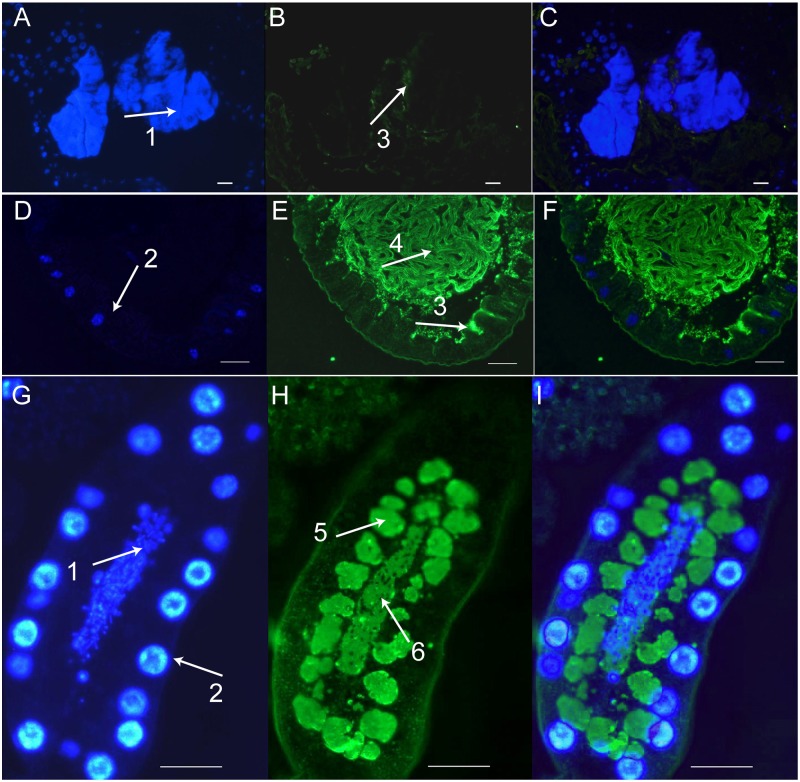
Our studies had shown that pgrp-lb transcripts are preferentially expressed in the bacteriome and female milk gland organ and that pgrp-lb expression level correlates with Wigglesworthia density (21). We performed immunohistochemical analysis to determine the cellular and subcellular localization of PGRP-LB protein relative to symbiont presence (Fig. 1 and Fig. S2). DAPI staining confirmed the presence of symbionts in the gut bacteriome, milk gland cells, and lumen (7, 9). PGRP-LB was also localized to the periphery of the bacteriome organ (Fig. 1 B and E), as well as in the storage reservoirs of milk gland cells and lumen (Fig. 1H), confirming its secretory nature and direct transfer from mother to her intrauterine larval progeny.
Fig. 1.
Localization of PGRP-LB by immunostaining. Sections were incubated with either anti–PGRP-LB antibody (A–I) or preimmune sera (Fig. S2). (A–C) Bacteriome. (D–F) Midgut. (G–I) Milk gland. A, D, and G show DAPI staining. B, E, and H show staining with PGRP-LB. C, F, and I show merged images. Arrows denote symbionts (1), host cell nuclei (2), PGRP-LB associated with gut matrix (3), gut lumen (4), milk gland storage reservoir (5), and milk gland lumen (6). (Scale bar: 25 μm.)
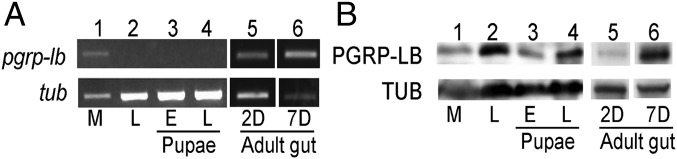
To determine the tissue and host developmental stage-specific regulation of PGRP-LB, RT-PCR and Western analysis were performed on milk glands, intrauterine larva, early pupae, late pupae, and adult guts (2 and 7 d after emergence) (Fig. 2). Both PGRP-LB transcripts and protein were detected in the milk gland and gut tissue of adults, and pgrp-lb levels in the gut increased after emergence. In contrast, no PGRP transcripts but PGRP-LB protein was detected in the juvenile stages—confirming the maternal transmission of PGRP-LB to the developing larva in mother’s milk.
Fig. 2.
PGRP-LB expression in different host developmental stages. Transcriptional analysis (A) and protein expression analysis (B) were conducted for milk gland and fat body fractions of adult females (M, lane 1); larva (L, lane 2); pupae at 24 h (E, lane 3) and 3 wk (L, lane 4) after deposition; and guts at 2 d (2D) and 7 d (7D) after emergence (lanes 5 and 6, respectively). Each sample represents three pooled individuals. Results from one of two independent experiments are shown. The PCR amplification cycle was 25. Tsetse tubulin RNA (gmmtub) and Tubulin protein (GmmTub) were used as internal controls.
PGRP-LB Influences Wigglesworthia Density and Host Fecundity.
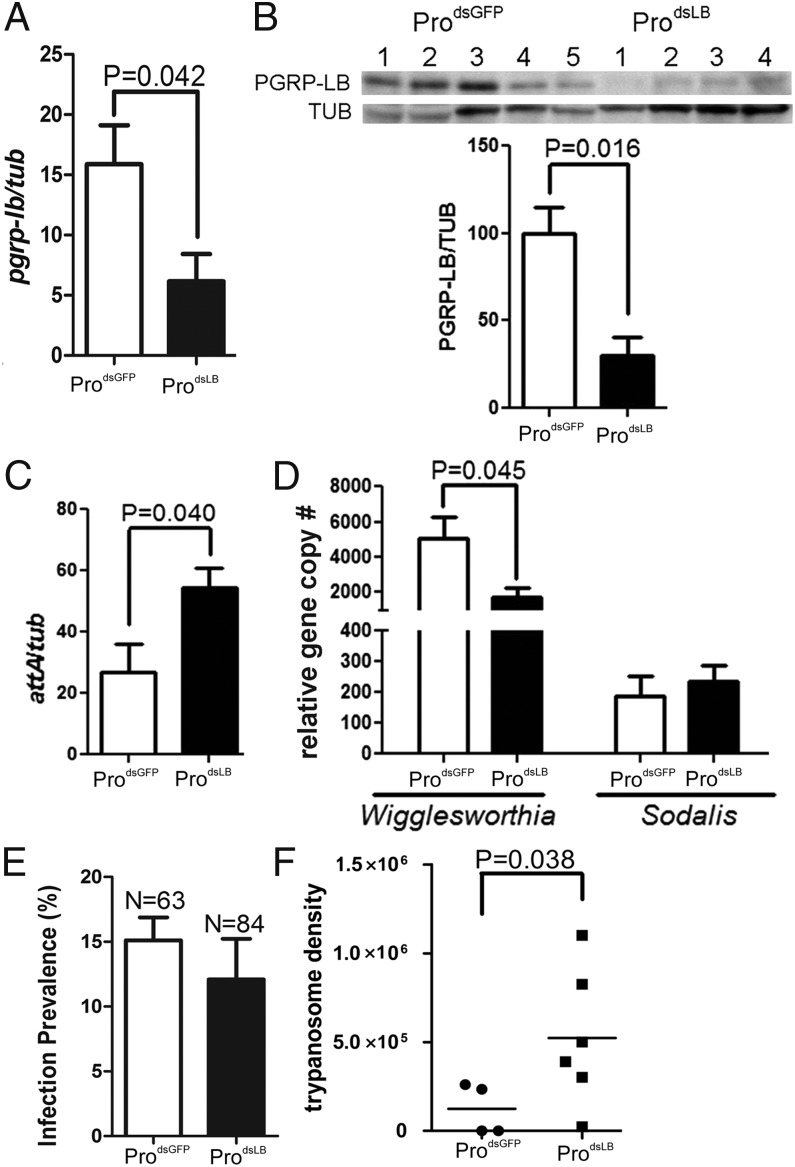
To understand PGRP-LB influences on symbiont density regulation in the maternal transmission process, fertile mothers were subjected to double-stranded PGRP-LB (dsLB) treatments. Comparative analysis of PGRP-LB transcript levels between the control dsGFP and dsLB treatment groups and protein levels in the milk gland reservoirs and lumen indicated a significant knockdown effect (Fig. S3). Milk gland sections from dsLB females stained with a different antibody (anti-Milk Gland Protein) (9) showed the presence of high levels of GmmMGP and confirmed the specificity of dsLB treatment (Fig. S3B3).
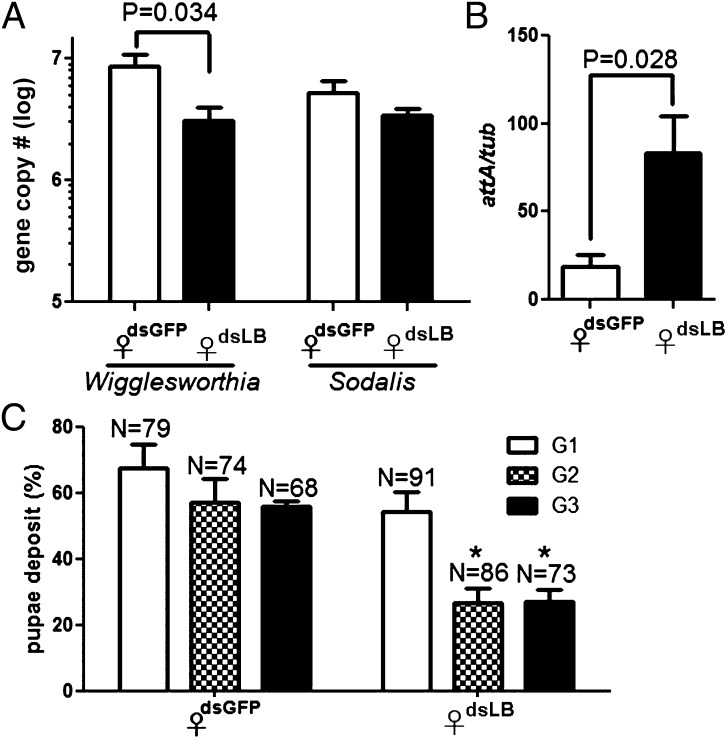
Wigglesworthia density in the milk gland of dsLB-females showed a significant decrease in comparison with dsGFP controls (Fig. 3A), whereas no significant difference was noted for Sodalis. We next measured the transcript levels for one of the AMPs (Attacin), expressed downstream of the Imd immune pathway. In agreement with our previous observations from whole dsLB females, attacin expression in the milk gland tissue was up-regulated in LB depleted flies (Fig. 3B). The effector AMPs (including Attacin) may be responsible for the decreased Wigglesworthia density we observed when PGRP-LB levels were reduced because silencing both PGRP-LB and -LC proteins could prevent damage to Wigglesworthia (21). Sodalis was shown to be resistant to the bacteriocidal activity of insect AMPs in vitro, which may explain the lack of density effects we observed for Sodalis in the presence of increased Attacin levels (25, 26).
Fig. 3.
Effect of PGRP-LB silencing on symbiont density, immune response, and fecundity of pregnant females. (A and B) Symbiont densities (Wigglesworthia and Sodalis) (A) and attacin levels (B) were measured from dsRNA-treated females. Error bars indicate SEM (n = 5). The levels of attacin were normalized against host tubulin. (C) Fecundity effects of dsRNA treatments on pregnant females. Pupal deposition percentage for each of the three GCs is given as total pupal deposition number per female. *Statistically significant values between dsGFP and dsLB treatments in second GC (G2) and third GC (G3), respectively (P < 0.05). Results are the mean of three independent experiments.
Because Wigglesworthia fitness is essential for host fecundity, we measured the effect of PGRP-LB silencing on female fecundity. The pupal deposition rate of dsGFP-treated flies was 57%, whereas that of dsLB was reduced to ∼26.7% in the second gonotrophic cycle (GC). The same trend was seen in the third GC, with 56.3% in dsGFP and 27.2% in dsLB flies (Fig. 3C). Loss of fecundity could be due to either decreased Wigglesworthia-mediated nutritional effects or to a tradeoff caused by increased host immune activity. A similar loss of fecundity was observed when tsetse were parasitized with trypanosome strains that resulted in host immune induction (22). Thus, these findings further support obligate Wigglesworthia as being indispensible for female fecundity and PGRP-LB as protecting Wigglesworthia from host damage by preventing immune activation.
Maternal PGRP-LB Influences on Larval Symbiosis and Immunity.
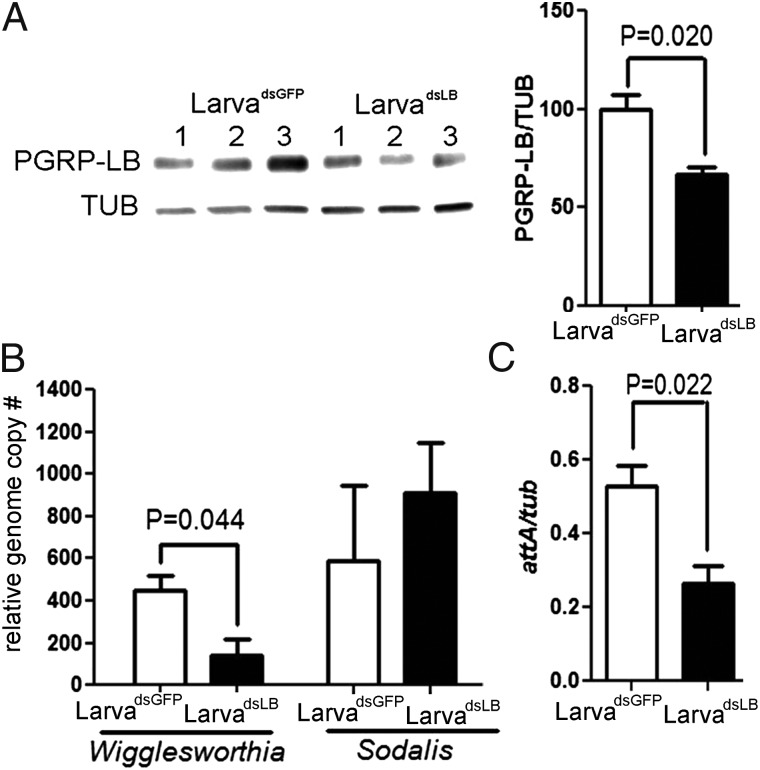
Larval progeny acquire Wigglesworthia through their mother’s milk, and maternal PGRP-LB levels in the milk can influence the symbiotic densities transferred. We measured the immune status and symbiont density from the intrauterine larva microscopically dissected from dsRNA-treated mothers (LarvadsLB). Both the PGRP-LB protein levels and Wigglesworthia density were significantly lower in LarvadsLB in comparison with control LarvadsGFP (Fig. 4 A and B, respectively). It appears that the maternal Wigglesworthia levels transferred to the larva are important for symbiotic infection densities, especially because our earlier studies had shown little to no proliferation of symbionts during the immature developmental stages (27). The attacin levels in LarvadsLB were also significantly lower than the controls (Fig. 4C). Because LarvadsLB has less Wigglesworthia, there may be less bacterial stimuli to activate the larval immune system. Given that the presence of Wigglesworthia in larval stages is essential for the development of adult immune system, we reasoned that LarvadsLB could give rise to adults with decreased fitness traits, particularly compromised immunity (28).
Fig. 4.
Effect of PGRP-LB silencing on symbiont density and larval immune response. (A) Three third-instar larva from each treatment group underwent Western analysis. PGRP-LB levels were normalized according to host β-Tubulin. (B) Wigglesworthia and Sodalis densities in LarvadsLB and LarvadsGFP were normalized according to host β-tubulin. Error bar indicates SE (n = 3). (C) Attacin levels were measured from the same samples and normalized to host β-tubulin. Error bar indicates SE (n = 3). Results from one of two independent experiments are shown.
Maternal Effects on Immune Status of Adult Progeny.
We evaluated the long-term effects of maternal PGRP-LB level modification on adult progeny fitness. We measured PGRP-LB levels, symbiont density, and immune response (attacin levels) from ProdsLB and control ProdsGFP flies 20 d after eclosion. Both the mRNA and protein levels of PGRP-LB in ProdsLB were approximately threefold lower than the controls (Fig. 5 A and B, respectively). Wigglesworthia density in ProdsLB was also significantly less, whereas Sodalis levels were not significantly different (Fig. 5D). The attacin levels, however, were approximately twofold higher in ProdsLB than in controls (Fig. 5C). We tested the consequences of decreased larval Wigglesworthia and PGRP-LB effects on vector competence of ProdsLB. When challenged with trypanosomes, there was no significant difference in the infection prevalence between ProdsLB and ProdsGFP (Fig. 5E). However, parasitized ProdsLB had fourfold higher trypanosome density than the control ProdsGFP (Fig. 5F). These results suggest that varying transmission dynamics of Wigglesworthia and PGRP-LB from mother to immature progeny can have long-lasting consequences on emerging adults. Flies that acquire fewer symbionts, and consequently less PGRP-LB, during juvenile development have immune defects and are at a disadvantage as young adults.
Fig. 5.
Long-term effects of reduced maternal PGRP-LB transmission on symbiont density and immune response of adult progeny. (A) pgrp-lb levels from ProdsGFP and ProdsLB adult females 20 d after eclosion normalized to β-tubulin (n = 5). (B) Western analysis of PGRP-LB from the same flies normalized to host β-Tubulin (n ≥ 4). (C) attacin levels normalized to host β-tubulin. Error bar indicates SE (n = 5). (D) Wigglesworthia and Sodalis genome copy numbers normalized to host β-tubulin (n = 5). (E) Midgut infection prevalence for T. b. rhodesiense in ProdsLB and ProdsGFP. (F) Parasite infection density in parasitized flies. Results show the combination of three independent experiments.
Antipathogenic Functions of recPGRP-LB.
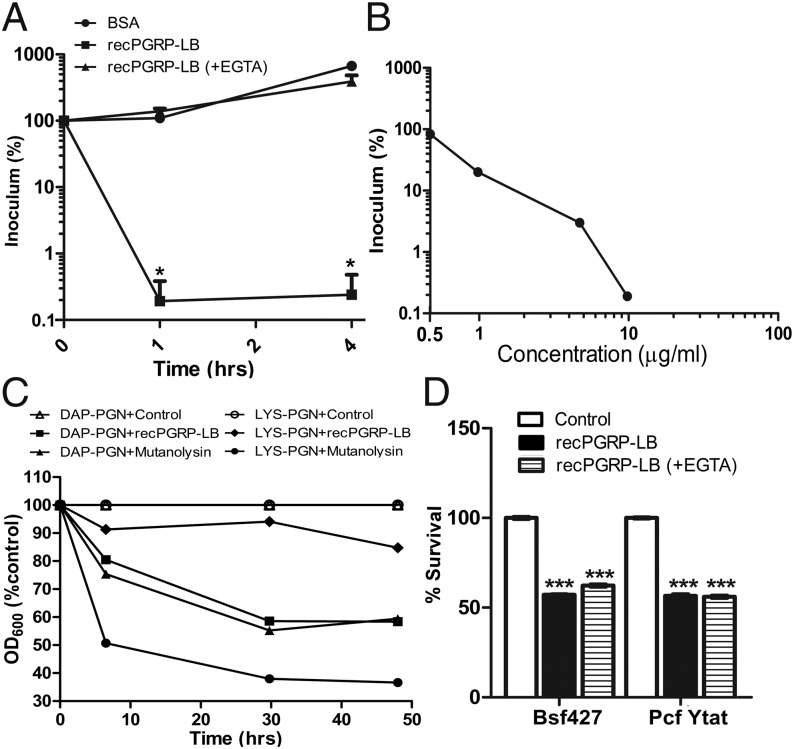
Our previous studies had incriminated both the Imd pathway and PGRP-LB for trypanosome resistance in tsetse, because independent experimental reduction of pgrp-lb and pgrp-lc led to higher parasite infection prevalence and density (21). This result was unexpected because increased levels of AMPs (including Attacin) in the presence of reduced PGRP-LB should have reduced parasite infections (25, 26). Thus, we speculated that PGRP-LB could have an antiparasitic activity, which could also harm parasites. Here, we expressed recPGRP-LB in S2 cells and tested its antimicrobial and amidase activities. When recPGRP-LB (10 μg/mL corresponding to 0.4 μM) was added to the growth medium in vitro, >99% of Escherichia coli was killed in 1 h (Fig. 6A). When Zn2+ was removed from rec-protein by EGTA treatment, recPGRP-LB lost its bactericidal activity, in agreement with the human and zebrafish PGRPs (29, 30). The bactericidal action of recPGRP-LB was dose-dependent with low concentrations (≤5 μg/mL) showing less killing activity (Fig. 6B). recPGRP-LB (at 5 μg/mL) was able to hydrolyze DAP-PGN as efficiently as mutanolysin (200 units/mL), but not LYS-PGN (Fig. 6C). Next, we tested the antiparasitic effects of recPGRP-LB. Both procyclic insect and cultured bloodstream form parasites showed ∼50% killing in vitro relative to control medium when supplemented with 10 μg/mL recPGRP-LB (Fig. 6D). The depletion of Zn2+ by EGTA treatment did not impair the trypanocidal activity of recPGRP-LB, which indicates that PGRP-LB killing of trypanosomes did not involve amidase activity but rather depended upon yet another unknown amidase-independent mechanism (Fig. 6D). It remains to be seen, however, whether the recPGRP-LB used in killing assays reflects physiological levels typically associated with PGRP-LB in the gut tissue. The antiparasitic activity of PGRP-LB may explain the higher trypanosome densities that we noted in parasitized ProdsLB adults, which have less PGRP-LB levels than corresponding control adults. The molecular aspects of tsetse’s PGRP-LB antitrypanosomal activity remain to be investigated. Because parasite infections and intensity can have a negative impact on host fecundity through immune induction (22), the antiparasitic activity of PGRP-LB can further enhance host fitness.
Fig. 6.
Bactericidal and trypanocidal activity of recombinant PGRP-LB. (A) E. coli was incubated with 10 μg/mL (0.4 μM) recPGRP-LB or EGTA-treated recPGRP-LB for indicated times. *P < 0.05. Results show the mean of four independent experiments. (B) E. coli was incubated with recPGRP-LB at different concentrations for 1 h. Results show one of two independent experiments. (C) Amidase activity of recPGRP-LB on DAP- and LYS-PGN. Data shown are representative of two independent experiments. (D) Trypanocidal activity of recPGRP-LB with and without EGTA treatment on bloodstream and procyclic cell cultures or with protein buffer (control), respectively. Results show one of two independent experiments. Error bar indicates SE (n = 4). The significance of differences was calculated by the Student t test. ***P < 0.0001.
Discussion
Symbiosis with beneficial microbes is widespread in animals (31). The functional roles of symbionts in host physiology, symbiont colonization processes, and host- or symbiont-mediated mechanisms that protect symbiosis from hostile host immune responses are of interest. Here we show that a host pathogen recognition protein (PGRP-LB) plays an essential role in tsetse symbiosis. Tsetse PGRP-LB is expressed only in adults and is maternally transferred to the intrauterine larva in mother’s milk. PGRP-LB protects Wigglesworthia symbiosis by suppressing the activation of symbiont-damaging host immune responses via its catalytic activity. Adult fecundity and immune system expression rely on a fine-tuned balance between Wigglesworthia density and maternal PGRP-LB levels acquired during juvenile development. In addition, PGRP-LB has an antiprotozoal activity to influence tsetse’s vector competence. Although antibacterial functions for PGRPs have been documented, this report describes an antipathogen function for PGRP against eukaryotic protozoa.
Tsetse has coevolved with its symbionts so that the fly does not mount a strong antimicrobial immune response. Symbiont densities, however, are tightly controlled throughout host development, with the exception of the teneral state immediately after eclosion when symbionts proliferate for a few days before being maintained at homeostatic levels (32). Wigglesworthia resides tightly packed in bacteriocytes in the gut bacteriome but has a free-living population in the lumen of the milk gland. Although the intracellular Wigglesworthia may be protected from hostile immune responses, the extracellular forms in the milk can induce host immune responses and can be adversely affected by host immune effectors. Our results show that tsetse PGRP-LB plays a key role in maintaining symbiotic homeostasis. Reduction of PGRP-LB induces the expression of the immune effector AMPs in the gut, which negatively impact Wigglesworthia densities. Loss of Wigglesworthia fitness, in turn, decreases the reproductive output of females by half in comparison with dsGFP-treated controls. PGRP-LB silencing also reduces Wigglesworthia densities in the milk and Wigglesworthia numbers transmitted to the intrauterine progeny. In the extreme case, when we had eliminated the extracellular population of Wigglesworthia, thus preventing its transmission to the larva, the emerging adult progeny were found to suffer major fitness losses, including total sterility, impaired cellular immune responses, and high susceptibility to trypanosome infections (7, 28). PGRP-LB synthesis is restricted to the adult state and is induced shortly after flies receive their first blood meal (21). The juvenile stages do not synthesize PGRP-LB, but rather acquire it in the milk. The abundant levels of PGRP-LB transferred to the juvenile stages can also prevent the induction of larval and pupal immunity in response to the presence of the symbiotic fauna. The impaired development of immune system during juvenile development when PGRP-LB levels in the milk are reduced has negative consequences for the establishment of symbiosis in the offspring and then later in the emerging adults. Our results indicate that fluctuations in symbiotic densities (and PGRP-LB levels) transferred from mother to larva can have long-lasting effects on emerging adult progeny. We find that reduced PGRP-LB levels in fertile mothers leads to progeny with lower Wigglesworthia densities and less PGRP-LB, even when analyzed in emerging adults 20 d after eclosion. The emerging adults also lack the ability to control trypanosome densities when parasitized. It is possible that the smaller seed of Wigglesworthia provisioned by the mother to her larva is unable to stimulate host immune development sufficiently during the immature stages. Our results suggest that tsetse innate immunity actively regulates symbiotic homeostasis throughout development largely through PGRP-LB, and PGRP-LB plays an important role in this regulation both directly as an antimicrobial effector or indirectly through modulation of host immune response activation.
The antimicrobial actions of PGRPs have been described in eukaryotes, but the antiprotozoal activity we describe here is unprecedented. Our results show that, in addition to the immune effector AMPs, PGRP-LB may regulate the success of trypanosome infections and parasite densities in tsetse. Although adult tsetse show high resistance to trypanosome infections, flies immediately after eclosion show greater susceptibility to parasitism (33). The inability of young tsetse to regulate parasite resistance may reflect the immature nature of its immune system, which after a few blood meals becomes fully active. Given that PGRP-LB expression is also induced following blood feeding, the increased resistance to trypanosomes may be due to the higher levels of PGRP-LB present in the gut of older flies. The levels of the maternally transmitted PGRP-LB present in the teneral gut could also influence the trypanosome susceptibility traits in young adults. Progeny that acquire higher maternal PGRP-LB during juvenile development may be more resistant to trypanosomes as a young adult than those that receive less. PGRP-LB may constitute the first line of defense against invading trypanosomes, followed by the effector AMPs produced once the immune signal cascades are activated.
Several studies have demonstrated that both insects and mammals deploy multiple negative regulatory mechanisms to prevent immune activation by reducing signal strength (6, 23, 34, 35). A tightly regulated immune system is important for the host to mount an effective immune response against the incoming pathogens and can also benefit symbiosis. Vertebrate PGRPs can directly kill invading microbes through their antimicrobial activity by triggering bacterial two-component system that cause membrane depolarization and [OH]• production in the cytoplasm (29, 36). Drosophila PGRP-LB has a catalytic activity that can help scavenge microbial PGN and prevent induction of Imd pathway (16). The catalytic functions of PGRP-LB may protect Wigglesworthia symbiosis by scavenging the released PGN to prevent host immune induction, and its bactericidal function may eliminate the invading bacteria directly, similar to the actions of vertebrate PGRPs. Unlike Wigglesworthia, tsetse’s immune effectors do not impact Sodalis adversely. Prior studies had also indicated that recAttacin is not affective against Sodalis despite its potent activity against E. coli and trypanosomes in vitro (26). Thus, it appears that, although tsetse actively protects the indispensable Wigglesworthia symbiosis, commensal Sodalis has undergone adaptations to survive in the hostile host environment.
Regulated expression of PGRP-LB is essential for protecting symbiosis in the adult to ensure host fecundity, for the success of symbiotic transfer to juvenile progeny in the milk, and for optimal symbiont colonization processes in juvenile development. PGRP-LB also has an antitrypanosomal activity that may influence the vector competence in adult tsetse. Trypanosome infections have been shown to reduce host fecundity due to the cost of host immune response activation. Thus, both as a direct immune effector and as the negative regulator of the Imd pathway, PGRP-LB plays a crucial role in symbiotic homeostasis for optimal fecundity. Ultimately, the evolutionary success of tsetse relies on optimal maternal transmission of Wigglesworthia and PGRP-LB to the intrauterine progeny. Similar long-term beneficial effects have also been described for maternal antibodies, which induce T-cell–dependent idiotypic responses during the neonatal period (3). In addition, transgenerational immune priming has been demonstrated in insects challenged with bacteria or viruses (37, 38). Maternally transmitted susceptibility for trypanosome infections has also been described in tsetse (39–41). It remains to be seen whether PGRP-LB levels may be responsible for transgenerational effects as such and whether parasitism with trypanosomes can lead to long-term maternal imprinting in tsetse.
Materials and Methods
Insects and Trypanosome Infection.
Glossina morsitans morsitans colony and trypanosomes were maintained as described (7, 42). For fly infections, 2 × 106 per milliliter of Bsf T. b. rhodesiense were provided in the second blood meal. Flies that did not feed were discarded. Fourteen days after challenge, midgut infections were microscopically scored, and parasite density was measured by quantitative RT-PCR for trypanosome β-tubulin.
dsRNA Treatments and Fecundity Measurements.
The dsRNAs (8 μg per fly) were introduced via thoracic microinjection as described (21). Third-instar larva from the second and third GCs were surgically obtained from dsLB- and dsGFP-treated mothers (denoted as LarvadsLB or LarvadsGFP), respectively. Adult progeny from treated mothers were collected 20 d after eclosion (denoted as ProdsLB or ProdsGFP). The experimental plan is schematically shown in Fig. S1. Fertile females treated with dsRNAs were monitored for three GC. Surviving mothers and the number of pupae deposited daily by each group was recorded. Fecundity is expressed as the percentage of number of pupae deposited per female for each of the three GC.
Gene Expression and Symbiont Density.
Gene expression (gmmatt and pgrp-lb) and symbiont numbers were analyzed by quantitative PCR. Values are represented as the mean (±SEM), and statistical significance was determined by using a Student t test and Microsoft Excel software. All primer sequences used in this study are listed in Table S1.
Western Blot Analysis and Immunohistochemistry.
Milk gland/fat body tissues of 40-d-old pregnant mothers and their intrauterine larvae were dissected. Deposited pupae were collected. For Western analysis, equal volumes of protein extract were combined from three flies as described (9), and rabbit polyclonal sera was generated as described in Fig. S2. recPGRP-LB and tsetse–β-Tubulin specific antisera were used at concentrations of 1:5,000 and 1:10,000, respectively. Carcasses of 30-d-old females, 15 d after dsGFP and dsLB treatments, respectively, were fixed and processed as described (9). Slides were incubated with anti–PGRP-LB (1:50), anti-GmmMGP (1:5,000), or preimmune sera (1:50), respectively. Secondary antibodies were Alexa Fluor 488 goat anti-rabbit IgG (for PGRP-LB) and Alexa Fluor 546 goat anti-rabbit IgG (for milk gland protein) (Invitrogen) at a concentration of 4 μg/mL.
Expression of Active recPGRP-LB in S2 Cells.
The complete pgrp-lb CDS without the signal peptide (GenBank accession no. DQ307160) was cloned into pMT/BiP/V5-His vector (Invitrogen) and transformed into S2 cells by using Effectene Transfection Reagent (Qiagen; Cat No: 301425). recPGRP-LB expression was induced with 500 mM CuSO4, purified from the supernatant by nickel-agarose (His-Bind Kit; Novagen) affinity chromatography, and dialyzed against buffers as described (30).
Amidase and Antipathogen Assay.
For amidase activity, 0.5 mg/mL insoluble DAP- and LYS-PGN (Sigma) were incubated with 5 μg/mL (0.2 μM, 10% vol/vol) recPGRP-LB in protein buffer (5 mM Tris, pH 7.6, 150 mM NaCl, 5 μM ZnSO4, 10% glycerol) at 37 °C for 48 h, respectively, and OD600 was measured as described (29). Mutanolysin (200 units/mL, 10% vol/vol) (Sigma) and protein buffer were used as positive and negative controls, respectively.
Antibacterial assay with E. coli was preformed as described (30), except that E. coli were incubated with recPGRP-LB or BSA in 10% LB for up to 4 h. Trypanocidal activity of recPGRP-LB was assayed as described (43). In a volume of 100 μL, 2 × 103 Pcf Ytat or 5 × 103 Bsf 427 was incubated with recPGRP-LB (10 μg/mL; 0.4 μM) for 66 h at 28 °C and 37 °C, respectively. recPGRP-LB treated with 100 μM EGTA were used as control to test whether PGRP-LB killed microbes through amidase activity. Protein buffer (as above but with 10 μM ZnSO4) was used as control.
Supplementary Material
Acknowledgments
We thank Yineng Wu for technical assistance and Geoffrey Attardo for editorial assistance. This work was supported by National Institute of Allergy and Infectious Diseases Grant AI051584 (to S.A.) and by grants from the Li Foundation (S.A.) and Ambrose Monell Foundation (S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116431109/-/DCSupplemental.
References
- 1.Bernardo J. Maternal effects in animal ecology. Am Zool. 1996;36:83–105. [Google Scholar]
- 2.Boulinier T, Staszewski V. Maternal transfer of antibodies: Raising immuno-ecology issues. Trends Ecol Evol. 2008;23:282–288. doi: 10.1016/j.tree.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Lemke H, Tanasa RI, Trad A, Lange H. Benefits and burden of the maternally-mediated immunological imprinting. Autoimmun Rev. 2009;8:394–399. doi: 10.1016/j.autrev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu JH, Ha EM, Lee WJ. Innate immunity and gut-microbe mutualism in Drosophila. Dev Comp Immunol. 2010;34:369–376. doi: 10.1016/j.dci.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Pais RR, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 9.Attardo GM, et al. Analysis of milk gland structure and function in Glossina morsitans: Milk protein production, symbiont populations and fecundity. J Insect Physiol. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma WC, Denlinger DL. Secretory discharge and microflora of milk gland in tsetse flies. Nature. 1974;247:301–303. [Google Scholar]
- 11.Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 12.Oliver TH, Leather R, Cook JM. Tolerance traits and the stability of mutualism. Oikos. 2009;118:346–352. [Google Scholar]
- 13.Weiss BL, Wu Y, Schwank JJ, Tolwinski NS, Aksoy S. An insect symbiosis is influenced by bacterium-specific polymorphisms in outer-membrane protein A. Proc Natl Acad Sci USA. 2008;105:15088–15093. doi: 10.1073/pnas.0805666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charroux B, Rival T, Narbonne-Reveau K, Royet J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009;11:631–636. doi: 10.1016/j.micinf.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Dziarski R, Gupta D. Review: Mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 2010;16:168–174. doi: 10.1177/1753425910366059. [DOI] [PubMed] [Google Scholar]
- 16.Zaidman-Rémy A, et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Byun M, Oh BH. Crystal structure of peptidoglycan recognition protein LB from Drosophila melanogaster. Nat Immunol. 2003;4:787–793. doi: 10.1038/ni952. [DOI] [PubMed] [Google Scholar]
- 18.Lefèvre C, et al. Endosymbiont phylogenesis in the dryophthoridae weevils: Evidence for bacterial replacement. Mol Biol Evol. 2004;21:965–973. doi: 10.1093/molbev/msh063. [DOI] [PubMed] [Google Scholar]
- 19.Troll JV, et al. Peptidoglycan induces loss of a nuclear peptidoglycan recognition protein during host tissue development in a beneficial animal-bacterial symbiosis. Cell Microbiol. 2009;11:1114–1127. doi: 10.1111/j.1462-5822.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang SM, Zeng Y, Loker ES. Characterization of immune genes from the schistosome host snail Biomphalaria glabrata that encode peptidoglycan recognition proteins and gram-negative bacteria binding protein. Immunogenetics. 2007;59:883–898. doi: 10.1007/s00251-007-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Wu Y, Yang G, Aksoy S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc Natl Acad Sci USA. 2009;106:12133–12138. doi: 10.1073/pnas.0901226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, et al. Infections with immunogenic trypanosomes reduce tsetse reproductive fitness: Potential impact of different parasite strains on vector population structure. PLoS Negl Trop Dis. 2008;2:e192. doi: 10.1371/journal.pntd.0000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Zaidman-Rémy A, et al. Drosophila immunity: Analysis of PGRP-SB1 expression, enzymatic activity and function. PLoS ONE. 2011;6:e17231. doi: 10.1371/journal.pone.0017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haines LR, Hancock RE, Pearson TW. Cationic antimicrobial peptide killing of African trypanosomes and Sodalis glossinidius, a bacterial symbiont of the insect vector of sleeping sickness. Vector Borne Zoonotic Dis. 2003;3:175–186. doi: 10.1089/153036603322662165. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem Mol Biol. 2005;35:105–115. doi: 10.1016/j.ibmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Rio RV, Wu YN, Filardo G, Aksoy S. Dynamics of multiple symbiont density regulation during host development: Tsetse fly and its microbial flora. Proc Biol Sci. 2006;273:805–814. doi: 10.1098/rspb.2005.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashyap DR, et al. Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems. Nat Med. 2011;17:676–683. doi: 10.1038/nm.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. Zebrafish peptidoglycan recognition proteins are bactericidal amidases essential for defense against bacterial infections. Immunity. 2007;27:518–529. doi: 10.1016/j.immuni.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 32.Snyder AK, Deberry JW, Runyen-Janecky L, Rio RV. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proc Biol Sci. 2010;277:2389–2397. doi: 10.1098/rspb.2010.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welburn SC, Maudlin I. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann Trop Med Parasitol. 1992;86:529–536. doi: 10.1080/00034983.1992.11812703. [DOI] [PubMed] [Google Scholar]
- 34.Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nat Immunol. 2009;10:936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- 35.Ruland J. Return to homeostasis: Downregulation of NF-κB responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- 36.Royet J, Dziarski R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 37.Sadd BM, Schmid-Hempel P. Facultative but persistent trans-generational immunity via the mother’s eggs in bumblebees. Curr Biol. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Tidbury HJ, Pedersen AB, Boots M. Within and transgenerational immune priming in an insect to a DNA virus. Proc Biol Sci. 2011;278:871–876. doi: 10.1098/rspb.2010.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maudlin I. Inheritance of susceptibility to Trypanosoma congolense infection in Glossina morsitans. Ann Trop Med Parasitol. 1982;76:225–227. doi: 10.1080/00034983.1982.11687531. [DOI] [PubMed] [Google Scholar]
- 40.Maudlin I, Dukes P. Extrachromosomal inheritance of susceptibility to trypanosome infection in tsetse flies. I. Selection of susceptible and refractory lines of Glossina morsitans morsitans. Ann Trop Med Parasitol. 1985;79:317–324. doi: 10.1080/00034983.1985.11811925. [DOI] [PubMed] [Google Scholar]
- 41.Welburn SC, Maudlin I. Rickettsia-like organisms, puparial temperature and susceptibility to trypanosome infection in Glossina morsitans. Parasitology. 1991;102:201–206. doi: 10.1017/s0031182000062491. [DOI] [PubMed] [Google Scholar]
- 42.Brun R, Schönenberger M. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- 43.Haines LR, et al. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl Trop Dis. 2009;3:e373. doi: 10.1371/journal.pntd.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.