Abstract
An imbalance of commensal bacteria and their gene products underlies mucosal and, in particular, gastrointestinal inflammation and a predisposition to cancer. Lactobacillus species have received considerable attention as examples of beneficial microbiota. We have reported previously that deletion of the phosphoglycerol transferase gene that is responsible for lipoteichoic acid (LTA) biosynthesis in Lactobacillus acidophilus (NCK2025) rendered this bacterium able to significantly protect mice against induced colitis when delivered orally. Here we report that oral treatment with LTA-deficient NCK2025 normalizes innate and adaptive pathogenic immune responses and causes regression of established colonic polyps. This study reveals the proinflammatory role of LTA and the ability of LTA-deficient L. acidophilus to regulate inflammation and protect against colonic polyposis in a unique mouse model.
Keywords: dendritic cells, regulatory T cells, anti-inflammatory
Identifying bacterial gene products that enhance protective versus pathogenic inflammation in the gut is critical to rebalance homeostasis in gastrointestinal (GI) chronic inflammatory diseases and malignancies. Commensal Lactobacillus species (i.e., Lactobacillus acidophilus) are normal inhabitants of the natural microbiota in the human GI tract (1, 2). L. acidophilus stimulates innate cells to produce inflammatory and regulatory cytokines through interaction of their surface layer proteins and other cell surface components (3–7). To investigate the potential role of lipoteichoic acid (LTA) of L. acidophilus in the induction of inflammatory signals, we deleted the phosphoglycerol transferase gene (LBA0447) that synthesizes LTA. LTA is a zwitterionic glycolipid found in the cell wall of several Gram-positive bacterial strains, including L. acidophilus, which stimulates dendritic cells (DCs) through Toll-like receptor 2, resulting in cytokine release (8, 9). Disruption of LTA synthesis generated a L. acidophilus derivative (NCK2025) that mitigates colitis in mice (10). Based on these observations and those of others (11, 12), it was proposed that LTA induces inflammation and that its absence significantly attenuates overt intestinal inflammation.
Inflammation has a tumor-promoting role in mice with polyposis and in human colon cancer (13–17). T regulatory cells (Tregs) critically regulate inflammation and play a protective role in polyposis (15, 18) and colon cancer (19–21). However, chronic interaction of Tregs with proinflammatory cells and their cytokines can reverse the anti-inflammatory properties of these cells and render them proinflammatory (15, 16, 22, 23). Consensus suggests that interactions between lymphocytes and myeloid cells regulate pro- vs. antitumor immunity (17, 24), and we hypothesized that the gut microbiota plays an essential role in control of this balance. We used L. acidophilus NCK2025, deficient in LTA, to investigate the moderation of pathogenic inflammation within the tumor microenvironment in a unique mouse model of colonic polyposis.
Results
Ablation of Polyps by LTA-Deficient L. acidophilus in TS4Cre × APClox468 Mice.
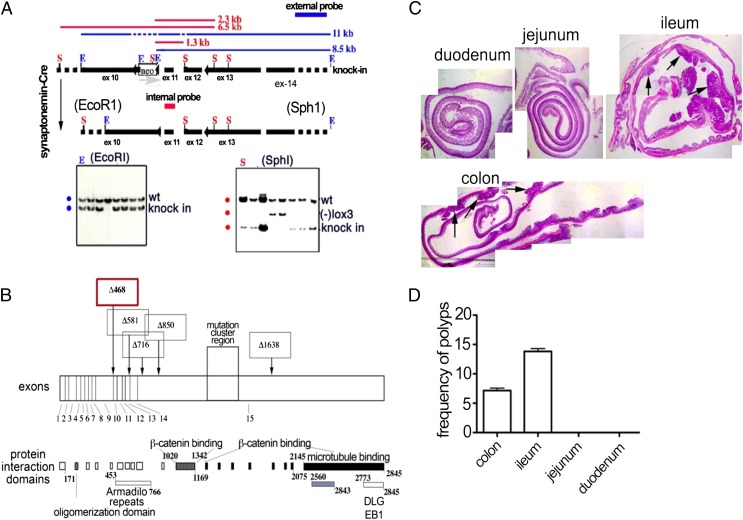
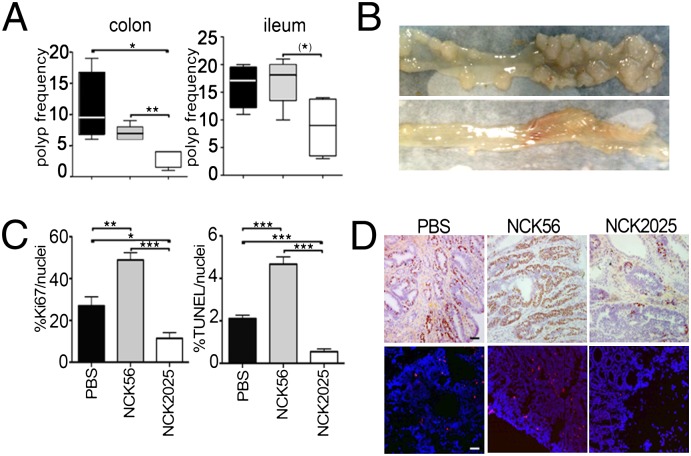
To target a key carcinogenic event in the colon, homologous recombination was used to introduce loxP sites on either side of exons 11 and 12 of the mouse adenomatous polyposis coli (APClox468) gene (Fig. 1 A and B). These mice (APClox468) were crossed to TS4-Cre transgenic mice (25) that express Cre specifically in the colon and distal ileum. Mice double heterozygous for conditional APClox468 and TS4-Cre were generated and aged. As early as 4 to 5 mo of age, extensive polyposis was detected throughout the colon and distal ileum, but not in the proximal ileum, jejunum, or duodenum (Fig. 1 C and D). To analyze the immunomodulatory properties of NCK2025, TS4Cre × APClox468 mice 5 mo of age were treated daily with oral doses of NCK2025 (5 × 108 cfu/mouse), or were fed water as a control for 4 wk. To specifically address the role of LTA, a third group of mice was treated in a similar manner with the parental LTA+ strain, L. acidophilus NCK56. After 4 wk of treatment, all mice were euthanized and analyzed. There was little to no change in polyposis in NCK56-treated mice compared with control PBS solution-treated mice. In contrast, NCK2025-treated mice had a reduced number of polyps in the distal ileum and significantly decreased polyps in the colon (Fig. 2 A and B). Mitotic and apoptotic, but not intranuclear, β-catenin activities (data not shown) were significantly reduced in polyps of NCK2025-treated mice compared with PBS solution- and NCK56-treated mice (Fig. 2 C and D). Mice treated with NCK56 had higher intrapolyp mitotic and apoptotic activities compared with untreated or NCK2025-treated mice (Fig. 2 C and D). These observations demonstrate the therapeutic properties of oral NCK2025 treatment in mice with preestablished colonic polyposis.
Fig. 1.
Conditional truncation of the APC gene at exon 468 to generate APClox468 mice. (A) A cosmid clone spanning exons 10 to 14 of the mouse APC gene was modified to encode a loxp flanked neoR gene cassette upstream of exon 11 and a third loxP sequence carrying an SphI site immediately downstream of APC exon 12. The entire cassette was excised and transfected into TC1 (129/SV) embryonic stem cells (46), and NeoR clones were picked. An external (thick blue line) and an internal (thick red line) probe were used to detect homologous recombination by DNA Southern blot, as revealed by SphI or EcoRI fragments spanning upstream sequences (2.3 kb and 6.5 kb for wt and recombinant alleles, respectively; red lines) or downstream (11 kb and 8.5 kb for wt and recombinant alleles, respectively; blue lines). Loss of the third loxP site produced a smaller SphI fragment of 1.3 kb. The neoR cassette was removed by crossing to synaptonemal-Cre mice and screening. Mice were backcrossed for more than 20 generations to C57BL/6J. (B) Schematic representation of the truncation at exon 468 compared with other truncations from previously reported mouse models of polyposis. (C) Distribution of polyps in the GI tract of TS4Cre × cAPClox468 mice. Pictures were taken at a magnification of ×16 and stitched by using Mosaic J plug-in of ImageJ. (D) Compiled frequencies of polyps from six TS4Cre × cAPClox468 mice at 4 mo of age.
Fig. 2.
Status of polyps in TS4Cre × cAPClox468 mice (n = 6) treated with PBS solution, NCK56, or NCK2025. (A) Polyps were counted in the ileum and colon, and results are presented in box-and-whisker plots. (B) Photographs of representative colons of mice treated with PBS solution (Upper) or NCK2025 (Lower). (C) Quantification of Ki-67+ and TUNEL+ cells in polyps of mice treated with PBS solution, NCK56, or NCK2025. (D) Staining of colonic polyp for Ki-67 (Upper) and TUNEL (Lower) of 6-μm sections from paraffin-embedded tissue of mice treated with PBS solution, NCK56, or NCK2025. (Scale bar: 50 μm.) Images were acquired by using Tissue Gnostics and analyzed with ImageJ. The data were statistically analyzed by unpaired t test (***P < 0.0001, **P < 0.004, and *P < 0.0176). Ki-67 (iHistochem) and TUNEL (Millipore) staining was performed per manufacturers’ instructions. Data are representative of three independent experiments.
Immune Regulation by LTA-Deficient L. acidophilus.
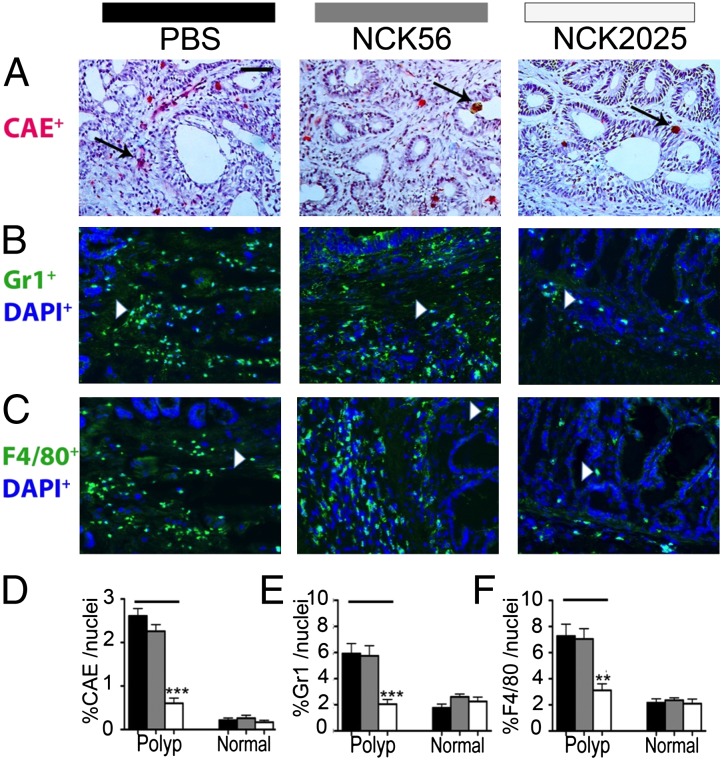
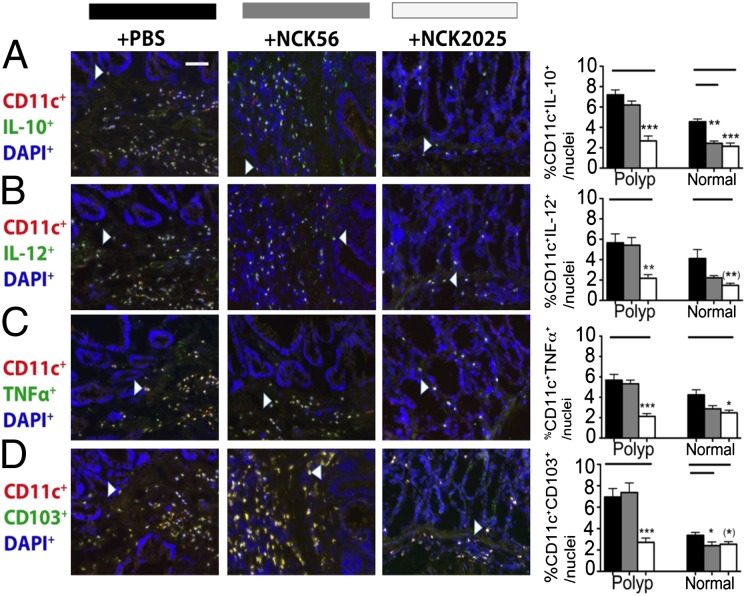
To provide evidence for dampened inflammation in NCK2025-treated mice, immunofluorescent staining was performed throughout the colon. Previously, the expansion and activation of mast cells in adenomatous polyps was reported, and the evidence supported their tumor-promoting role (13, 17, 26). Therefore, we quantified intrapolyp mast cell densities in TS4Cre × APClox468 NCK2025-treated mice compared with mice fed the parental NCK56. Significant decreases in the intrapolyp mast cell counts in NCK2025-treated mice were observed; however, no differences were observed between NCK56- and PBS solution-treated mice (Fig. 3 A and D). Polyps were infiltrated with relatively high densities of CD11b+F4/80+ macrophages and CD11b+Gr1+ myeloid cells that based on their differential expression of Ly6C and Ly6G markers, we had defined earlier to be a mixture of monocytic and granulocytic myeloid derived suppressor cells (14, 27). Therefore, the impact of treatments on intrapolyp densities of these cells was quantified. Treatment with NCK2025 resulted in significant decreases in the intrapolyp densities of F4/80+ and Gr1+ cells, approaching levels typical of the healthy gut (Fig. 3 B, C, E, and F). Densities of these cells did not change in NCK56-treated mice. Mucosal immunity is imprinted by DCs (28–31). To reveal the impact of the treatments, we determined the intrapolyp expression of IL-10, IL-12, or TNF-α by resident DCs. Overall, numbers of intrapolyp DCs tended to be higher compared with healthy neighboring tissue, but this difference was not statistically significant (Fig. 4 A–C), with the exception of CD103+ DCs, which were significantly elevated within the polyps (Fig. 4D). Treatment with NCK2025 lowered the DC counts to levels approaching those of healthy WT mice, whereas NCK56 had little impact (Fig. 4 A–D). Thus, reductions in intrapolyp densities of DCs and proinflammatory cells suggest that NCK2025 had successfully reset the pathogenic immune environment of the gut in polyp-ridden mice back toward its balanced physiological state.
Fig. 3.
Attenuation of intrapolyp inflammation in TS4Cre × cAPClox468 by NCK2025 treatment. (A) CAE-positive mast cells, (B) Gr1+ granulocytes, (C) F4/80+ macrophages, (D) frequencies of intrapolyp CAE+ cells (***P < 0.0001), (E) frequencies of intrapolyp Gr1+ cells (***P < 0.0005), and (F) frequencies of intrapolyp F4/80+ cells (**P < 0.006). (Scale bar: 50 μm.) Black arrows show mast cells, and white arrowheads show F4/80+ or Gr1+ cells for PBS solution-, NCK56-, and NCK2025-treated mice (n = 5 per group). Error bars show SEM. Data were analyzed by two-tailed Student t test, type 2, compared with untreated mice. Sections (5 μm) of frozen colon tissues were stained with CAE-specific antibodies as described previously (13). For immunostaining, primary antibodies used were rat anti-mouse F4/80 (Abcam), biotin anti-mouse Gr1, and hamster anti-mouse CD11c (BD Biosciences); secondary antibodies (DAPI; Invitrogen) were anti-hamster Alexa Fluor 594 and anti-rat Alexa Fluor 488. Tissues were then reacted with streptavidin 488 and DAPI to reveal nuclei. Images were acquired by using a TissueGnostics Tissue/Cell High Throughput Imaging and Analysis System and analyzed by using ImageJ software. Data are representative of three independent experiments.
Fig. 4.
Representation and quantification of infiltration in the polyps and normal adjacent tissues by DCs expressing immune-suppressive and proinflammatory cytokines. (A) CD11c+IL-10+ cells (***P < 0.0001 and **P < 0.001), (B) CD11c+IL-12+ cells (**P < 0.006 and **P < 0.004), (C) CD11c+TNFα+ cells (***P < 0.0005 and *P < 0.01), and (D) CD11c+CD103+ cells (***P < 0.0009 and *P < 0.05). Significant increase in intrapolyp compared with extrapolyp CD103+ DCs (**P < 0.0014). (Scale bar: 50 μm.) White arrowheads show double-positive cells. Data were analyzed by two-tailed Student t test, type 2, compared with untreated mice. Immunostaining, acquisition and analyses were performed as described for Fig. 2. Antibodies were hamster anti-mouse CD11c (BD Biosciences); rat anti-mouse IL-10, IL-12, IFN-γ, and TNF-α (BioLegend); anti-hamster Alexa Fluor 594; and anti-rat Alexa Fluor 488. Black bars indicate PBS solution treatment; gray bars, NCK56; and white bars, NCK2025. Data are representative of three independent experiments.
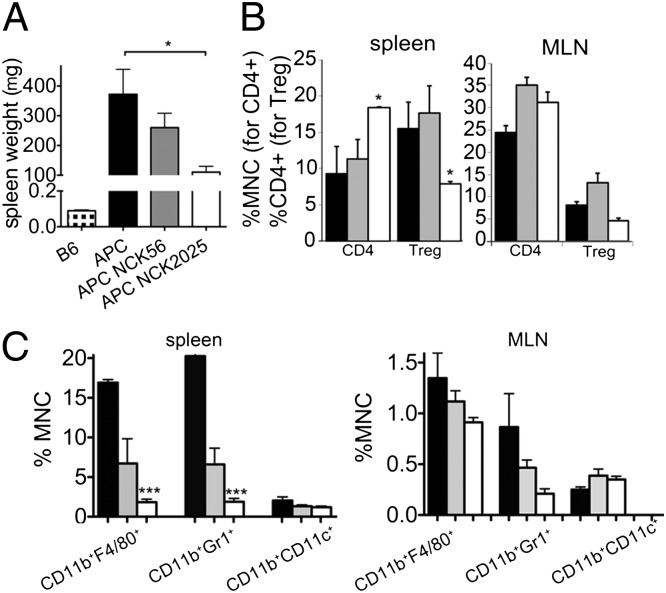
Previously, we reported that, in the hereditary polyposis mouse model, inflammation associated with polyposis becomes systemic and mice develop splenomegaly and elevated levels of serum proinflammatory cytokines (14). Aged TS4Cre × APClox468 mice also developed splenomegaly (Fig. 5A). Treatment with NCK2025 induced significant reductions in splenic size, whereas NCK56 treatment showed similar, although statistically insignificant, trends (Fig. 5A). Reduction in splenic size correlated with increased relative frequencies of CD4+ effector T cells and reduced Tregs (Fig. 5B), as well as reduced macrophages and granulocytes in the spleen (Fig. 5C). Similar but not significant trends were also seen in the mesenteric lymph nodes (MLNs; Fig. 5 B and C). These changes coincided with significant decreases in serum levels of IL-10 as well as proinflammatory cytokines, but with increases in IL-22; both the NCK2025 and parental NCK56 strains caused decreases in IL-6 and IL-10, and showed a trend to decreased IFN-γ (Fig. S1). Together, these findings demonstrate that administration of NCK2025 to mice with established polyps down-regulates inflammation and resets both local and systemic immunity, whereas the parental L. acidophilus NCK56 elicits an intermediate response.
Fig. 5.
Attenuation of systemic inflammation in TS4Cre × cAPClox468 mice by NCK2025 treatment. (A) Splenic weights in TS4Cre × cAPClox468 mice (n = 5 per group) that were untreated or treated with L. acidophilus strains (*P < 0.026). (B) Frequencies of CD4+Foxp3− effector T cells and CD4+Foxp3+ Tregs in the spleen and MLN of untreated and treated TS4Cre × cAPClox468 mice; splenic CD4+ T cells (*P < 0.065) and splenic Tregs (P < 0.026). (C) Frequencies of CD11b+F4/80+ macrophages, CD11b+Gr1+ granulocytes, and CD11b+CD11c+ DCs among spleen-derived leukocytes of untreated or treated mice; splenic CD11b+F4/80+ cells (***P < 0.0001) and splenic CD11b+Gr1+ cells (***P < 0.0004). (C) Same frequencies among MLN-derived leukocytes. Histograms show compiled results with SEM; trends shown were not statistically significant. Student t test was performed with two tails, type 2 between untreated and treated groups. Single cell suspensions were filtered (40 μm), and red blood cells were lysed by using Ack Lysing Buffer (BioWhittaker). Cells were washed and then incubated with Fc block (BD Bioscience). Dead cells were excluded (LIVE/DEAD Violet Dead cell Stain kit; Invitrogen). Antibodies used were CD11c FITC (HL3), GR1 APC (RB6-8C5), CD4 Percp (RM4-5), CD25 biotin (7D4), and streptavidin FITC (BD Pharmingen); and F4/80 Percp (BM8) and CD11b PE-Cy7 (M1/70; Biolegend). Data were acquired with a FACSCanto II device (BD) and analyzed by using FlowJo software (Tree Star). Black bars indicate untreated mice, gray bars NCK56-treated mice, and white bars NCK2025-treated mice. Data are representative of two independent experiments.
Sustained Phenotype of Foxp3+ Regulatory T Cells by LTA-Deficient L. acidophilus.
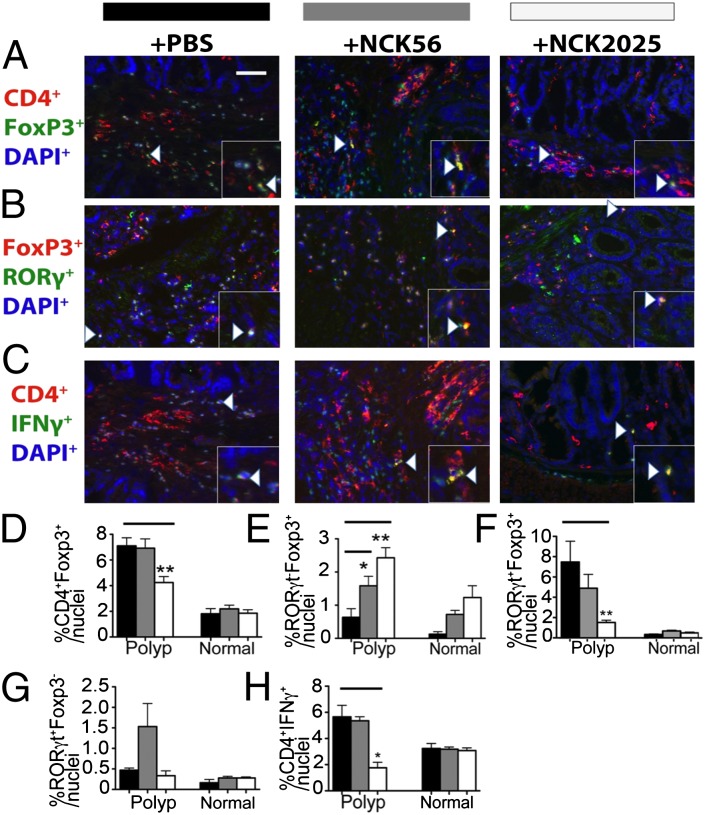
Tregs play a dual role in cancer, increasing in total number and suppressing protective T-cell immunity as well as protecting against cancer through suppression of inflammation. In cancer and chronic inflammation, Tregs can lose their anti-inflammatory properties. This results via expression of RORγt and loss of Foxp3, leading to conversion to TH17 cells (32–35). Recently, we reported that, in polyposis and colon cancer, Tregs can gain proinflammatory TH17-like properties while maintaining their ability to suppress T cells (15, 16, 36, 37). This subset of Tregs critically contributes to escalation of cancer-associated inflammation and promotes tumor growth (15, 16, 22). In consideration of the reduced inflammation in mice treated with NCK2025, we assessed the frequencies of bona fide Foxp3+ Tregs defined as Foxp3+RORγt− stained cells vs. Tregs with TH17 characteristics, defined as Foxp3+RORγt+ cells. The overall densities of Foxp3+ cells decreased in polyps of mice treated with NCK2025, but not in mice treated with the parental NCK56 (Fig. 6 B and D). Interestingly, double staining for Foxp3 and RORγt. revealed a critical change in the quality of polyp-infiltrating Tregs with differential changes in densities of bona fide vs. proinflammatory Tregs. Thus, polyps of NCK2025-treated mice showed significant increases in densities of bona fide Foxp3+ Tregs (Fig. 6 B and E), but decreases in densities of Foxp3+RORγt+ proinflammatory Tregs (Fig. 6 B and F); mice treated with NCK56 showed intermediate responses (Fig. 6 B, E, and F). Levels of RORγt-expressing non-Tregs did not change with NCK2025, whereas proinflammatory CD4+IFNγ+ T cells were reduced (Fig. 6 B and G). These observations illustrate a shift in balance from pro- to anti-inflammatory Tregs in the polyp microenvironment of NCK2025-treated mice, with a corresponding reduction in inflammatory helper T cells. These findings highlight the importance of analyzing Treg subsets separately, and implicate NCK2025 in the improvement of Treg protective function in mice with polyposis. Treatment with NCK2025 reduced the frequencies of IFN-γ–producing CD4+ T-cells, in line with the attenuation of inflammation in these mice (Fig. 6 C and H).
Fig. 6.
Change in quality of polyp-infiltrating Tregs and helper CD4+ T cells. Representations of polyp-infiltrating (A) CD4+Foxp3+ Tregs, encompassing anti- and proinflammatory subsets; (B) cells expressing RORγt and/or Foxp3, encompassing anti- and proinflammatory Tregs, as well as proinflammatory T cells. (C) CD4+IFN-γ+ proinflammatory T-cells: mice (n = 5 per group) were untreated or treated with PBS solution, NCK56, or NCK2025 as indicated. Quantification in the polyps and normal adjacent tissue of (D) CD4+Foxp3+ Tregs, encompassing anti- and proinflammatory subsets (*P < 0.004); (E) RORγt−Foxp3+ anti-inflammatory Tregs (**P < 0.002, n = 5; and *P < 0.04, n = 5); (F) RORγt+Foxp3+ proinflammatory Tregs (**P < 0.008, PBS solution and NCK2025); (G) RORγt+Foxp3− proinflammatory T cells (trend not statistically significant); and (H) CD4+IFN-γ+ proinflammatory effector T cells (*P < 0.016). (Scale bar: 50 μm.) White arrowheads represent F4/80+ or Gr1+ cells. Data were analyzed by two-tailed Student t test, type 2, compared with untreated mice. Immunostaining, acquisition, and analyses were performed as described for Fig. 3. Antibodies used were mouse anti-mouse CD4 (Abcam), rat anti-mouse IFN-γ (BioLegend), rat anti-mouse Foxp3 (eBiosciences), rabbit anti-mouse RORγt, anti-rat Alexa Fluor 488, anti-mouse Alexa Fluor 594, and anti-rabbit Alexa Fluor 488. Black bars indicate PBS treatment, gray bars NCK56, and white bars NCK2025. Insets in corners of each image show higher magnifications. Data are representative of three independent experiments.
Discussion
Colorectal cancer is the second most common cause of cancer deaths in the United States and other developed countries, despite important advances in detection, surgery, and chemotherapy (38). In the past few decades, the link between induced pathogenic inflammation and colonic tumorigenesis has been well established, with significant supporting evidence from immunogenetic, pharmacological, and epidemiological studies. Inflammation and its establishment are known to be complex processes in response to changes in the homeostatic condition. It has been postulated that, although inflammation is beneficial for the host, it can often progress beyond manageable levels with deleterious results. Thus, with the initiation of inflammatory responses, simultaneous commencement of anti-inflammatory signals is triggered to reestablish a balanced homeostasis.
Insufficient levels of these anti-inflammatory or regulatory signals can lead to chronic pathogenic inflammation that is believed to promote carcinogenesis, including colon cancer. To address whether the LTA-deficient L. acidophilus strain NCK2025 could regulate induced detrimental inflammation in polyposis, as has previously been demonstrated in a murine colitis model, we generated a polyposis model whereby polyps could be developed in the colons of these mice.
The data reported here demonstrate that pathologic inflammation in mice with precancerous colonic polyps can be reset to a protective mode by oral treatment with a probiotic microbial strain with anti-inflammatory properties, L. acidophilus NCK2025. Healthy intestinal immunity is positioned in a state of equilibrium that permits accurate and rapid protective responses against pathogens while curtailing pathogenic induced inflammatory processes. This balance is achieved through microbiota-driven inflammation (39) and IL-10–dependent suppression of inflammation by Tregs (40–43). Proinflammatory and anti-inflammatory CD4+ T cells are differentiated and activated via their interactions with innate immune cells, including DCs. Regulatory or proinflammatory DCs are recognized by their expression of cytokines that induce generation of extrathymic Tregs and by proinflammatory cytokines that commit T cells to TH1 or TH17 lineages (44, 45). Gut-derived DCs play a significant role in imprinting gut homing properties to these T cells in a CD103/retinoic acid-dependent manner (28–31). In this study, the absence of LTA in the L. acidophilus strain NCK2025 results in immunomodulatory changes that alter inflammation and immunity that may oppose further progression of established colonic polyps.
Cancer inflammation is regulated by Tregs, which, in polyposis and cancer, tend to revert to a proinflammatory phenotype (15, 16). An interesting outcome of oral treatment of mice with NCK2025 was the change in polyp-infiltrating Treg subsets. Although densities of proinflammatory cells in the gut as well as in the spleen were down-regulated, relative densities of effector Foxp3+RORγt− Tregs increased, whereas Foxp3+RORγt+ proinflammatory Tregs decreased. These changes appeared to reset healthy immunity in the NCK2025-treated mice, as evidenced by a reduced number of polyps in the distal ileum and significantly decreased polyps in the colon.
Beyond emphasizing the important role of the gut microbiota in mucosal immune regulation and in cancer initiation and progression, this work demonstrates the reversibility of preneoplasia. Genetically modified L. acidophilus strains may provide new opportunities for regulating intestinal immunity to protect against pathogenic inflammation and the susceptibility to colon cancer. Additional studies are required to further characterize bacterial gene products which induce regulatory signals in innate immune cells (e.g., DCs, macrophages) that, in turn, affect intestinal homeostasis and induced inflammation, all of which play critical roles in colon cancer.
Materials and Methods
Generation of TS4Cre × APClox468 Mice.
A cosmid clone spanning exons 10 to 14 of the mouse APC gene was modified to encode a loxp flanked neoR gene cassette upstream of exon 11 and a third loxP sequence carrying an SphI site immediately downstream of APC exon 12. The entire cassette was then excised and transfected into TC1 (129/SV) embryonic stem cells (46), and NeoR clones were picked. To facilitate selection of transfected ES cells, a neoR cassette flanked by loxP sites was introduced upstream of exon 11. An external and an internal probe were used to detect homologous recombination by DNA Southern blot, as revealed by SphI or EcoRI fragments spanning upstream sequences; 2.3 kb and 6.5 kb for wt and recombinant alleles, respectively, or downstream (11 kb and 8.5 kb for wt and recombinant alleles, respectively). The neoR cassette proved to interfere with gene expression and was removed by crossing the initial founder mouse to mice expressing Cre universally but inefficiently (synaptonemal-Cre mice) (47). In the final founder, only two loxP sides remained flanking exons 11 and 12. Cre-mediated recombination truncated the APC gene at exon 468. The remaining sequences missed all β-catenin binding sites as well as the armadillo repeats, and encoded the most severely truncated APC gene product reported to date. Loss of the third loxP site resulted in a smaller SphI fragment of 1.3 kb. Subsequently, the neoR cassette was removed by crossing to synaptonemal-Cre mice and screening. Mice were backcrossed for more than 20 generations to C57BL/6J. Mice were maintained in microisolator cages under specific pathogen-free, Helicobacter-free conditions at the animal care facility. Experiments were performed in an accredited establishment according to National Institutes of Health Guidelines for the Guide for Care and Use of Laboratory Animals (NIH-72-23), and the local ethics committee approved animal protocols.
Bacterial Strains.
L. acidophilus NCK56 (NCFM) and NCK2025 were inoculated at 1% and propagated in de Man, Rogosa, and Sharpe broth (Difco) at 37 °C for 15 h. Subsequently, 1 mL of each culture was transferred to 10 mL of fresh de Man, Rogosa, and Sharpe broth and incubated at 37 °C for 18 h. In preparation for oral treatment, the concentration of each L. acidophilus strain was adjusted to 1 × 109 cfu/mL based on OD600 readings that had previously been correlated with cfu numbers (24). Each mouse was orally treated with 5 × 108 cfu bacterial strain; therefore, 500 μL of 1 × 109 cfu/mL suspension was centrifuged, pelleted, and then resuspended in 100 μL of PBS solution. Each mouse was then treated with 100 μL PBS solution (control group) or 5 × 108 cfu of either L. acidophilus strain orally in100 μL sterile PBS solution.
Immunofluorescence.
Colons of mice treated with NCK56, NCK2025, or PBS solution alone were fillet-opened, rolled, and snap frozen at −80 °C in optimal cutting temperature compound. Sections (5 μm) were cut, fixed in ice-cold methanol (−20 °C) for 15 min, and blocked with 1% BSA. Subsequently, sections were incubated overnight at 4 °C with purified hamster anti-mouse CD11c (BD Biosciences), CD4, FoxP3, RoRγ, IFN-γ, TNF-α, IL-12, GR1, F4/80, CD103, and rat anti-mouse IL-10 (BioLegend), followed by two washes with PBS solution and incubation with anti-hamster Alexa Fluor 594 and anti-rat Alexa Fluor 488 (Invitrogen) for 1 h. Sections were then washed two times with PBS solution and incubated for 10 min with DAPI (Invitrogen), washed with PBS solution two times, and mounted with antifade mounting medium as described previously (14). Images were acquired by using a Tissue/Cell High Throughput Imaging and Analysis System (TissueGnostics) and analyzed by using ImageJ software. In some experiments, mice were killed, and colon cross-sectional Swiss rolls were fixed in 10% (vol/vol) formaldehyde and embedded in paraffin. Tissue sections (4 μm) were stained for chloracetate esterase (CAE) and subsequent H&E.
Flow Cytometry.
Single-cell suspensions of spleens and MLNs of TS4Cre × cAPClox468 mice that were treated with PBS solution, NCK56, or NCK2025 were filtered (40 μm), and red blood cells were lysed by using Ack Lysing Buffer (BioWhittaker). Cells were washed and then incubated with Fc block (BD Bioscience). To exclude dead cells, a LIVE/DEAD Violet Dead cell Stain kit was used (Invitrogen). Subsequently, cells were stained with the following antibodies: CD11c FITC (HL3), GR1 APC (RB6-8C5), CD4 Percp (RM4-5), CD25 biotin (7D4), and streptavidin FITC (BD Pharmingen); and F4/80 Percp (BM8) and CD11b PE-Cy7 (M1/70; Biolegend). Data were acquired with an FACSCanto II device (BD) and analyzed by using FlowJo software (Tree Star).
Multiplex ELISA.
Sera of PBS solution-, NCK56-, or NCK2025-treated TS4Cre × cAPClox468 mice were collected and analyzed for cytokines. Multiplex ELISA was conducted according to the manufacturer’s instructions (Millipore) on filtered (0.22 μm) serum. Subsequently, results were acquired with a Luminex 100 instrument and analyzed by using xPONENT software (Luminex).
Statistical Analysis of Data.
For paired comparisons, we used a paired Student t test ran with two tails, type 2 between untreated and treated groups. For nonpaired comparisons, we used an unpaired Student t test.
Supplementary Material
Acknowledgments
We thank Dr. Paul Okunieff, Dr. David Nelson, and Dr. Glen Morris for fruitful scientific discussions. This work was supported in part by National Institutes of Health Grant 1R01AI098833-01, DoD CA111002, NIH/NCRR clinical & Translational Science Award to the University of Florida (UL1 RR029890), and the by North Carolina Agricultural Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207230109/-/DCSupplemental.
References
- 1.Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008;7:163–174. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- 2.Ouwehand AC, Salminen S, Isolauri E. Probiotics: An overview of beneficial effects. Antonie van Leeuwenhoek. 2002;82:279–289. [PubMed] [Google Scholar]
- 3.Mohamadzadeh M, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantinov SR, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci USA. 2009;106:4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh YJ, et al. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2009;75:3093–3105. doi: 10.1128/AEM.02502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh YJ, Klaenhammer TR. Genomic features of Lactobacillus species. Front Biosci. 2009;14:1362–1386. doi: 10.2741/3313. [DOI] [PubMed] [Google Scholar]
- 8.Mayer ML, Phillips CM, Townsend RA, Halperin SA, Lee SF. Differential activation of dendritic cells by Toll-like receptor agonists isolated from the Gram-positive vaccine vector Streptococcus gordonii. Scand J Immunol. 2009;69:351–356. doi: 10.1111/j.1365-3083.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 9.Mayer ML, Phillips CM, Stadnyk AW, Halperin SA, Lee SF. Synergistic BM-DC activation and immune induction by the oral vaccine vector Streptococcus gordonii and exogenous tumor necrosis factor. Mol Immunol. 2009;46:1883–1891. doi: 10.1016/j.molimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Mohamadzadeh M, et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2011;108(suppl 1):4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grangette C, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claes IJ, et al. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol. 2010;162:306–314. doi: 10.1111/j.1365-2249.2010.04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gounaris E, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gounaris E, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS ONE. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gounaris E, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blatner NR, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA. 2010;107:6430–6435. doi: 10.1073/pnas.0913683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khazaie K, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 18.Erdman SE, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 19.Correale P, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinicrope FA, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosolini M, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 22.Colombo MP, Piconese S. Polyps wrap mast cells and Treg within tumorigenic tentacles. Cancer Res. 2009;69:5619–5622. doi: 10.1158/0008-5472.CAN-09-1351. [DOI] [PubMed] [Google Scholar]
- 23.Piconese S, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 24.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- 26.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheon EC, et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res. 2011;71:1627–1636. doi: 10.1158/0008-5472.CAN-10-1923. [DOI] [PubMed] [Google Scholar]
- 28.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 29.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villablanca EJ, Cassani B, von Andrian UH, Mora JR. Blocking lymphocyte localization to the gastrointestinal mucosa as a therapeutic strategy for inflammatory bowel diseases. Gastroenterology. 2011;140:1776–1784. doi: 10.1053/j.gastro.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kryczek I, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 38.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 39.Niess JH, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 40.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhry A, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443–449. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 47.Vida F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol Reprod Dev. 1998;51:274–280. doi: 10.1002/(SICI)1098-2795(199811)51:3<274::AID-MRD6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.