Abstract
Infection by carcinogenic human papillomaviruses (HPV) results in precancers [cervical intraepithelial neoplasia (CIN)] and cancers near the ectoendocervical squamocolumnar (SC) junction of the cervix. However, the specific cells targeted by HPV have not been identified and the cellular origin of cervical cancer remains elusive. In this study, we uncovered a discrete population of SC junctional cells with unique morphology and gene-expression profile. We also demonstrated that the selected junctional biomarkers were expressed by a high percentage of high-grade CIN and cervical cancers associated with carcinogenic HPVs but rarely in ectocervical/transformation zone CINs or those associated with noncarcinogenic HPVs. That the original SC junction immunophenotype was not regenerated at new SC junctions following excision, not induced by expression of viral oncoproteins in foreskin keratinocytes, and not seen in HPV-related precursors of the vagina, vulva, and penis further support the notion that junctional cells are the source of cervical cancer. Taken together, our findings suggest that carcinogenic HPV-related CINs and cervical cancers are linked to a small, discrete cell population that localizes to the SC junction of the cervix, expresses a unique gene expression signature, and is not regenerated after excision. The findings in this study uncover a potential target for cervical cancer prevention, provide insight into the risk assessment of cervical lesions, and establish a model for elucidating the pathway to cervical cancer following carcinogenic HPV infection.
Keywords: gynecology, oncology, embryogenesis
Carcinogenic human papillomaviruses (HPVs) cause cervical cancer and its precursor, cervical intraepithelial neoplasia (CIN), at the squamocolumnar (SC) junction (1–6). Because of both cervical anatomy and hormonal status, the position of this junction varies. In most women, vaginal pH acidification occurring during adolescence induces the replacement of a portion of the endocervical columnar epithelium by a metaplastic squamous epithelium. This area of replacement [the transformation zone (TZ)] leads to the proximal migration of the SC junction.
In the last decade, the relationship between HPV infection and neoplasia has spawned new paradigms of cervical cancer prevention via HPV screening and Pap smear triage (7, 8). Moreover, successful construction of papilloma virions in vitro has resulted in vaccines that prevent many CINs and presumably, cervical cancer (9).
Although infection with HPV is necessary for the development of CIN, the components of early cervical carcinogenesis have yet to be fully assembled. HPV infection presumably initiates when the virus enters basal keratinocytes via defects in the epithelial covering, attachment to the basement membrane, and viral capsid conformational change (10). Models of HPV-mediated oncogenesis use HPV-immortalized keratinocytes or transgenic mice (11, 12). These systems have uncovered important molecular pathways; however, they have not shed light on why cervical neoplasms are topographically restricted to the SC junction (5, 6). Rather, it was generally assumed that the TZ squamous epithelium was the site of neoplastic change. Recently, it was reported that Barrett esophagus, a precursor of esophageal adenocarcinoma, is derived from a discrete population of embryonic cells residing at the gastroesophageal SC junction (13). In this report, we describe a similar, and previously unreported, population of SC junction cells in the cervix with a unique expression profile, embryonic characteristics, and relationship to carcinogenic HPV-associated cervical neoplasia.
Results
Identification and Transcriptional Analysis of SC Junction Cells.
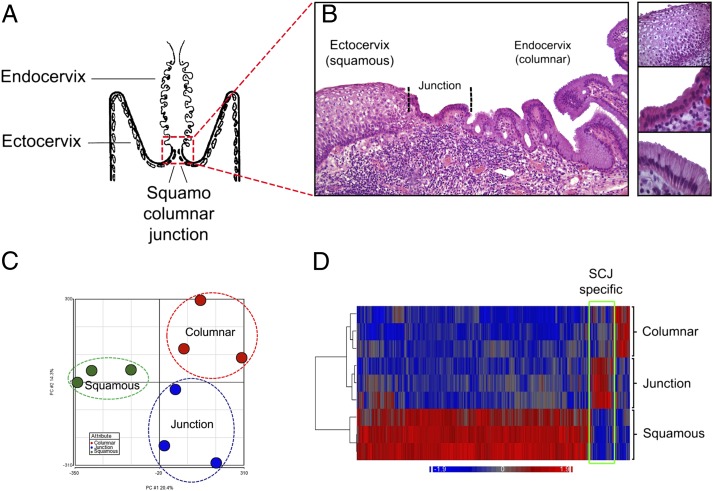
Histologic analysis of the SC junction from adults (schematic in Fig. 1A) revealed a discrete population of cuboidal epithelial cells at the interface of either the ectocervical or TZ squamous epithelium (depending on whether metaplastic replacement of columnar epithelium has taken place) and endocervix. Numbering ∼40 (38 ± 6; n = 15) in cross-section, these cells displayed a unique morphology and were designated as the SC junctional cell population (Fig. 1B). The number of cells was constant irrespective of patient age. By laser capture microdissection, three cases each of HPV-uninfected ecto- and endocervical epithelium and SC junction were sampled, and the gene expression profiles of these three populations compared. Pair-wise comparisons of Affymetrix exon arrays revealed the three sites to be distinct (Fig. 1C) and unsupervised correlation of the expression profiles of the three sites revealed discrete differences in expression, segregating the SC junction cells from the squamous and columnar cells (bracketed in the heat map in Fig. 1D). The genes from each group are tabulated in Tables S1 and S2. Seventy-seven genes were up-regulated by 2.0-fold or greater in the SC junction cells.
Fig. 1.
Identification and transcriptional analysis of SC junction cells. (A) Schematic representation of the human cervix. (B) Histology of adult cervix, with squamous (Top), junctional (Middle), and columnar (Bottom) cells. (C) Two-dimensional representation of a principle component analysis of expression microarray data derived from the three cell populations. (D) Heat map of expression microarray data anchored by a comparison between the three groups; SC junction-specific expression is bracketed.
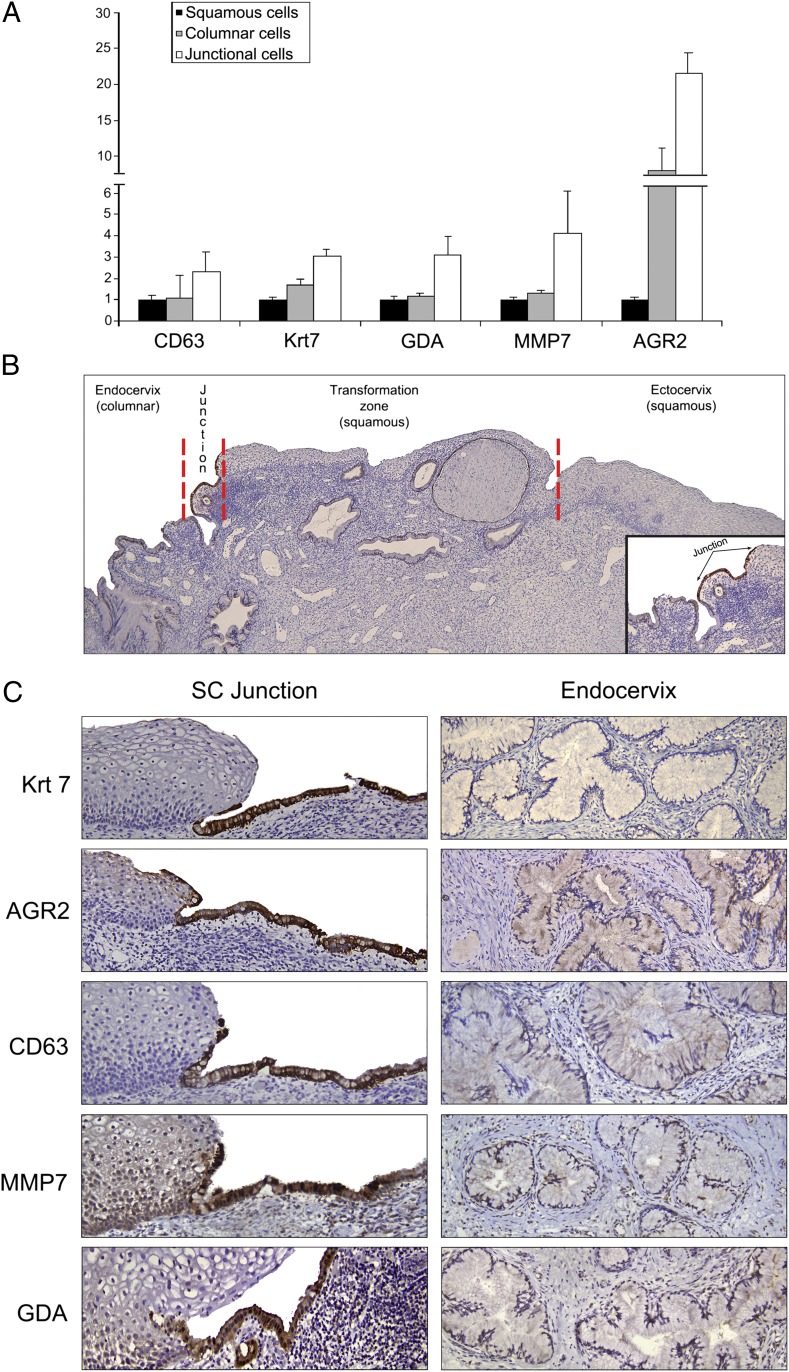
To validate some of the gene expression differences identified by microarray analysis, serial sections of adult cervix were stained with antibodies corresponding to five junction cell-specific transcripts [keratin (Krt)7, anterior gradient (AGR)2, cluster differentiation (CD)63, matrix metalloproteinase (MMP)7, guanine deaminase (GDA)] (Fig. 2A). This five-gene panel highlighted cuboidal epithelial cells immediately proximal to the mature keratinocytes. A small number of the positively staining cells extended over the surface of the adjacent stratified squamous epithelium (Fig. 2 B and C). Antibodies in this panel did not stain either the ectocervical/TZ squamous cells or the mature endocervical columnar cells (Fig. 2 B and C).
Fig. 2.
Characterization of several SC junction-specific biomarkers. (A) Fold differences in expression of five genes within the squamous, columnar, and junctional cells. These results are derived from microarray analysis. (B) Low-magnification image showing the entire cervix. Krt7 staining highlights a discrete population of cuboidal cells at the interface of the transformation zone squamous epithelium and endocervix. (C) Antibodies stain an identical cell population at the SC junction. The positive staining extends from the surface of the stratified squamous epithelium onto the adjacent basement membrane. Note the absence of staining of either the mature keratinocytes or the endocervical columnar cells.
Expression of Junction-Specific Genes in Carcinogenic HPV-Associated CINs and Carcinomas.
CINs and cancers were stained with the junction-specific antibodies to ascertain their relationship to junctional cells. CINs were divided into those caudal to the SC junction (ectocervical/TZ) and those in direct continuity with the SC junction (junctional).
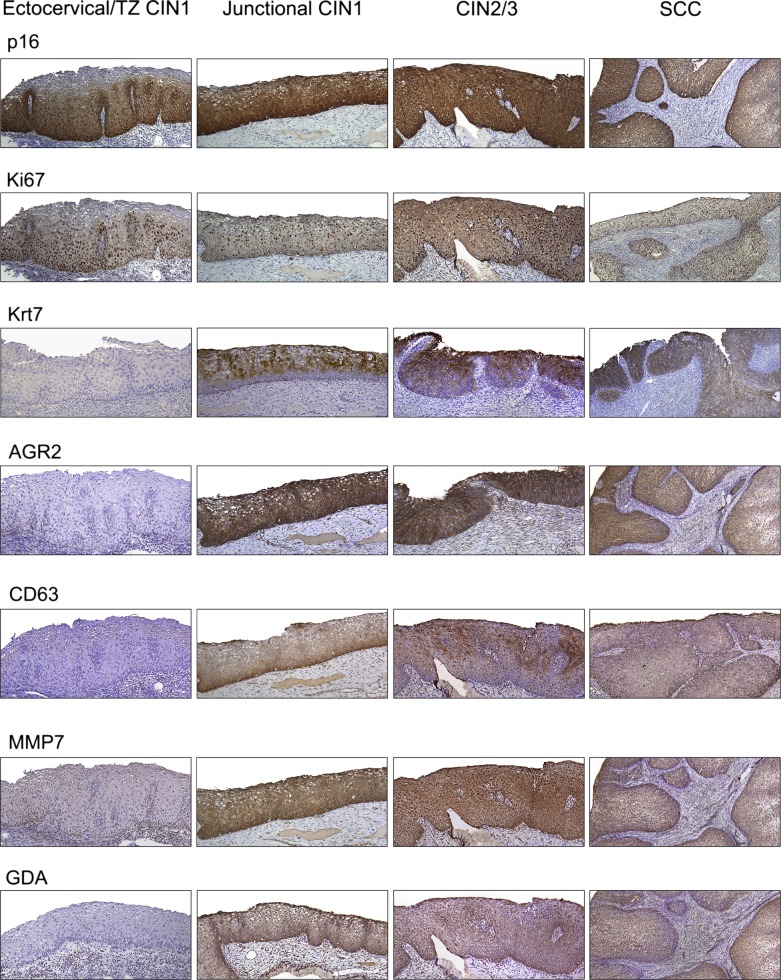
CINs are generally classified as low (CIN1) and high (CIN2/3) grade, reflecting their putative risk of progressing to malignancy. All 120 CINs and cancers tested scored positive for HPV (Table 1). All 58 high-grade CINs (CIN2s and CIN3s) and squamous carcinomas contained carcinogenic HPVs. Moreover, all involved the SC junction and displayed the immunological markers common to the junction-specific cells (Table 1 and Fig. 3). All adenocarcinomas stained positive for the SC junction-specific markers as well (Fig. S1). In contrast, 34 of 42 low-grade CINs (81%) were located either in the ectocervix or TZ and did not express junction-specific markers (Fig. 3). Of these ectocervical/TZ low-grade CINs, 10 had noncarcinogenic HPVs, and 24 had carcinogenic or “probably carcinogenic” HPVs (Table 1). Similarly to ectocervical/TZ CIN1, HPV-associated vaginal, vulvar, and penile (pre)neoplastic lesions were junction-specific marker-negative (Fig. S2). For the eight junctional CIN1s, all contained carcinogenic HPVs and all stained positive for junction-specific markers (Table 1 and Fig. 3). The patterns of p16ink4 staining in ectocervical/TZ CIN1s included both diffuse and patchy, whereas a strong and diffuse staining, continuous from basement membrane was invariably observed in all junctional CIN1s, CIN2/3s, and cancers (Table 1).
Table 1.
Immunostaining evaluation of p16ink4a and SC Junction biomarker expression and HPV genotypes in cervical specimens
| N (%) | ||||||
| Ectocervical/TZ CIN 1 (n = 34) | Junctional CIN 1 (n = 8) | CIN 2/3 (n = 48) | SCC (n = 10) | Adenocarcinoma in situ (n = 11) | Invasive adenocarcinoma (n = 9) | |
| Protein expression | ||||||
| SC junction-specific genes | ||||||
| Krt7 | 0/34 (0) | 8/8 (100) | 48/48 (100) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| AGR2 | 0/34 (0) | 8/8 (100) | 48/48 (100) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| CD63 | 0/34 (0) | 8/8 (100) | 48/48 (100) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| MMP7 | 0/34 (0) | 8/8 (100) | 48/48 (100) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| GDA | 0/34 (0) | 8/8 (100) | 48/48 (100) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| p16ink4a | ||||||
| Negative | 0/34 (0) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| Patchy | 16/34 (47) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| Diffuse basal | 8/34 (23.5) | 1/8 (12.5) | 2/48 (4.2) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| Diffuse full thickness | 10/34 (29.5) | 7/8 (87.5) | 46/48 (95.8) | 10/10 (100) | 11/11 (100) | 9/9 (100) |
| HPV type | ||||||
| Carcinogenic | ||||||
| 16 | 8/34 (23.6) | 6/8 (75) | 29/48 (60.4) | 6/10 (60) | 7/11 (63.6) | 5/9 (55.6) |
| 18 | 3/34 (8.8) | 1/8 (12.5) | 5/48 (10.4) | 1/10 (10) | 3/11 (27.3) | 3/9 (33.3) |
| 31 | 3/34 (8.8) | 0/8 (0) | 5/48 (10.4) | 1/10 (10) | 0/11 (0) | 0/9 (0) |
| 33 | 1/34 (2.9) | 0/8 (0) | 3/48 (6.2) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 35 | 0/34 (0) | 1/8 (12.5) | 1/48 (2.1) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 45 | 1/34 (2.9) | 0/8 (0) | 0/48 (0) | 1/10 (10) | 1/11 (9.1) | 0/9 (0) |
| 51 | 0/34 (0) | 0/8 (0) | 1/48 (2.1) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 52 | 2/34 (5.9) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 58 | 2/34 (5.9) | 0/8 (0) | 2/48 (4.2) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| “Probably carcinogenic” | ||||||
| 53 | 4/34 (11.8) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 66 | 0/34 (0) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| Noncarcinogenic | ||||||
| 6 | 4/34 (11.8) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 11 | 2/34 (5.9) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 40 | 1/34 (2.9) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 42 | 1/34 (2.9) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 43 | 0/34 (0) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 44 | 0/34 (0) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 54 | 2/34 (5.9) | 0/8 (0) | 0/48 (0) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| Multiple | ||||||
| 16 and 18 | 0/30 (0) | 0/8 (0) | 0/48 (0) | 1/10 (10) | 0/11 (0) | 1/9 (11.1) |
| 35 and 6 | 0/30 (0) | 0/8 (0) | 1/48 (2.1) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
| 52 and 6 | 0/30 (0) | 0/8 (0) | 1/48 (2.1) | 0/10 (0) | 0/11 (0) | 0/9 (0) |
Fig. 3.
Immunohistochemistry of sections of human cervix with CIN and squamous carcinoma for p16, Ki67, and the five SC junction-specific antibodies. Note the absence of staining of the ectocervical/TZ CIN1s and uniform staining of junctional CINs of all grades and squamous carcinoma. The expression of SC junction-specific markers was assessed in 34 ectocervical/TZ CIN1, 8 junctional CIN1, 48 CIN2/3, and 10 cancers.
Topographic Specificity of the SC Junction Immunophenotype.
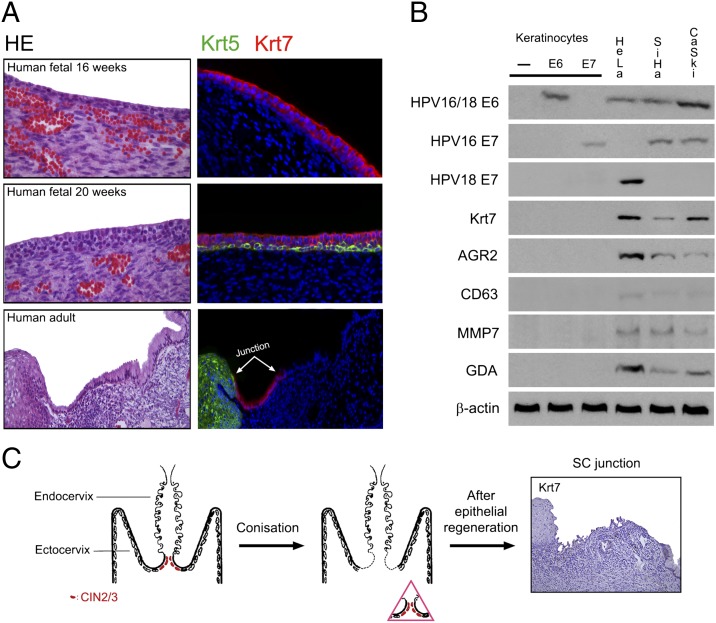
In the 16-wk human fetus, Krt7-positive cells were widely distributed throughout the cervix (Fig. 4A, Top), before the onset of squamous (Krt5) differentiation. The fetal cervical epithelium also stained positively with the other SC junction markers (Fig. S3). At ∼20 wk of gestation, basal Krt5 expression emerged, and the primitive Krt7-positive epithelial cells maintained their apical position above the stratifying squamous epithelium (Fig. 4A, Middle). In the adult, the Krt7-positive cells were confined to the SC junction (Fig. 4A, Bottom) and were distinct from the Krt5-positive ectocervical/TZ squamous epithelium.
Fig. 4.
Topographic specificity of the SC junction immunophenotype. (A) Fluorescence micrograph of human cervix at 16 wk (Top) showing diffuse Krt7 immunopositivity. At 20 wk of gestation, basal Krt5 expression emerges (Middle). In the adult cervix (Bottom), the Krt7 staining is limited to the SC junction. For each case, a corresponding histology image [hematoxylin–eosin staining (HE)] is shown. (B) Western blots of lysates of control (−), HPV16 E6- or E7-expressing primary human keratinocyte cultures, cervical adenocarcinoma (HeLa), and squamous carcinoma (SiHa, CaSki) reacted with antibodies specific for the SC junctional cells. Only cervix-derived tumor cells (HeLa, SiHa, and CaSki) score positive. (C) Schematic illustration of the squamocolumnar junction before and after LEEP (Left and Center). Absence of Krt7 staining in a “new” SC junction following LEEP (Right).
To address the possibility that transformation of basal keratinocytes by carcinogenic HPVs could induce up-regulation of SC junction-specific genes and mimic the SC junction immunophenotype, protein lysates from HPV16 E6- or E7-expressing primary human keratinocyte cultures and cervix-derived HPV18-associated adenocarcinoma (HeLa) and HPV16-related squamous carcinoma (SiHa, CaSki) cells were isolated and immunoblotted with SC junction-specific antibodies. As shown in Fig. 4B, viral oncoproteins did not induce SC junction marker expression in vitro, and SC junction-specific staining was limited to the cervical tumors.
To determine whether the SC junction-specific immunophenotype is regenerated following excision of the SC junction, we identified new SC junctions in one or more tissue blocks from 11 of 20 hysterectomy specimens following cone biopsy or loop electrical excision procedure (LEEP). Where recorded, hysterectomies were performed from 1 to 23 mo following the excision procedure and half were performed within 3 mo. New SC junctions were defined by the juxtaposition of mature ectocervical squamous epithelium and endocervical or lower uterine segment mucosa and were immunostained with SC junction-specific markers. None of these new SC junctions displayed the SC junction immunophenotype in contrast to all of the original SC junctions (Fig. 4C and Fig. S4).
Discussion
This study reveals a discrete population of cells at the squamocolumnar junction of the cervix that could be responsible for most, if not all, HPV-associated cervical carcinomas. We show that this group of junctional cells has a unique gene expression profile that is different from that of the adjacent endocervical and ectocervical/TZ epithelium and that SC junction markers are maintained in both squamous cell carcinomas and adenocarcinomas that emanate from this region. Although we cannot rule out de novo emergence of this cuboidal cervical cell type during adult life, the SC junction marker expression displayed by fetal cervical epithelium (Fig. 4 and Fig. S3) supports the embryonic origin of these SC junction cells. The fact that these cells are either within or in close proximity to the SC junction is consistent with the historically supported assumption that cervical cancer and its precursors originate in this site (5, 6). These observations, combined with the overlap in immunophenotypic identity between SC junction cells and HPV-induced squamous and columnar neoplasms, suggest that this unique population of cells at the SC junction forms a core group that can spawn multiple tumor phenotypes (Table 1).
The cell of origin for cervical cancer has been subject to speculation for some time but has traditionally been linked to the squamous epithelium in the TZ. Interestingly, we found that the SC junction population is not a stratified squamous epithelium (the prototype for most models of HPV infection in the TZ) but a single layer of cuboidal epithelial cells (Figs. 1, 2, and 4). The observation in this report that SC junction cells are the prime target for cervical carcinogenesis does not deny the prevailing theory that epithelial disruption and basal keratinocyte infection lead to HPV infection (10), because it is likely the mechanism for HPV-related lesions developing in the TZ, ectocervix, and other mucosal sites. Moreover, columnar or reserve cells have also been proposed as targets of infection (14). However, their broader topographic distribution argues against a unique role in cancer development.
An important opportunity from this study would be to exploit the SC junction immunophenotype to more precisely understand cancer risk in early cervical neoplasia. At present, three parameters provide risk information, including carcinogenic HPV, expression of p16ink4, and histologic grade (7, 8, 15–19). Carcinogenic HPVs and strong lesional expression of p16ink4 are consistently found in all high grade CINs and carcinomas (7, 8, 15–19); nevertheless, they will not discriminate this collective group from most CIN1s because the latter frequently harbor carcinogenic HPVs (17, 19). Moreover histologic estimates of lesion grade are subjective and subject to observer error. Although their biological role in the context of cervical carcinogenesis is still unknown, SC junction-specific markers provide three important perspectives that could be relevant to the role of this region in tumor development. First, they seem to be a constant in all high-grade CINs and cancers, supporting their common origin. Second, they are virtually absent in most low-grade CINs, irrespective of HPV type, implying the latter are derived from the ectocervix/TZ, and thus pose a lower risk of progression (Table 1 and Fig. 3). Third, SC junction markers highlight a minority of CIN1s that shares a SC junction location, a high frequency of HPV16 positivity, and strong p16ink4 immunostaining with high grade CINs and cancers. Thus, in nearly all of the cases that we studied, the SC junction markers described in this report precisely predicted the cervical cancer precursors with the highest likelihood of harboring the most carcinogenic HPV (HPV16), irrespective of their histologic grade. However, the exact predictive value of these biomarkers for CIN1s that will progress in grade needs to be confirmed by determining the risk of a CIN2/3 outcome, which, in itself, would require a prospective study.
Recently, Wang et al. uncovered a similarly unique cell population at the SC junction of esophagus and stomach proposed as the cellular origin of Barrett’s esophagus, the precursor of esophageal adenocarcinoma (13). Wang et al. suggest that at the esophagogastric junction, the significance of this population lies in its potential to attach to and expand along the basement membrane when esophageal squamous cells are experimentally damaged (13). In the cervix, although some cells score positive for Ki67 without evidence of mitosis (Fig. S5), expansion of the SC junction cells presumably occurs prior to or in concert with carcinogenic HPV infection, and the end result is a range of phenotypes, including both columnar and squamous differentiation. The requirement of the SC junction cells for this to occur in the context of carcinogenic HPV infection is underscored by two observations in this study. The first is the fact that SC junction markers were specific only for cell lines from cervical carcinomas (HeLa, CasKi, SiHa). In contrast, human foreskin keratinocytes expressing HPV oncoproteins were SC junction marker-negative (Fig. 4B). The second is the lack of SC junction markers in lesions arising in other genital sites, including vagina, vulva, and penis (Fig. S2).
An intriguing observation was the loss of the SC junction immunophenotype following excision. There is a high cure rate for CIN following successful excision or ablation of the SC junction (20, 21). Cure is usually attributed to the combination of removing the preinvasive neoplasm and a secondary immune system that prevents reinfection by HPV. This study introduces the additional possibility that the recurrence risk is also low because susceptible SC junction cells are not regenerated following excision (Fig. 4C and Fig. S4), one not inconsistent with the traditional link between the SC junction and cancer risk (5, 6). This implies that preemptive ablation of the SC junction would remove the embryonic cell population and possibly prevent cervical cancer. Whether this approach would actually work remains unclear, and the possibility that the SC junction cells expand during cervical remodeling must be taken into account and resolved by comparing SC junctions across a wide age range.
The mechanisms by which viral–host cell interactions lead to malignancy in the SC junction population remain to be elucidated. However, the SC junction expression data provide a unique template to study SC junction-specific transgenic models of HPV carcinogenesis. Whereas several transgenic mouse models of cervical cancer have been established using krt14 promoter-driven HPV oncogenes in the murine cervix, SC junction-specific promoters can deliver a more precise mouse model of which the HPV oncogenes are expressed in a cell type that is uniquely vulnerable to carcinogenic HPVs. A final question is whether similar populations of SC junctional cells are responsible for neoplasms tied to carcinogenic HPV infections in other sites, such as the anus and oropharynx (22–24). The observation of a discrete population of epithelial cells at the anorectal SC junction (Fig. S6) opens the door to this possibility.
Materials and Methods
This study was approved by the Human Investigation Committee of the Brigham and Women’s Hospital.
Case Material and Tissue Classification.
Cervical tissues were obtained from discarded fresh and archival material in the Women’s and Perinatal Pathology Division. Sections of 15 normal cervices from women who underwent total hysterectomy for non-cervical-related neoplasms or benign uterine disease were examined for cells at the SC junction that were morphologically distinct from ecto- and endocervical epithelium. One hundred consecutive CINs and squamous cell carcinomas from biopsies or excisions in the pathology archive were reviewed by two observers and classified as CIN1 or CIN2/3, and invasive carcinoma, without knowledge of their HPV or biomarker status (25). When the diagnoses were not concordant between the first two observers, a third pathologist reviewed the slide, and the majority diagnosis (two of three) was assigned. Twenty HPV-related adenocarcinoma specimens were also analyzed and classified as in situ or invasive cancer. To avoid misclassification of benign epithelial abnormalities, each biopsy (including cancers) was HPV typed with the HPV type-specific primers (Table S3). Moreover, each case was immunostained for p16ink4, a biomarker highly expressed in cervical carcinogenesis and KI67, a DNA proliferation marker increased in CINs (Fig. 3) (15–17). Immunostaining and designation of lesion location was performed by another observer not involved in the pathology review. A separate set of 20 hysterectomy specimens, from patients ranging in age from 32 to 69 y, performed after an excisional procedure (LEEP or cone biopsy) for cervical neoplasia was assessed for regeneration of the SC junction cells. The expression of junctional biomarkers was finally analyzed in 6 vaginal, 15 vulvar, and 4 penile (pre)neoplastic lesions, as well as in fetal tissues obtained from voluntary pregnancy terminations with patient consent.
HPV Detection.
Each DNA sample was subjected to PCR amplification with HPV type-specific primers targeting carcinogenic HPVs 16, 18, 31, 33, 35, 45, 51, 52, and 58; “probably carcinogenic” HPVs 53 and 66; and noncarcinogenic HPVs 6, 11, 40, 42, 43, 44, and 54. Amplified products were typed according to predicted molecular mass of the reaction product and compared with controls (26). Amplification of a fragment of the b-globin gene was used to assess the quality of the target DNA. All of the primer sequences are listed in Table S3.
Immunohistochemistry and Western Blotting.
Immunohistochemistry, immunofluorescence, and Western blot analysis were performed as described previously and imaged at the Nikon Imaging Facility at the Harvard Medical School (17, 27). Results were reviewed and verified by two observers. The primary antibodies used in this study were mouse anti-cytokeratin 7 (clone RCK105; Thermo Scientific), rabbit anti-AGR2 (Proteintech), mouse anti-CD63 (Abcam), goat anti-MMP7 (R and D systems), rabbit anti-GDA (Sigma-Aldrich), mouse anti-p16ink4 (Santa Cruz Biotechnology), rabbit anti-Ki67 (Abcam), rabbit anti-cytokeratin 5 (Clone EP1601Y; Thermo Scientific), mouse anti-HPV16/18 E6 (clone C1P5; Abcam), mouse anti-HPV16 E7 (clone 8C9; Invitrogen), mouse anti-HPV18 E7 (Santa Cruz Biotechnology), and rabbit anti-β-actin (Santa Cruz Biotechnology) antibodies. Each antibody yields a staining pattern that is consistent with gene/protein characterization data. Mouse, rabbit, and goat control IgG (Santa Cruz Biotechnology) were used as negative control.
Immunostaining Assessment.
p16ink4 immunolabeling was evaluated by using a semiquantitative score based on the extent of the staining according to an arbitrary scale. The scoring of p16ink4 included both nuclear and cytoplasmic staining and was graded as 0 (negative), 1 (rare singly dispersed positive cells), 2 (strong staining but discontinuous or limited to basal layers), and 3 (strong and diffuse staining, uniform from basal layer to epithelial surface). Regarding cytokeratin 7, AGR2, CD63, MMP7, and GDA expression, these junction-specific markers were either expressed by the large majority (>90%) of the dysplastic/cancerous cells (positive staining) or were not expressed (negative staining).
Transcription Analysis.
Fresh cervical specimens from three independent women (41, 44, and 52 y old) who underwent total hysterectomy for noncervical or benign uterine disease were embedded in OCT, sectioned on a cryostat, and stained with hematoxylin to morphologically identify ectocervical squamous, endocervical columnar, and junctional cells. To exclude the possibility that the tissue specimens were infected by HPV, each case was both immunostained for p16ink4 and HPV typed by PCR. Twelve serial frozen sections (6 μm thick) of each tissue sample were microdissected using a PALM microbeam instrument (Zeiss), and each selected cell population (squamous, columnar, or junctional cells) from different slides but from a same patient were pooled. Total RNAs were extracted using the Pico Pure RNA extraction kit (Arcturus) and were amplified using the WT Pico RNA Amplification System, WT-Ovation Exon Module and Encore Biotin Module (NuGEN Technologies) and hybridized onto GeneChip Human Exon 1.0 ST Array (Affymetrix) following the instructions of the manufacturer. GeneChip operating software was used to process all of the cell intensity (CEL) files and calculate probe intensity values. To validate sample, quality probe hybridization ratios were calculated using Affymetrix Expression Console software. The intensity values were log2-transformed and imported into the Partek Genomics Suite 6.5(beta). Exons were summarized to genes, and a one-way ANOVA was performed to identify differentially expressed genes. P values and fold-change were calculated for each analysis. Specific genes for each group (up-regulated by 2.0-fold or greater in one population of cells compared with the others) were classified according to P values. Heat maps were generated using Pearson’s correlation or Euclidean method, and Principal Component Analysis was conducted using all probe sets.
Supplementary Material
Acknowledgments
We thank the Division of Gynecologic Oncology in the Department of Obstetrics and Gynecology and Reproductive Biology at Brigham and Women’s Hospital for their cooperation. This work was supported by Defense Advanced Research Projects Agency (DARPA) Project N66001-09-1-2121; National Institutes of Health Grants RC1 HL100767, R01-GM083348, and R21CA124688; the Singapore–Massachusetts Institute of Technology Alliance for Research and Technology; the European Research Council; Agence de Nationale; the Institute of Medical Biology; and the Genome Institute of Singapore of the Agency for Science, Technology and Research. This work was also supported by Department of Defense Grant W81XWH-10-1-0289 (to C.P.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202684109/-/DCSupplemental.
References
- 1.Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshart M, et al. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3:1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crum CP, Ikenberg H, Richart RM, Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310:880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- 4.Lorincz AT, Lancaster WD, Temple GF. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J Virol. 1986;58:225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–328. [PubMed] [Google Scholar]
- 6.Marsh M. Original site of cervical carcinoma; topographical relationship of carcinoma of the cervix to the external os and to the squamocolumnar junction. Obstet Gynecol. 1956;7:444–452. [PubMed] [Google Scholar]
- 7.de Sanjose S, et al. Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman M, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koutsky LA, et al. Proof of Principle Study Investigators A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 10.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci USA. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens JE, et al. Distribution pattern and marker profile show two subpopulations of reserve cells in the endocervical canal. Int J Gynecol Pathol. 2009;28:381–388. doi: 10.1097/PGP.0b013e31819932f8. [DOI] [PubMed] [Google Scholar]
- 15.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaes R, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 17.Keating JT, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–891. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Wright TC, Jr, et al. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 19.Galgano MT, et al. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–1087. doi: 10.1097/PAS.0b013e3181e8b2c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reich O, Pickel H, Lahousen M, Tamussino K, Winter R. Cervical intraepithelial neoplasia III: Long-term outcome after cold-knife conization with clear margins. Obstet Gynecol. 2001;97:428–430. doi: 10.1016/s0029-7844(00)01174-1. [DOI] [PubMed] [Google Scholar]
- 21.Richart RM, et al. An analysis of “long-term” follow-up results in patients with cervical intraepithelial neoplasia treated by cryotherapy. Am J Obstet Gynecol. 1980;137:823–826. doi: 10.1016/0002-9378(80)90892-3. [DOI] [PubMed] [Google Scholar]
- 22.Mork J, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza G, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 24.Palefsky JM, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 25.Crum CP, Cibas ES, Rose PG, Peters WA., III . Cervical squamous neoplasia. In: Crum CP, Nucci MR, Lee KR, editors. Diagnostic Gynecologic and Obstetric Pathology. Philadelphia: Elsevier; 2011. pp. 269–298. [Google Scholar]
- 26.Tate JE, et al. A comparison of early (E7) and late (L1) primer-mediated amplification of papillomaviral DNA in cervical neoplasia. Mol Cell Probes. 1996;10:347–351. doi: 10.1006/mcpr.1996.0047. [DOI] [PubMed] [Google Scholar]
- 27.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.