Abstract
Declining CO2 over the Cretaceous has been suggested as an evolutionary driver of the high leaf vein densities (7–28 mm mm−2) that are unique to the angiosperms throughout all of Earth history. Photosynthetic modeling indicated the link between high vein density and productivity documented in the modern low-CO2 regime would be lost as CO2 concentrations increased but also implied that plants with very low vein densities (less than 3 mm mm−2) should experience substantial disadvantages with high CO2. Thus, the hypothesized relationship between CO2 and plant evolution can be tested through analysis of the concurrent histories of alternative lineages, because an extrinsic driver like atmospheric CO2 should affect all plants and not just the flowering plants. No such relationship is seen. Regardless of CO2 concentrations, low vein densities are equally common among nonangiosperms throughout history and common enough to include forest canopies and not just obligate shade species that will always be of limited productivity. Modeling results can be reconciled with the fossil record if maximum assimilation rates of nonflowering plants are capped well below those of flowering plants, capturing biochemical and physiological differences that would be consistent with extant plants but previously unrecognized in the fossil record. Although previous photosynthetic modeling suggested that productivity would double or triple with each Phanerozoic transition from low to high CO2, productivity changes are likely to have been limited before a substantial increase accompanying the evolution of flowering plants.
Keywords: paleoecology, photosynthesis, tracheophyte
Role of CO2 in Plant Evolution and Productivity over Geological Time Scales
Atmospheric CO2 concentrations have fluctuated greatly over the past 400 million years: CO2 levels are thought to have decreased by a factor of 10 or more as a result of the Devonian evolution of deep rooting plants and, subsequently, to have varied between levels somewhat less than and fivefold greater than preindustrial levels (1, 2). Just as land plant evolution has been a primary driver of these changes, in turn, CO2 has often been assigned a dominant role in plant evolution. CO2 changes have been implicated for the radiation of vascular plants, seed plants, and flowering plants; for the spread of C4 photosynthesis and grasslands; and for the evolution of arborescence and both laminate leaves in general and the high vein density leaves of angiosperms in particular (3–12). Furthermore, models of plant function have repeatedly predicted that large swings in terrestrial productivity of 200–300% accompany these changes in atmospheric CO2 (13–16).
Plants require CO2, and productivity can be adversely affected by the CO2 minima of the recent geological past that have approached the CO2 compensation point for plant growth (17, 18), but did the positive relationship with CO2 carry to a doubling or tripling of current productivity during the Mesozoic highs in CO2 concentration? This suggestion raises a number of complications. For example, it is in conflict with a variety of hypotheses regarding the angiosperm radiation that are dependent on high productivity being unique to the flowering plants and not a general trait of all plants in a high-CO2 world (19–22). Furthermore, if productivity were highly CO2-dependent, that dependence would carry through even to the availability of different architectural and ecological possibilities. For example, occupation of frequently disturbed habitats requires high enough productivity to complete a life cycle between successive disturbance events (23); thus, the accessibility of disturbed habitats as a viable environment would have fluctuated tremendously through time for all plant lineages. Finally, if the correlation between CO2 concentrations and productivity is tightly and strongly positive, that would even have an impact on our understanding of atmospheric CO2 itself, because the standard modeling of vegetation, atmospheric, and geological processes to determine CO2 concentrations includes only modest increases in productivity as CO2 increases. The temporal variability of CO2 would be dampened if CO2 fertilization effects were greater because of the feedbacks of the additional plant productivity on CO2 drawdown through increased silicate weathering (figure 10 in ref. 24). Paradoxically, the models of plant function suggesting that large swings in productivity accompany the large changes in CO2 concentrations would also make those large changes in CO2 difficult to achieve because of these feedbacks.
Models of plant function in the geological past are difficult to test experimentally because modern plants have evolved under low-CO2 conditions and greenhouse tests at elevated CO2 levels cannot be carried out over macroevolutionary time scales (15), but a solution may be available for the testing of their accuracy and completeness by comparing predictions against the fossil record. Here, we use leaf fossils to test morphological predictions derived from models of plant function under elevated CO2 regimes to evaluate previous hypotheses regarding the role of CO2 fluctuations in driving angiosperm evolution and primary productivity.
Leaf Vein Density
The high density of veins found in the leaves of many flowering plants is unique. Nonangiosperms average about 2.5 mm of vein length per square millimeter of leaf area and rarely reach higher than 5 mm mm−2, but angiosperms average around 10 mm mm−2 and can reach higher than 25 mm mm−2, with high vein densities appearing independently in at least three angiosperm lineages: the magnoliids, monocots, and eudicots (14, 25, 26). This abundance of vasculature was shown to correlate with much higher physiological activity, including a fourfold increase in transpiration capacity and more than a doubling of assimilation capacity, that resulted in important implications both for tropical climate and vegetation feedbacks and for the rise to ecological dominance of angiosperms over the Cretaceous and early Paleocene (14, 27–29). However, those correlations of leaf vein density with transpiration and assimilation capacities are based on empirical measurements of living plants grown under the low ambient CO2 concentrations of the modern world (14, 25, 30).
Modeling of leaf physiology at the 1,000-ppm CO2 concentrations that are believed to have been exceeded during the Cretaceous suggests that nearly all plants would have been much more productive than they are today (13–15) and that the advantage of increased vein density would saturate at low vein density levels that are accessible by all plant groups (14) (results reproduced in Fig. 1B). Thus, it has been argued that the Cretaceous initiation of an overall trend in atmospheric CO2 reduction toward modern levels was essential for the emergence of angiosperm leaf characteristics because high vein density provides no advantage with high CO2 (14).
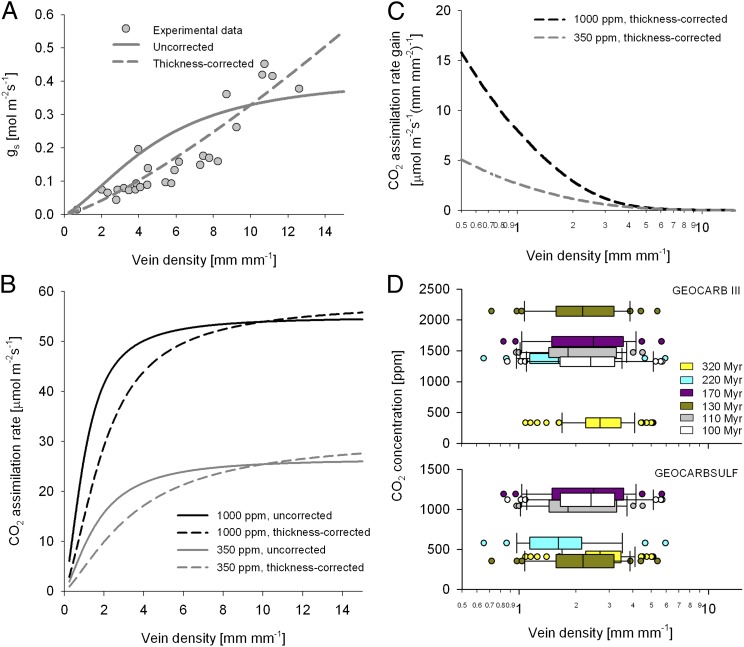
Fig. 1.
Expected relationships between vein density and assimilation capacity and fossil leaf vein density distributions relative to CO2 concentrations. (A) Comparison of empirical measurements (circles) of relation between leaf vein density and stomatal conductance [digitized from the study by Brodribb and Feild (14)], with alternative models relating vein density and assimilation capacities, including a previously used model with fixed leaf thickness (14) and a previously undescribed corrected model allowing thickness to vary with vein density (36). (B) Modeled effects of vein density on assimilation capacities at high (1,000 ppm) and low (350 ppm) atmospheric CO2 concentrations for uncorrected and vein density/leaf thickness-corrected models. (C) Photosynthetic rate gain for change in vein density at high and low CO2 calculated with the corrected model. (D) Distribution of vein densities at different times in Earth history plotted at GEOCARBIII (24) and GEOCARBSULF (55) estimates of contemporaneous CO2. Myr, million years. Specific data are provided in Table S1.
Emphasis has been on the lack of advantage for high vein density in a high-CO2 world (14), but perhaps the more important conclusion to draw from this physiological model is that possession of leaf vein densities much lower than 3 mm mm−2 would present enormous disadvantages because it is associated with dramatic loss of CO2 assimilation capabilities. Although the modern low-CO2 world can be permissive of a broad morphological diversity because only moderate gains in assimilation rate accompany increasing vein density, this should not be expected in a world of 1,000 ppm CO2, where plants with a vein density of 2 mm mm−2 were modeled to assimilate almost twice as fast as plants with a vein density of 1 mm mm−2 (figure S4 in ref. 14). This modeling of plant function in high CO2 thus suggests that an absolute difference in assimilation capacities as large as that between modern ferns and angiosperm tropical trees and crops would be compressed between two vein densities that would both be exceedingly low by modern angiosperms. This range of very low vein densities is equally achievable by any lineage of vascular plants and in any environment: High vein densities may require ample water supply, but low vein densities are available anywhere from the rainforest understory to desert succulents.
Testing the hypothesized relationship between CO2 and angiosperm leaf evolution is complicated by high leaf vein density being unique to the angiosperms; however, all other plants should respond similarly within their potential range even if they lack the developmental and physiological capacities for the very high vein densities exhibited by angiosperms. Thus, at times of high CO2 when very low vein densities should be maladaptive, few fossil leaves should have had vein densities less than 3 mm mm−2 regardless of phylogenetic affinity.
Results and Discussion
Fossil History of Leaf Vein Densities.
When leaf fossils are sampled from high-CO2 intervals, the prediction of decreasing prevalence of low vein densities below 3 mm mm−2 proves to be false (Table S1; full ANOVA statistical results are provided in SI Text). Vein densities change little through time, and high proportions of nonangiosperms have vein densities lower than this threshold throughout Earth history and over a wide range of CO2 concentrations (Fig. 1D). This abundance of low vein density leaves is too great to be accounted for by plants that are independently constrained to low productivity by deep shade or other environmental stressors. First, the vein densities in question are low regardless of environment: Shade-requiring angiosperms can have vein densities greater than 5 mm mm−2 (26). More generally, the paleobotanical record is biased toward preservation of woody plants of forest canopies and more open environments because herbs and shrubs of the shaded forest floor produce fewer leaves and their leaves have less opportunity for transport to waterway-based sites of deposition and are more prone to decay (31, 32). The cases in which all plants are preserved equally are rare (33, 34). Thus, a few of the lower bound outliers of vein density may well represent tolerators of deep shade, but the median vein density for each time interval is sometimes less than 2 mm mm−2 and is never more than 2.6 mm mm−2 for each high-CO2 time interval (Fig. 1D). Thus, if more than 50% of the taxa have very low vein densities, it can be safely inferred that a high proportion of the canopy plants had such vein densities. Furthermore, the bias toward preservation of plants growing along lakes and rivers that has been problematic for leaf-based paleoclimate proxies (35) does not explain the prevalence of low vein density plants through time because riparian and nonriparian plants have similar vein densities (Fig. S1 and Table S2). Our results indicate that plants with very low densities grew side by side with plants of higher vein density regardless of CO2 concentrations, contrary to the prediction that those plants would be subject to enormous disadvantages when atmospheric CO2 was high.
Reconciling Physiology and the Fossil Record.
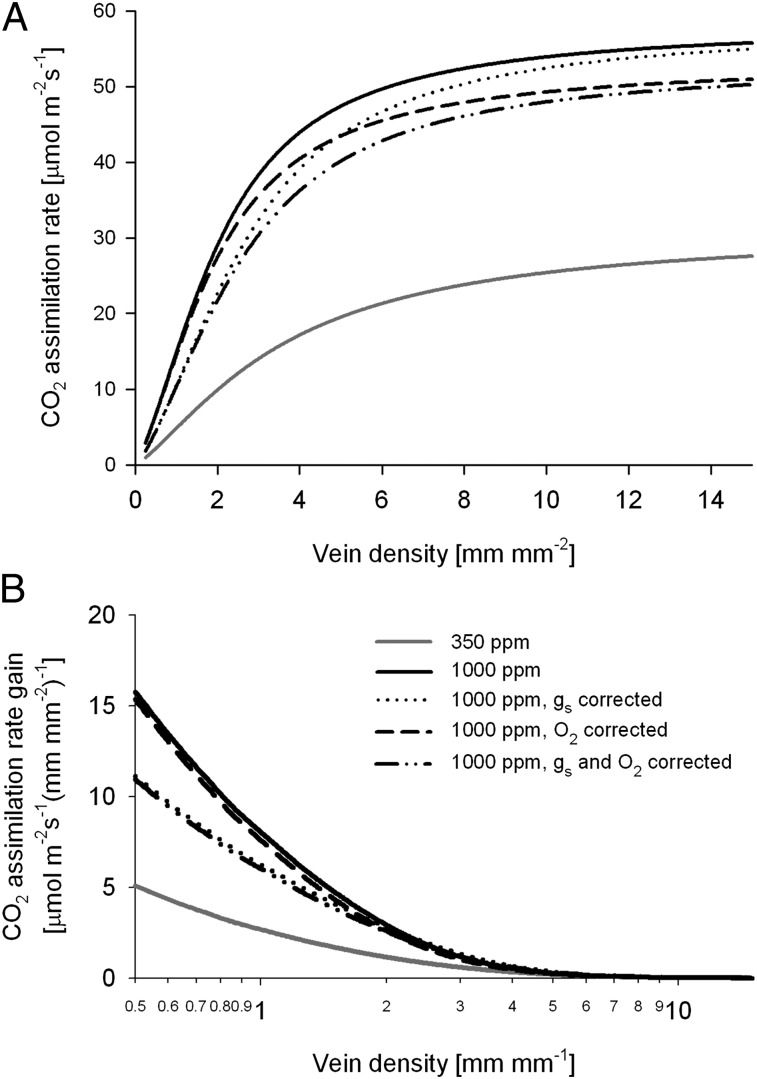
The prediction derived from physiological modeling that very low leaf vein densities should be rare during times of elevated CO2 is not met by the fossil record, and no trivial correction is available to bring these two approaches into concordance (full details on model corrections are provided in SI Text). First, the original model (14) only allowed for very thin leaves, which is adequate only for high vein densities. The distance between veins should roughly equal the distance from vein to stomata for optimal water delivery (36), such that low vein density plants in exposed environments would require much thicker leaves to avoid partial desiccation of the leaf lamina. A more accurate scaling of leaf thickness to vein density provides a better fit to experimental data (Fig. 1A), but this more accurate depiction increases the distance of high-resistance hydraulic transport through the mesophyll in low vein density leaves, and thereby accentuates their potential disadvantages (Fig. 1 B and C). Second, although poorly constrained, stomatal conductivity should have been lower with the reduced stomatal density seen at times of high CO2 in the geological past (37–40). Reducing stomatal conductivity does increase the vein density at which productivity plateaus but without altering the overall conclusion that the penalty of low vein density is far larger when CO2 is high (Fig. 2). Third, the O2/CO2 ratio can have an impact on maximum photosynthetic rates through photorespiration, but elevated oxygen does not alter expectations (Fig. 2), and neither does consideration of a more complete range of potential CO2 concentrations (Fig. 3). The expectation would remain that very low vein densities should be strongly disfavored during high-CO2 regimes because even small increases in the vein density would dramatically improve photosynthetic capacity (Fig. 3), an expectation rejected by the fossil record even though these small changes would be equally available to all plant lineages.
Fig. 2.
Impact on assimilation of stomatal conductance and atmospheric oxygen. (A) Changes in predicted assimilation rate in relation to vein density caused by introducing decreased stomatal conductance in response to high-CO2 concentrations (16) and/or changes attributable to the elevated O2 concentrations expected for the Cretaceous (52, 55) applied to the model corrected for leaf thickness. (B) Photosynthetic rate gain for change in vein density as predicted for inclusion of model corrections for stomatal conductance and O2 concentrations. gs, stomatal conductance to water
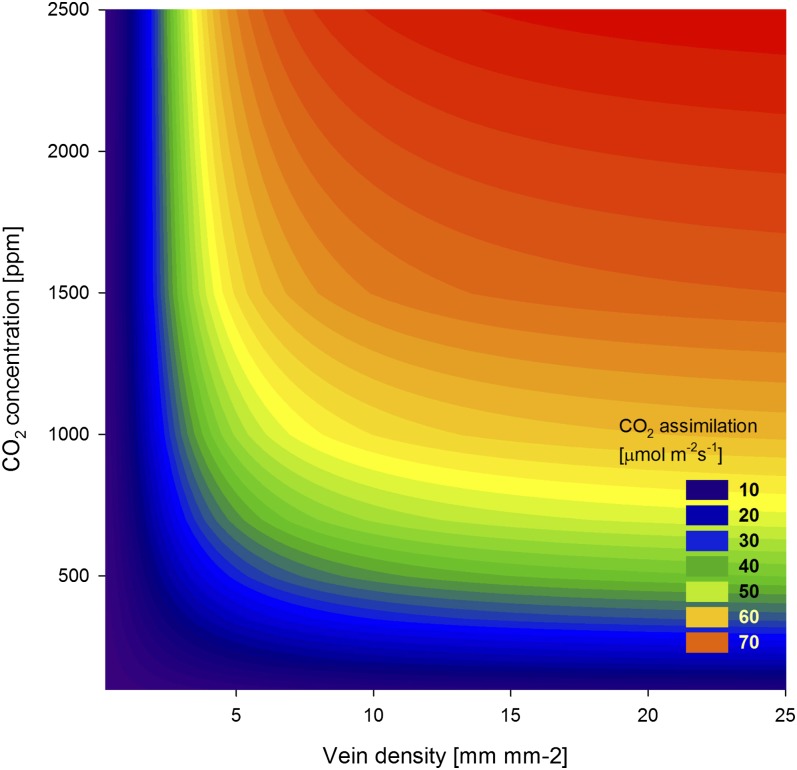
Fig. 3.
Model expectations for the relationship between productivity and the full range of potential leaf vein densities and CO2 concentrations. Modern O2 concentrations, as well as corrections to the original model for variable leaf thickness and stomatal conductance, were used.
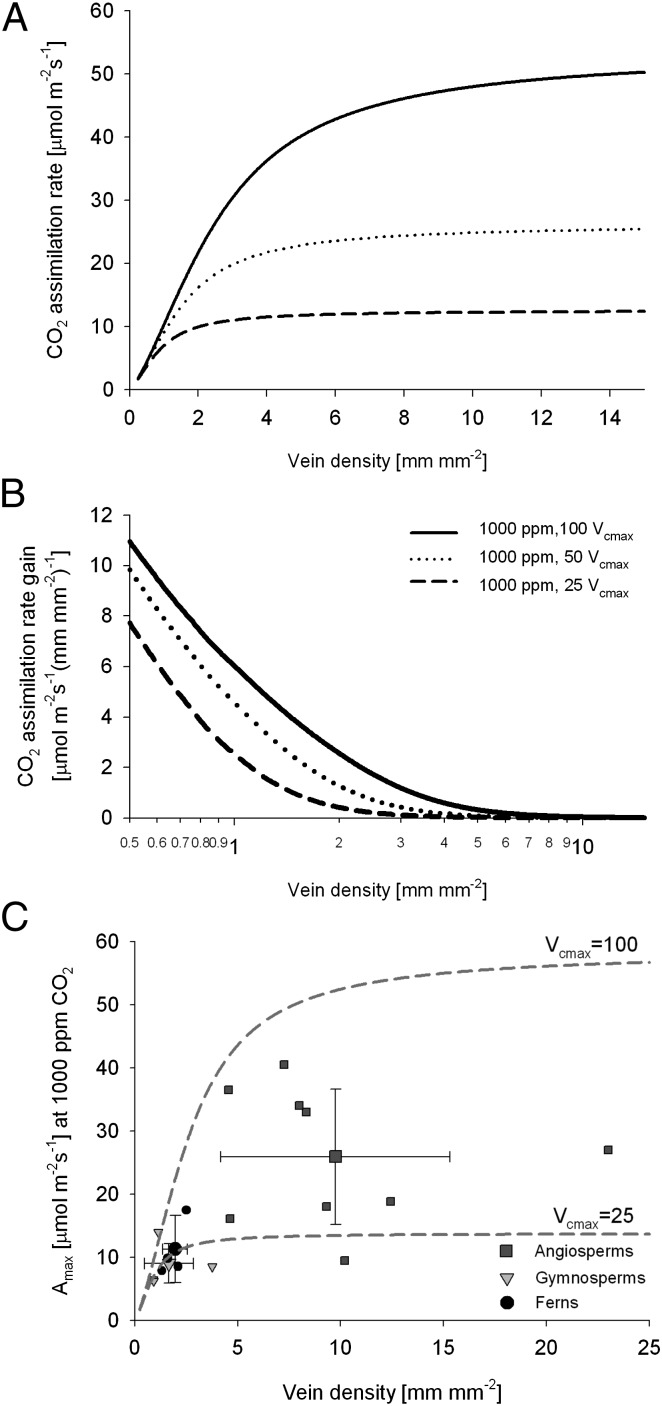
The steep disadvantages to very low vein density suggested by photosynthetic models derive from the assumption that given enough CO2, any fern exposed to adequate light could be more productive than angiosperm crops, such as sunflower or maize, are now with modern CO2 concentrations (e.g., figure S4 in ref. 14). Only lower maximum assimilation rates for nonangiosperms could explain the lack of a correlation between vein density and CO2 concentration: A lower plateau for assimilation capacities would also lower the vein density at which that plateau is achieved, potentially encompassing the entire range of available vein densities (Fig. 4 A and B). Vein density would then be free to vary in response to environmental and ecological specialization with little impact on assimilation capacities.
Fig. 4.
Impact of maximum rate of Rubisco carboxylation, Vcmax, on assimilation capacity at 1,000 ppm of CO2. (A) For the fully corrected model, changes in predicted assimilation rate in relation to vein density are shown for different values of Vcmax (μmol⋅m−2⋅s−1). (B) Photosynthetic rate gain with changing vein density for different values of Vcmax. (C) Empirical measurement of lineage-specific relationships between vein density and assimilation at 1,000 ppm of CO2 in living ferns, gymnosperms, and angiosperms plotted along with modeled relationships between vein density and assimilation capacity at two Vcmax levels. Amax, light-saturated photosynthesis. A plant species list is provided in Table S3.
In this scenario, angiosperms would have advantages over other plant groups regardless of CO2 concentration, as would be consistent with other differences in angiosperm vegetative biology, including leaf traits and stomatal conductivity through time (16, 25, 41–43). In the modern low-CO2 world, individual nonangiosperms, particularly some conifers, can overlap with less productive flowering plants, but both the typical and maximum photosynthetic capacities of angiosperms are substantially higher (42–45). Although it is always unclear how literally such experiments should be extrapolated up to macroevolutionary time scales, angiosperm advantages do persist and substantial lineage-specific differences in CO2 fertilization are seen when modern plants are exposed to 1,000 ppm CO2 (Fig. 4C).
Further support for physiological distinctions between angiosperms and other plants is that angiosperm leaf fossils do conform to the model expectations that nonangiosperms violate. The earliest angiosperms share the same range of very low vein densities as nonangiosperms; however, as they evolve the higher vein densities indicative of higher photosynthetic capacities over the high-CO2 Late Cretaceous, they also vacate the vein densities less than 3 mm mm−2 in a way that is not seen among nonangiosperms (figure 1 in ref. 26), where very low vein densities account for almost 50% of angiosperms in the pre-Albian Early Cretaceous, 7% in the Albian, and no more than 4% in the Late Cretaceous and Paleocene. This provides additional evidence that the patterns seen among nonangiosperm leaf fossils cannot be dismissed as an artifact of the perpetual existence of plants environmentally limited to low productivity. Any such ecological caveat should have applied equally to the angiosperm record, particularly because obligate shade plants are both ancestral and continuously abundant for angiosperms (46).
Although modeled in terms of limitations on the maximum rate of carboxylation, actual lineage-specific limitations on maximum photosynthetic capacity could take any number of forms. Rubisco activity is well known as a CO2-dependent limit on photosynthesis that suggests highly elevated productivity as CO2 increases (5, 15, 47). However, other limitations provide more opportunity for lineage-specific differences that are much less CO2-dependent, such as investment in photoreceptors or the Calvin cycle regeneration of RuBP (47, 48), or may actually be exacerbated by increasing CO2, such as the nitrate assimilation (49) and inorganic phosphate recycling that are also necessary for primary production (48, 50). Without constraining which factors may be involved, the stability of vein density distributions throughout the fossil record outside of the angiosperms suggests substantially lower photosynthetic capacities in most nonangiosperms regardless of CO2 concentrations. Rather than productivity potentially ranging from 10 to more than 40 μmol⋅m−2s−1 through time (13, 15), no more than the bottom half of that range may have been available before angiosperm evolution. Previous workers have been careful to acknowledge that those values are maxima that may not be reached because of other limitations, such as water availability. However, those elevated levels of productivity are reached by flowering plants in many extant environments, including rainforests and more temperate environments where moisture is adequate (14), and we argue no plant could achieve those levels seen in the modern world before the advent of angiosperms regardless of CO2 concentrations.
Plant Productivity Through Time and Environmental Implications.
If most nonangiosperms do indeed have limited capacity for assimilation much greater than their modern levels, the large swings in productivity through time predicted by modeling vegetation responses to CO2 are unlikely to have happened, except for a potential increase over the Cretaceous and Cenozoic with the spread of angiosperms. This would be more consistent with carbon cycle modeling than the doubling or tripling of assimilation rates that has been predicted with elevated CO2 and would have been likely to prevent elevated CO2 in the first place through silicate weathering feedbacks. This hypothesis of relative stability for plant productivity through vascular plant history at least up to the Cretaceous would be an important grounding for consideration of the evolution of terrestrial animals and ecosystems. Contradictory views of how productivity has changed since the Cretaceous exist in the literature (8, 13–16, 19–22, 25). Have flowering plants brought flourishing abundance that also benefited animal life (perhaps even in the oceans), or are angiosperms simply the best at enduring the deprivations of a diminished, carbon-starved world? Consideration of photosynthetic models in light of the fossil record suggests that productivity has not declined with angiosperm evolution and is likely to have increased.
The potential of increased productivity with angiosperm evolution is bolstered by a final discrepancy between model output and plant morphology: Although flowering plant leaf vein densities average around 10 mm mm−2 and can surpass 25 mm mm−2, photosynthetic models suggest there is never an advantage to densities greater than about 8 mm mm−2 regardless of CO2 concentrations. This expectation is based on the modeling of maximum instantaneous assimilation rates dependent on the maximum flux of CO2 through the stomata when they are open. Along with other aspects of angiosperm hydraulic physiology, such as vessels and reduced safety margins (51), high vein densities greater than 10 mm mm−2 may instead be important for maximizing the proportion of stomata that are not closed. Thus, the expectation is further bolstered that angiosperms have productivity advantages over other plants whether CO2 is high or low.
Future tests regarding changes in productivity through time with CO2 fluctuations and angiosperm evolution may be challenging but could come from a variety of directions. Increased photosynthetic capacities in angiosperms may not have had much impact on the carbon isotopic values of plants: For a given stomatal conductivity, the lower internal CO2 concentrations that might be expected to accompany higher assimilation rates would decrease isotopic discrimination, but internal CO2 concentrations might not have been lower if angiosperm hydraulic modifications allowed stomata to be open a greater proportion of the time. Increased Cretaceous abundance of charcoal has been argued to reflect increased angiosperm assimilation rates and fuel production (20), although over longer time periods, charcoal abundance has been considered more as an indicator of atmospheric oxygen levels than of plant productivity (20, 52, 53). Tests resulting from plant paleoecology may be the most informative. Of all plant growth forms, annuals are the most dependent on high growth rates. If productivity was tightly correlated with CO2 concentrations, this might be expected to carry over to the abundance and diversity of annual plants through time as well. Annuals are effectively absent outside of the angiosperms throughout earth history (54), regardless of CO2 concentrations.
Materials and Methods
All vein density measurements were performed with ImageJ (National Institutes of Health). Extant leaf vein density measurements were performed on a minimum of five leaves for each taxon. Fossil leaf vein density was measured from individual figured specimens or compiled from previously published studies (details are provided in Table S1). Angiosperm vein density measurements were based on three subsamples of each leaf at a magnification of 20× to 40×; however, this is not necessary for the thick veins and lower vein densities of nonflowering plants, for which the vein density of the entire lamina was measured. The photosynthetic model was derived from a previous source (14) with some corrections of parameter values as explained in SI Materials and Methods. Empirical measurements of photosynthetic rates in living plants were collected using a photosynthesis measuring system (LiCor 6400; with photosynthetically active radiation = 2,000 μE and CO2 concentration = 1,000 ppm; leaves were allowed 20 min of acclimation time in the chamber before measurements).
Supplementary Material
Acknowledgments
We thank Joe Berry, Dana Royer, Patrick MacGuire, and Michael Foote for helpful discussion or recitation. This work was supported by National Science Foundation Grant EAR-1024041 (to C.K.B. and M.A.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203769109/-/DCSupplemental.
References
- 1.Beerling DJ, Berner RA. Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci USA. 2005;102:1302–1305. doi: 10.1073/pnas.0408724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berner RA. The rise of plants and their effect on weathering and atmospheric CO2. Science. 1997;276:544–546. [Google Scholar]
- 3.Barrett PM, Willis KJ. Did dinosaurs invent flowers? Dinosaur-angiosperm coevolution revisited. Biol Rev Camb Philos Soc. 2001;76:411–447. doi: 10.1017/s1464793101005735. [DOI] [PubMed] [Google Scholar]
- 4.Becker P. Competition in the regeneration niche between conifers and angiosperms: Bond’s slow seedling hypothesis. Funct Ecol. 2000;14:401–412. [Google Scholar]
- 5.Beerling DJ. In: A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Ehleringer JR, Cerling TE, Dearing MD, editors. New York: Springer; 2005. pp. 114–132. [Google Scholar]
- 6.Beerling DJ, Osborne CP, Chaloner WG. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature. 2001;410:352–354. doi: 10.1038/35066546. [DOI] [PubMed] [Google Scholar]
- 7.Cerling TE, et al. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 8.McElwain JC, Willis KJ, Lupia R. In: A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Ehleringer JR, Cerling TE, Dearing MD, editors. New York: Springer; 2005. pp. 133–166. [Google Scholar]
- 9.Osborne CP, Beerling DJ, Lomax BH, Chaloner WG. Biophysical constraints on the origin of leaves inferred from the fossil record. Proc Natl Acad Sci USA. 2004;101:10360–10362. doi: 10.1073/pnas.0402787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JM. Speculations on carbon dioxide starvation, late Tertiary evolution of stomatal regulation and floristic modernization. Plant Cell Environ. 1994;17:345–354. [Google Scholar]
- 11.Willis KJ, McElwain JC. The Evolution of Plants. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 12.Woodward FI. Do plants need stomata? J Exp Bot. 1998;49:471–480. [Google Scholar]
- 13.Beerling DJ, Woodward FI. Changes in land plant function over the Phanerozoic: Reconstructions based on the fossil record. Bot J Linn Soc. 1997;124:137–153. [Google Scholar]
- 14.Brodribb TJ, Feild TS. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett. 2010;13:175–183. doi: 10.1111/j.1461-0248.2009.01410.x. [DOI] [PubMed] [Google Scholar]
- 15.Franks PJ, Beerling DJ. CO(2)-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology. 2009;7:227–236. doi: 10.1111/j.1472-4669.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 16.Franks PJ, Beerling DJ. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci USA. 2009;106:10343–10347. doi: 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagani M, Caldeira K, Berner RA, Beerling DJ. The role of terrestrial plants in limiting atmospheric CO(2) decline over the past 24 million years. Nature. 2009;460:85–88. doi: 10.1038/nature08133. [DOI] [PubMed] [Google Scholar]
- 18.Ward JK, et al. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc Natl Acad Sci USA. 2005;102:690–694. doi: 10.1073/pnas.0408315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bond WJ. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biol J Linn Soc Lond. 1989;36:227–249. [Google Scholar]
- 20.Bond WJ, Scott AC. Fire and the spread of flowering plants in the Cretaceous. New Phytol. 2010;188:1137–1150. doi: 10.1111/j.1469-8137.2010.03418.x. [DOI] [PubMed] [Google Scholar]
- 21.Vermeij GJ, Grosberg RK. The great divergence: When did diversity on land exceed that in the sea? Integr Comp Biol. 2010;50:675–682. doi: 10.1093/icb/icq078. [DOI] [PubMed] [Google Scholar]
- 22.Vermeij GJ. The energetics of modernization: the last 100 million years of biotic evolution. Paleontological Res. 2011;15:54–61. [Google Scholar]
- 23.Grime JP. Plant Strategies, Vegetation Processes, and Ecosystem Properties. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 24.Berner RA, Kothavala Z. GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic time. Am J Sci. 2001;301:182–204. [Google Scholar]
- 25.Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc Biol Sci. 2009;276:1771–1776. doi: 10.1098/rspb.2008.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feild TS, et al. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc Natl Acad Sci USA. 2011;108:8363–8366. doi: 10.1073/pnas.1014456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyce CK, Lee J-E. An exceptional role for flowering plant physiology in the expansion of tropical rainforests and biodiversity. Proc Biol Sci. 2010;277:3437–3443. doi: 10.1098/rspb.2010.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyce CK, Lee J-E, Feild TS, Brodribb T, Zwieniecki MA. Angiosperms helped put the rain in the rainforests: The impact of plant physiological evolution on tropical biodiversity. Ann Mo Bot Gard. 2010;97:527–540. [Google Scholar]
- 29.Lee J-E, Boyce CK. Impact of the hydraulic capacity of plants on water and carbon fluxes in tropical South America. J Geophys Res. 2010;115:D23123. [Google Scholar]
- 30.Brodribb TJ, Feild TS, Jordan GJ. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson DK. The origin of leaf-assemblages- new light on an old problem. Rev Palaeobot Palynol. 1985;46:117–188. [Google Scholar]
- 32.Spicer RA. In: Taphonomy: Releasing the Data Locked in the Fossil Record. Allison PA, Briggs DEG, editors. Vol 9. New York: Plenum; 1991. pp. 71–113. [Google Scholar]
- 33.Wang J, Pfefferkorn HW, Zhang Y, Feng Z. Permian vegetational Pompeii from Inner Mongolia and its implications for landscape paleoecology and paleobiogeography of Cathaysia. Proc Natl Acad of Sci USA. 2012;109:4927–4932. doi: 10.1073/pnas.1115076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing SL, Hickey L J, Swisher CC. Implications of an exceptional fossil flora for Late Cretaceous vegetation. Nature. 1993;363:342–344. [Google Scholar]
- 35.Burnham RJ, Pitman NCA, Johnson KR, Wilf P. Habitat-related error in estimating temperatures from leaf margins in a humid tropical forest. Am J Bot. 2001;88:1096–1102. [PubMed] [Google Scholar]
- 36.Noblin X, et al. Optimal vein density in artificial and real leaves. Proc Natl Acad Sci USA. 2008;105:9140–9144. doi: 10.1073/pnas.0709194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beerling DJ, Chaloner WG. Atmospheric CO2 changes since the last glacial maximum: Evidence from the stomatal density record of fossil leaves. Rev Palaeobot Palynol. 1994;81:11–17. [Google Scholar]
- 38.McElwain JC. Do fossil plants signal palaeoatmospheric CO2 concentration in the geological past? Philos Trans R Soc Lond B Biol Sci. 1998;353:83–96. [Google Scholar]
- 39.McElwain JC, Chaloner WG. Stomatal density and index of fossil plants track atmospheric carbon dioxide in the Paleozoic. Ann Bot (Lond) 1995;76:389–395. [Google Scholar]
- 40.Royer DL. Stomatal density and stomatal index as indicators of paleoatmospheric CO(2) concentration. Rev Palaeobot Palynol. 2001;114:1–28. doi: 10.1016/s0034-6667(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 41.Ida K. Eco-physiological studies on the response of taxodiaceous conifers to shading, with special reference to the behavior of leaf pigments. 1. Distribution of carotenoids in green and autumnal reddish brown leaves of gymnosperms. Bot Mag Tokyo. 1981;94:41–54. [Google Scholar]
- 42.Karst AL, Lechowicz MJ. Are correlations among foliar traits in ferns consistent with those in the seed plants? New Phytol. 2007;173:306–312. doi: 10.1111/j.1469-8137.2006.01914.x. [DOI] [PubMed] [Google Scholar]
- 43.Ripullone F, Grassi G, Lauteri M, Borghetti M. Photosynthesis-nitrogen relationships: Interpretation of different patterns between Pseudotsuga menziesii and Populus x euroamericana in a mini-stand experiment. Tree Physiol. 2003;23:137–144. doi: 10.1093/treephys/23.2.137. [DOI] [PubMed] [Google Scholar]
- 44.Wullschleger SD. Biochemical limitations to carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves from 109 species. J Exp Bot. 1993;44:907–920. [Google Scholar]
- 45.Zhang S, Dang Q-L. Effects of soil temperature and elevated atmospheric CO2 concentration on gas exchange, in vivo carboxylation and chlorophyll fluorescence in jack pine and white birch seedlings. Tree Physiol. 2005;25:523–531. doi: 10.1093/treephys/25.5.523. [DOI] [PubMed] [Google Scholar]
- 46.Feild TS, Arens NC, Doyle JA, Dawson TE, Donoghue MJ. Dark and disturbed: A new image of early angiosperm ecology. Paleobiology. 2004;30:82–107. [Google Scholar]
- 47.Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot. 2003;54:2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- 48.Stitt M. Limitation of photosynthesis by carbon metabolism: I. Evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant Physiol. 1986;81:1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bloom AJ, Burger M, Rubio Asensio JS, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328:899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- 50.Sage RF. Acclimation of photosynthesis to increasing atmospheric CO2: The gas exchange perspective. Photosynth Res. 1994;39:351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- 51.Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: A comparison of coexisting ferns and angiosperms. New Phytol. 2004;162:663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- 52.Belcher CM, McElwain JC. Limits for combustion in low O2 redefine paleoatmospheric predictions for the Mesozoic. Science. 2008;321:1197–1200. doi: 10.1126/science.1160978. [DOI] [PubMed] [Google Scholar]
- 53.Scott AC, Glasspool IJ. The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentration. Proc Natl Acad Sci USA. 2006;103:10861–10865. doi: 10.1073/pnas.0604090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyce CK, Leslie AB. The paleontological context of angiosperm vegetative evolution. Int J Plant Sci. in press. [Google Scholar]
- 55.Berner RA. GEOCARBSULF: A combined model for Phanerozoic atmospheric O2 and CO2. Geochim Cosmochim Acta. 2006;70:5653–5664. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.