Abstract
The microenvironment of the cochlea is maintained by the barrier between the systemic circulation and the fluids inside the stria vascularis. However, the mechanisms that control the permeability of the intrastrial fluid–blood barrier remain largely unknown. The barrier comprises endothelial cells connected to each other by tight junctions and an underlying basement membrane. In a recent study, we found that the intrastrial fluid–blood barrier also includes a large number of perivascular cells with both macrophage and melanocyte characteristics. The perivascular-resident macrophage-like melanocytes (PVM/Ms) are in close contact with vessels through cytoplasmic processes. Here we demonstrate that PVM/Ms have an important role in maintaining the integrity of the intrastrial fluid–blood barrier and hearing function. Using a cell culture-based in vitro model and a genetically induced PVM/M-depleted animal model, we show that absence of PVM/Ms increases the permeability of the intrastrial fluid–blood barrier to both low- and high-molecular-weight tracers. The increased permeability is caused by decreased expression of pigment epithelial-derived factor, which regulates expression of several tight junction-associated proteins instrumental to barrier integrity. When tested for endocochlear potential and auditory brainstem response, PVM/M-depleted animals show substantial drop in endocochlear potential with accompanying hearing loss. Our results demonstrate a critical role for PVM/Ms in regulating the permeability of the intrastrial fluid–blood barrier for establishing a normal endocochlear potential hearing threshold.
Keywords: mouse cochlea, paracellular permeability, tight junction, capillary
The intrastrial fluid–blood barrier separates the stria vascularis (SV) from peripheral circulation. The integrity of the barrier is critical for maintaining inner ear homeostasis, especially for sustaining the endocochlear potential (EP), an essential driving force for hearing function (1–4). Disruption of the barrier is closely associated with a number of hearing disorders, including autoimmune inner ear disease, noise-induced hearing loss, age-related hearing loss, and several genetically linked diseases (5–10). Despite the importance of the intrastrial fluid–blood barrier, little is understood about regulation of the barrier and the mechanisms that control its permeability.
In the classic view, the intrastrial fluid–blood barrier comprises basement membrane and endothelial cells (ECs) that connect to each other with tight junctions (11) to form a diffusion barrier that prevents most blood-borne substances from entering the ear (2). In a recent study, we found that the intrastrial fluid–blood barrier also includes a large number of pericytes and perivascular-resident macrophage-like melanocytes (PVM/Ms) (12, 13). The PVM/Ms are not observed in other capillary regions such as in capillary beds of the spiral ligament. The PVM/Ms are in close contact with vessels through cytoplasmic processes. The structural complexity of PVM/Ms’ capillary morphology suggests functional complexity. The cell types of the intrastrial fluid–blood barrier have yet to be characterized functionally, and the role of PVM/Ms in the intrastrial fluid–blood barrier is particularly elusive. In an earlier study (13), we characterized the PVM/Ms as resident macrophages, because the cells are positive for several macrophage surface molecules, including F4/80, CD68, and CD11b. In this study, we further characterize the PVM/Ms as expressing several melanocyte markers. Overall, our results show that PVM/Ms are a hybrid cell type and differ from the classical macrophage. Given their morphological similarities to astrocytes and glial cells, we speculate the PVM/Ms might have a functional role similar to that of astrocytes in the blood–brain-barrier and glial cells in the blood–retina barrier. Astrocytes and glial cells are known to have essential roles in regulation of barrier integrity and tissue-oriented functions (14, 15). Barriers without these cells lose tight-junction proteins, become leaky, and are associated with tissue edema (16). In our study, we tested whether communication between ECs and PVM/Ms is essential for the barrier function and, more specifically, whether such signaling controls microvessel permeability. The hypotheses were tested in both in vitro and in vivo models. Primary endothelial and PVM/M cell lines from mouse cochlea were established for in vitro tests, and the results were validated in vivo by ablating PVM/Ms. A transgenic mouse model with a diphtheria toxin (DT) receptor under control of a human integrin αM promoter (CD11b) enabled transient depletion of the PVM/Ms (17–19). Our results demonstrate that PVM/M regulation of intrastrial fluid–blood barrier integrity, mediated by pigment epithelial-derived factor (PEDF), affects the expression of tight junction-associated proteins. The experiment provides evidence that signaling between PVM/Ms and ECs regulates intrastrial fluid–blood barrier integrity and that regulation of permeability is instrumental in maintaining a normal EP and hearing threshold.
Results
Resident Macrophages with Melanocyte Characteristics Ensheath Strial Capillaries Through Cytoplasmic Processes.
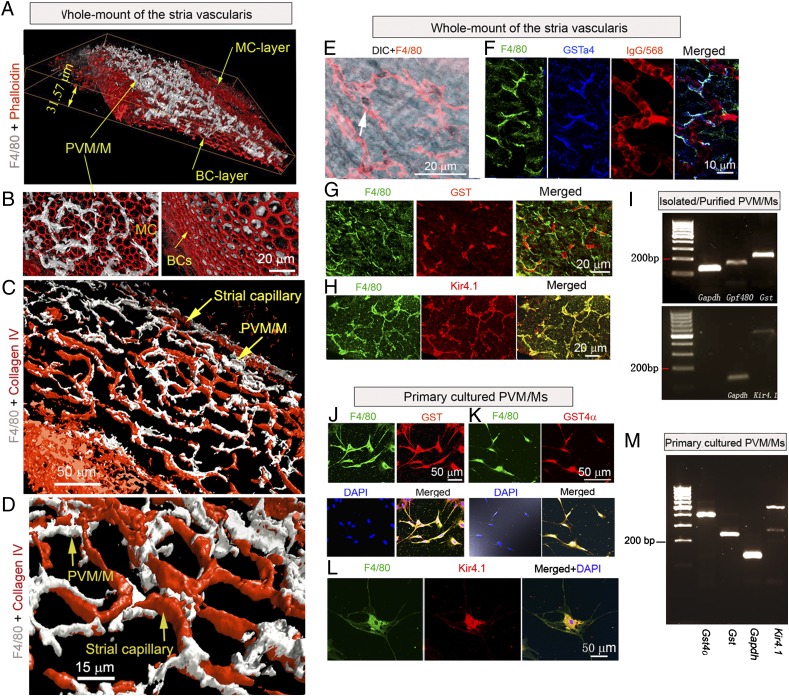
Confocal images show a large population of PVM/Ms sandwiched between marginal and basal cell layers of the SV (Fig. 1A). A 3D reconstructed confocal image of the SV shows that PVM/Ms are situated in or under subepithelial marginal cells (Fig. 1B, Left) but do not appear to have direct contact with basal cells (Figs. 1B, Right). The PVM/Ms are highly invested on the abluminal surface of capillaries (Fig. 1C) and are structurally intertwined with ECs with dendritic processes (Fig. 1D) that also interface with the capillary wall (Fig. S1 A and B). The PVM/Ms contain melanin pigment granules in the cytoplasm (Fig. 1E) and express melanocyte marker proteins, including cytosolic GSTα4 (Fig. 1F) (20, 21), GST (Fig. 1G), and Kir4.1 (Fig. 1H). mRNA for Gst, Gpf480, and Kir4.1 are detected by RT-PCR in isolated and purified PVM/Ms (Fig. 1I). Consistent with the findings from whole-mounted tissue, GST, GSTα4, and Kir 4.1 also are expressed in primary cultured PVM/Ms (Fig. 1 J–L), along with Gst, Gst4α, and Kir4.1 mRNA (Fig.1M). Hence, PVM/Ms have characteristics of melanocytes.
Fig. 1.
Perivascular-resident cells identified as melanocyte-like macrophages are in close contact with capillaries through cytoplasmic processes. (A) 3D reconstructed confocal image of the SV. PVM/Ms are labeled with an antibody for F4/80 (white), and the cytoskeleton is labeled with phalloidin (red). The PVM/Ms are sandwiched between marginal (MC) and basal cell (BC) layers of the SV. (B) 3D reconstructed images at different angles show that PVM/Ms are situated in or under subepithelial marginal cells (Left) and have less contact with basal cells (Right). (C and D) An overview (C) and a close-up view (D) of the 3D rendering of confocal stacks show the ramified processes of PVM/Ms interfacing with the endothelial tube. Capillaries are labeled with antibody for collagen IV. (E) PVM/Ms contain melanin pigment granules (arrow). (F) Triple labeling for F4/80 (green), GSTα4 (blue), and capillaries (red) in whole-mount SV shows that PVM/Ms express GSTα4. (G) Double-labeling for F4/80 (green) and GST (red) in the whole-mount SV shows that PVM/Ms express GST. (H) Double-labeling for F4/80 (green) and Kir4.1 (red) in the whole-mount SV shows that PVM/Ms express Kir4.1. (I) mRNA for Gpf480, Gst, and Kir4.1 was detected in isolated and purified PVM/Ms by RT-PCR analysis. (J and K) Primary cultured PVM/Ms are triple-labeled for GST (red) or GST4a (red), F4/80 (green), and nuclei (blue). (L) Primary cultured PVM/Ms are double-labeled for F4/80 (green) and Kir4.1. (M) mRNA for Gpf480, Gst, Gst4α, and Kir4.1 was found in primary cultured PVM/Ms by RT-PCR analysis.
EC Monolayers Are Highly Permeable in the Absence of PVM/Ms.
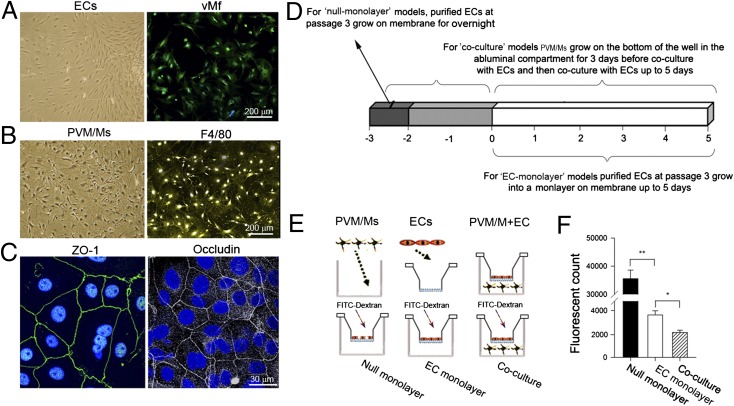
The permeability of the endothelial barrier was measured directly in a cell-culture model with an EC (Fig. 2A) and PVM/M (Fig. 2B) interface. The endothelial monolayer was verified for expression of various cell–cell tight-junction proteins, including zonula occludens 1 (ZO-1) (Fig. 2C, Left) and occludin (Fig. 2C, Right). Permeability was assessed by measuring the flux of 70-kDa fluorescent dextran across the endothelial monolayer for both the coculture model and two control models (null endothelial monolayer and confluent EC monolayer) (Fig. 2 D and E). The degree of EC monolayer leakage was determined by measuring the intensity of FITC-dextran fluorescence in the basolateral chamber. Our results show that the permeability of the EC monolayer increases markedly in the absence of PVM/Ms as compared with the presence of PVM/Ms (n = 5, P < 0.05), indicating that PVM/Ms strengthen the integrity of the endothelial barrier (Fig. 2F).
Fig. 2.
EC monolayers in vitro are more permeable in the absence of PVM/Ms. (A and B) Primary cultured ECs and PVM/Ms under differential interference contrast (DIC) microscopy (Left) are identified for marker proteins vMf (ECs) and F4/80 (PVM/Ms) under confocal fluorescent microscopy (Right). (C) The EC monolayer expresses tight-junction proteins ZO-1 and occludin. (D and E) Schematics of the in vitro models and experimental setup. (F) Quantitative analysis of monolayer permeability in the absence and presence of PVM/Ms. *P < 0.05; **P < 0.01.
Transgenic Ablation of PVM/Ms Results in Significant Leakage from Vessels and in Hearing Loss.
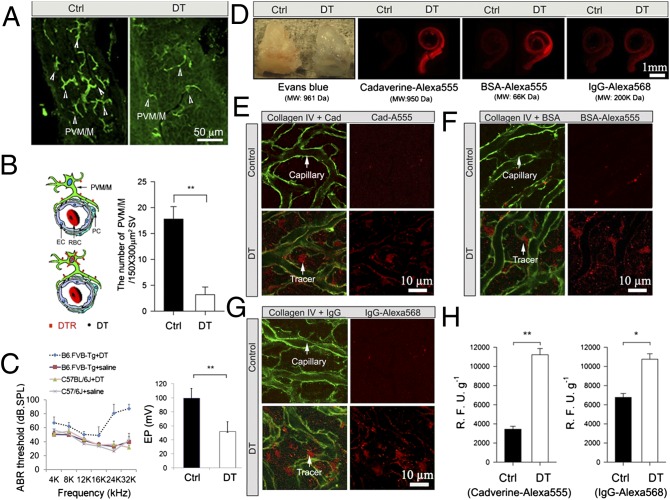
To validate the in vitro results, we performed an in vivo study in mice in which a transgene encoding a DT receptor was used for transient depletion of PVM/Ms. The mice were assigned randomly to receive DT or control (saline) injections, with an equal volume of saline administered to the control mice. A 5-d regimen of i.v. injections of DT caused a substantial reduction in the number of PVM/Ms (n = 10; P < 0.01) (Fig. 3 A and B). The PVM/M-ablated mice showed a significant drop in EP (B6.FVB-Tg + saline, n = 4; B6.FVB-Tg + DT, n = 6, P < 0.01) (Fig. 3C, Right) as well as hearing loss. The hearing loss was not seen in either of the control groups (C57BL/6J + saline, n = 10; B6.FVB-Tg + saline, n = 10) or in DT-treated mice not expressing the receptor (nC57BL/6J + DT, n = 10; P < 0.05 at 4–16 kHz; P < 0.01 at 24 kHz and 32 kHz) (Fig. 3C, Left). Consistent with the results observed in our in vitro model, we found that ablation of PVM/Ms results in marked vascular leakage. Immediately following the 5-d DT treatment, permeability assays using a range of low- and high-molecular-mass fluorescent tracers, including Evans blue, cadaverine Alexa Fluor-555, BSA-Alexa Fluor-555, and IgG-Alexa 568, were found to accumulate in the cochlear lateral wall tissue of PVM/M-ablated animals (Fig. 3D, Right), but not in control animals (Fig. 3D). Immunohistochemical staining in combination with confocal microscopy further demonstrated extravasation of various fluorescent tracers, including cadaverine Alexa Fluor-555 (Fig. 3E, Lower), BSA-Alexa Fluor-555 (Fig. 3F, Lower), and IgG Alexa 568 (Fig. 3G, Lower) in the PVM/M-ablated mice but not in the control mice (Fig. 3 E–G, Upper). The capillaries displayed significant leakiness in the absence of PVM/Ms (n = 5; P < 0.05) (Fig. 3H). The in vivo results corroborate in vitro results showing that absence of PVM/Ms weakens the endothelial barrier. This finding also confirms previous findings that disruption of the intrastrial fluid–blood barrier results in significant hearing loss (7, 10).
Fig. 3.
Transgenic ablation of PVM/Ms results in leaky capillaries. (A) Confocal images show the distribution of PVM/Ms (arrowheads) before (Left) and after (Right) DT treatment. (B) (Left) The illustration characterizes the depletion of PVM/Ms depletion from DT binding its receptor (DTR). (Right) The PVM/M population was reduced significantly by 5 d of DT treatment. **P < 0.01. (C) Depletion of PVM/Ms causes hearing loss (Left) and drop in EP (**P < 0.01) (Right). (D) Fluorescent tracers accumulated in the cochlear lateral wall of PVM/M-ablated animals (Right) but not in the control animals (Left). (E–G) Extravasation of different size tracers from the capillaries, visualized by collagen IV immunostaining (green), was observed in DT-treated mice (Left) but not in the control mice (Right). Arrows point to tracer fluorescence (red), which leaked from the capillaries. (H) Capillary leakage to lower-molecular-weight Cadaverine Alexa Fluor-555 (Left) and to high-molecular-weight IgG-Alexa568 (Right) is significant in the PVM/M-ablated mice but not in the control mice. *P < 0.05; **P < 0.01.
PVMs Control Barrier Permeability by Affecting Global Expression of Tight Junction-Associated Proteins.
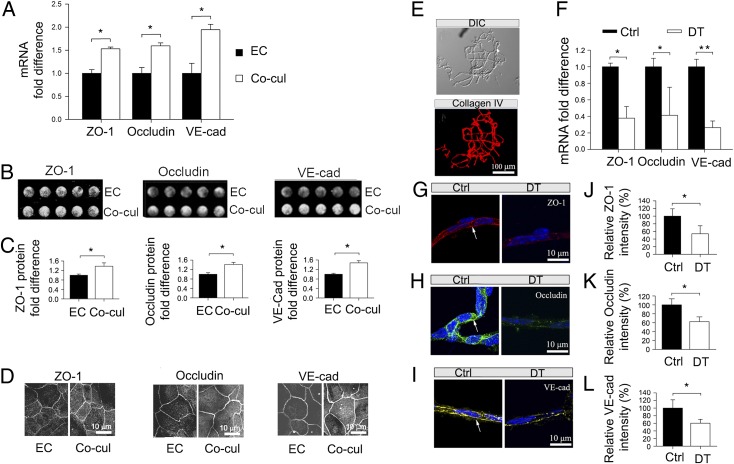
The permeability properties of the intrastrial fluid–blood barrier are largely a function of the tightness of the intercellular junction. The major tight junction-associated proteins in the intrastrial fluid–blood barrier are occludin, claudins, zonula occludens, and adherens-junction proteins (22). Several tight- and adherens-junction proteins, including ZO-1, occludin, and vascular endothelial cadherin (ve-cadherin), have been found in the intrastrial fluid–blood barrier (23, 24). In the in vitro models, mRNA levels for zo-1, occludin, and ve-cadherin, assessed with quantitative RT-PCR (qRT-PCR), were decreased dramatically in the absence of PVM/Ms (n = 3; P < 0.05) (Fig. 4A). Concurrent with decreased mRNA expression, protein levels for ZO-1, occludin, and ve-cadherin, analyzed by in-cell Western blotting, also showed a marked decrease ( n = 5; P < 0.05) (Fig. 4 B and C). Furthermore, immunohistochemical examination by confocal microscopy clearly showed a less dense distribution of tight- and adherens-junction proteins between ECs in the absence of PVM/Ms (Fig. 4D). The data indicate that PVM/Ms have broad effects on expression of junction proteins, which link directly to endothelial barrier permeability.
Fig. 4.
PVM/Ms control barrier integrity by affecting the expression of tight-junction proteins. (A) qRT-PCR shows mRNA for zo-1, occludin, and ve-cadherin in the presence and absence of PVM/Ms. *P < 0.05. (B) In-cell Western blot analysis of the protein expression. (C) Quantitative analysis of the proteins in the presence and absence of PVM/Ms. *P < 0.05. (D) Immunofluorescent labeling of the proteins. Co-cul, co-cultured. (E) Isolated capillaries from the SV. (Upper) A DIC image of the isolated capillaries. (Lower) Isolated capillaries labeled with antibody for collagen IV. (F) qRT-PCR results compare mRNA for zo-1, occludin, and ve-cadherin in isolated capillaries of control and DT-treated animals. (G–I) Immunofluorescent labeling for ZO-1 (red), occludin (green), and ve-cadherin protein (yellow) in isolated capillaries (DAPI counterstain, blue) of control (Left) and DT-treated animals (Right). (J–L) Expression of the proteins is significantly reduced in PVM/M-depleted animals. *P < 0.05; **P < 0.01.
The impact of PVM/Ms on the expression of junction proteins was validated further with qRT-PCR analysis and immunofluorescent analysis on isolated strial capillaries from control and PVM/M ablated animals. A previously established sandwich-dissociation method (25) was used to isolate and separate capillaries (Fig. 4E) and tight junction-associated proteins from adjoining marginal and basal cell layers with minimal contamination. Tight- and adherens-junction expression was assessed in the control and PVM/M -ablated animal groups. Consistent with the results of the in vitro study, mRNA for zo-1, occludin, and ve-cadherin, determined by qRT-PCR, was reduced significantly in the absence of PVM/Ms (Fig. 4F). Isolated capillaries from the control mice displayed strong patches of immunohistochemical staining for in situ-expressed ZO-1, occludin, and ve-cadherin protein (Fig. 4 G–I, Left); the staining was decreased dramatically in the PVM-depleted cochlear capillaries of PVM/M-ablated animals (Fig. 4 G–I, Right). Ablation of PVM/Ms significantly reduced expression of ZO-1, occludin, and ve-cadherin (n = 3; P < 0.05) (Fig. 4 J–L). The in vivo result further validates our hypothesis that PVM/Ms control barrier integrity by affecting the expression of tight junction-associated proteins.
PVMs Exert an Effect on Barrier Integrity Through Focal Secretion of PEDF.
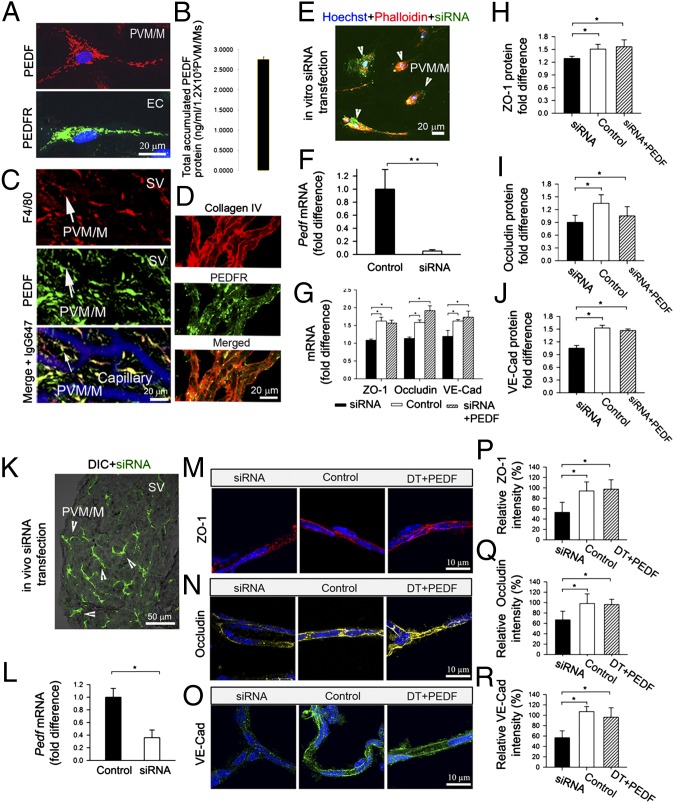
Next we examined the mechanism by which PVM/Ms the affect expression of junction proteins. PEDF is a 50-kDa glycoprotein previously found expressed in the rat inner ear, including in the SV (25). In this study, we found that PEDF is expressed in primary cultured PVM/Ms (Fig. 5A, Upper), whereas PEDF receptor (PEDFR) is expressed in primary cultured ECs (Fig. 5A, Lower). With an ELISA, we measured 2.8 ± 0.1 ng/mL PEDF protein produced by 1.2 × 105 PVM/Ms in a 4-d culture medium (Fig. 5B). In a whole-mount SV preparation, we also found that the PEDF was expressed most strongly in PVM/Ms (Fig. 5C), whereas its receptor, PEDFR, was expressed primarily in ECs (Fig. 5D). Given previous reports that PEDF is a potent endogenous inhibitor of vasopermeability (26, 27), PEDF/PEDFR signaling may mediate the effect of PVM/Ms on barrier permeability.
Fig. 5.
PVM/M control of barrier integrity is mediated by PEDF. (A) Cultured PVM/Ms express PEDF (Upper), whereas ECs express its receptor PEDFR (Lower). (B) PEDF production in a 4-d PVM/M culture medium. (C) Triple-labeled whole-mount SV shows PEDF (Top) strongly expressed in PVM/Ms (Middle). (Bottom) Merged image displaying capillary staining with antibody for IgG. (D) (Top) Isolated capillaries labeled with antibody for collagen IV. (Middle) PEDFR is expressed in the capillary wall. (Bottom) Merged image. (E) In vitro siRNA-transfected cells (green) double labeled for PVM/M marker protein F4/80 (red) and nuclei (DAPI, blue). (F) qRT-PCR analysis shows down-regulated mRNA expression of Pedf in the transfected PVM/Ms. (G) qRT-PCR analysis shows levels of mRNA for zo-1, occludin, and ve-cadherin in siRNA-transfected, control, and PEDF-treated EC monolayers. (H–J) In-cell Western blot protein analysis for ZO-1, occludin, and ve-cadherin in siRNA-transfected, control, and PEDF treated EC monolayers. (K) The confocal and DIC image shows siRNA-transfected cells (green) in the SV in vivo. (L) qRT-PCR shows down-regulated mRNA expression for Pedf in PVM/Ms in vivo. (M–O) Expression of the proteins in isolated capillaries of siRNA-transfected (Left), vector control (Center), and PEDF-treated PVM/M-depleted animals (Right). (P–R) Quantitative analysis of the proteins. *P < 0.05; **P < 0.01.
We tested the hypothesis in both in vitro and in vivo models using an siRNA-targeting vector to suppress Pedf expression. The in vitro-cultured PVM/Ms were transfected with siRNA for 48 h before co-culture with ECs (Fig. 5E). qRT-PCR analysis of Pedf transcripts from the in vitro samples showed the siRNA suppressed the Pedf gene in PVM/Ms by 80% (Fig. 5F). Down-regulation of Pedf gene expression resulted in a significant decrease in the expression of ZO-1, occludin, and ve-cadherin protein at both the transcriptional (Fig. 5G) and protein levels in cocultured ECs as compared with a vector control group (Fig. 5 H–J). Conversely, exposure of the monolayer to PEDF effectively ameliorates siRNA-induced down-regulation of ZO-1, occludin, and ve-cadherin protein expression (Fig. 5 H–J). In the in vivo animal model, PEDF expression was suppressed in PVM/Ms by a 5-d injection of a 5-μL solution of siRNA through the tympanic membrane. A fluorescent signal for siRNA strongly accumulated in the PVM/Ms (Fig. 5K). In our earlier studies, we found PVM/Ms to be phagocytic, engulfing blood-borne proteins such as serum protein (15). In this in vivo transfection experiment, we assume that the accumulation of siRNA in PVM/Ms was the result of phagocytotic activity, which also served our purpose for siRNA targeting PVM/Ms. qRT-PCR analysis showed a 60% down-regulation of Pedf gene expression in vivo by siRNA (Fig. 5L). Consistent with the down-regulation of ZO-1, occludin, and ve-cadherin seen in vitro, down-regulation of Pedf gene expression by siRNA resulted in dramatically decreased protein expression in capillaries (Fig. 5 L–N, Left) as compared with a vector control group (Fig. 5 M–O, Center). Application of PEDF effectively ameliorated siRNA-induced suppression of ZO-1, occludin, and ve-cadherin protein expression (Fig. 5 M–O, Right), whereas inhibition of PEDF significantly reduced the expression of tight- and adherens-junction proteins (Fig. 5 P–R, experiments in triplicate, P < 0.05). The results implicate PEDF signaling between PVM/Ms and ECs as an important mediator of the effect PVM/Ms have on expression of tight- and adherens-junction proteins.
Discussion
Our experiments show that perivascular-resident cells, identified here as melanocyte-like macrophages, have an important role in regulating the integrity of the intrastrial fluid–blood barrier and for maintaining normal hearing thresholds. In vitro, the absence of PVM/Ms increases the permeability of an EC monolayer. The results are confirmed in vivo, where ablation of PVM/Ms causes increased capillary leakage with an accompanying substantial drop in EP and hearing loss. These effects are mediated through PEDF, which directly affects the expression of tight junction-associated proteins such as occludin, ZO-1, and ve-cadherin.
The intrastrial fluid–blood barrier is one of the tightest blood–tissue barriers known in mammals. It is situated between blood flow and the intrastrial region of the SV. This region is critical for maintaining inner ear homeostasis, especially for maintaining the EP, an essential driving force for hearing function. At the cellular level, the intrastrial fluid–blood barrier comprises ECs lining cochlear microvessels and an associated basement membrane (2). Like other blood–tissue barriers, the intrastrial fluid–blood barrier is formed by tight junctions between ECs with the absence of fenestrations (11). Results from this study highlight the morphological complexity of the barrier in the SV, which includes surrounding basal lamina as well as a second line of support in PVM/Ms.
In a previous study, a large population of perivascular cells was found in the vicinity of the intrastrial fluid–blood barrier in the SV of normal adult cochlea. The cells were identified as perivascular-resident macrophages, because they were positive for several macrophage surface molecules, including F4/80, CD68, and CD11b. The macrophages were closely associated with microvessels and structurally were intertwined with ECs and pericytes. In this study, we found that the perivascular-resident macrophages are positive for melanocyte marker proteins, contain significant amounts of melanin, and express Kir 4.1, the fiduciary marker of intermediate cells (28). In the standard view, the SV comprises three cell layers (marginal, intermediate, and basal cell) and a rich capillary network. Earlier work had distinguished two subclasses of intermediate cells (29). We suspect the dark, melanocyte subclass of these intermediate cells is the equivalent of PVM/Ms. As a matter of fact, Conlee et al. (30) provide evidence for a distinction between melanocytes and intermediate cells in cats. Likewise, our studies show that PVM/Ms are a hybrid cell type, including characteristics of both macrophage and melanocyte phenotypes. Given their high density on capillary walls, an understanding of their function in the intrastrial fluid–blood barrier is critically important.
We hypothesize that the integrity of the intrastrial fluid–blood barrier is dependent on the structural association between PVM/Ms and ECs, with the process endings integral to the restrictiveness of the barrier. Although the in vitro model provides direct measurement of the interaction between ECs and PVM/Ms, some features of the in situ intrastrial fluid–blood barrier may be missing. Therefore we validated our findings with a transgenic (Tg) mouse model in which a DT receptor was expressed under the control of a human integrin αM promoter (CD11b). This mouse strain provided a system for transient depletion of PVM/Ms without toxic side effects (17, 18). In addition to leakage, PVM/M-ablated mice had hearing loss, demonstrating that a functional intrastrial fluid–blood barrier is required for normal hearing. When DT was administered to control mice, no side effects or toxicity was found. In mice not expressing the receptor, DT injection had no effect on hearing thresholds. However, in the DT receptor-fused mice, we found PVM/M depletion caused significant leakage in capillaries. In addition to leakage, PVM/M-ablated mice showed a substantial drop in EP accompanied by significant hearing loss. The EP, a voltage gradient essential for hearing, requires a functional capillary network. Hearing loss with PVM/M depletion may be the direct result of low EP from either breakdown of the barrier or loss of melanocytes. Melanocytes are essential for EP generation (31, 32), whereas disruption of the barrier results in an intrastrial electric shunt (7).
We next investigated possible mechanisms by which PVM/Ms effect changes in vascular permeability. Two transport pathways between the blood compartment and irrigated tissue are known (20): transcellular and paracellular passage. Transcellular passage through ECs requires cell fenestration or a complex system of trafficking vesicles, whereas the paracellular pathway depends on coordinated opening and closing of EC junctions. The permeability of the endothelium is largely a function of the tightness of the intercellular junctions.
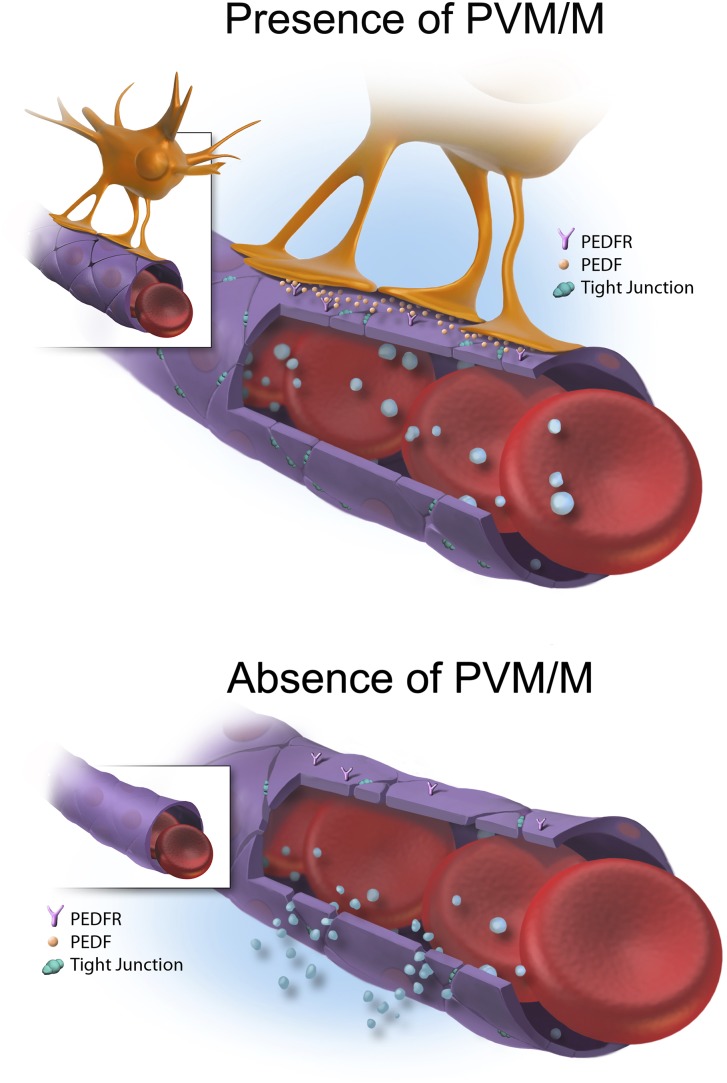
In an earlier mass spectrometry study, strial capillaries were found rich in tight-junction and cell-adhesion proteins (24). The major tight junction-associated proteins in the strial capillaries are occludins, claudins, ZOs, and adherens-junction proteins (23, 24). The present study found that tight-junction and adhesion proteins were down-regulated substantially in the absence of PVM/Ms. The down-regulation was associated with PEDF production. Application of PEDF to the tissue dramatically ameliorated the effect of PVM/M absence on leakage and attenuated the deregulation of junction proteins induced by absence of PVM/Ms (illustrated schematically in Fig. 6).
Fig. 6.
Working model of the PEDF/PEDFR signaling pathway regulating the expression of junction proteins. Capillary ECs are coupled by tight and adherens junctions that limit paracellular transport between adjacent cells. Circulating large molecules are extravasated from the capillary when PVM/Ms are absent. PVM/Ms control vascular permeability by affecting the expression of tight- and adherens-junction proteins through a PEDF/PEDFR signaling pathway.
PEDF is a 50-kDa secreted glycoprotein of the noninhibitory serpin family that exerts diverse physiological effects, including antiangiogenetic, antivasopermeability, antitumor, and neurotrophic effects (21). Studies have shown that PEDF binding to its receptor counteracts VEGF-induced vascular permeability (33, 34). Other studies point to PEDF suppression of VEGF-induced vascular permeability by targeting the PI3K/Akt signaling pathway (34) or phosphorylating adherens-junction proteins by a γ-secretase–mediated pathway (33). Our findings demonstrate that secretion of PEDF by PVM/Ms has direct and broad effects on the expression of several tight junction-associated proteins including occludin, ZO-1, and ve-cadherin.
In conclusion, this study has shown that PVM/Ms, a hybrid phenotype with macrophage and melanocyte characteristics, are important for hearing and that they control the integrity of the intrastrial fluid–blood barrier in the SV by affecting the expression of tight- and adherens-junction proteins. We established the important role of PVM/Ms in stabilizing the intrastrial fluid–blood barrier and identified PEDF as an essential signaling molecule.
Materials and Methods
Male C57BL/6J and B6.FVB-Tg (ITGAM-DTR/EGFP) 34Lan/J mice that encode a DT receptor fused with GFP under the control of a human integrin αM (ITGAM) promoter (CD11b) were used in this study. The Tg mice provide a DT-inducible system for transient depletion of macrophages. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University (IACUC approval number: B11265). All reagents and detailed experimental procedures used in this study, including primary PVM/M and EC cultures, isolation of strial capillaries, measurement of endothelial barrier permeability in vitro and in vivo, in-cell Western blot analysis, immunohistochemistry, confocal microscopy and immunofluorescence analysis, ELISA, qRT-PCR, transfection with siRNA, PVM/M counts, drug treatment, measurements of EP and auditory brain-stem response, electron microscopy, and statistical analyses, are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank John Henry for input and guidance with the development of work-flows for 3D rendering. This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grants DC008888-02A1 (to X.S.), DC008888-02S1 (to X.S.), R01-DC010844 (to X.S.), and NIHP30-DC005983.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205210109/-/DCSupplemental.
References
- 1.Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- 2.Juhn SK, Rybak LP, Prado S. Nature of blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol Laryngol. 1981;90:135–141. doi: 10.1177/000348948109000208. [DOI] [PubMed] [Google Scholar]
- 3.Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–991. [PubMed] [Google Scholar]
- 4.Takeuchi S, Ando M, Sato T, Kakigi A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hear Res. 2001;155:103–112. doi: 10.1016/s0378-5955(01)00252-0. [DOI] [PubMed] [Google Scholar]
- 5.Lin DW, Trune DR. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–534. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Mori N, Matsunaga T. Adenylate cyclase modulation of ion permeability in the guinea pig cochlea: A possible mechanism for the formation of endolymphatic hydrops. Acta Otolaryngol. 1992;112:667–673. doi: 10.3109/00016489209137457. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Salmon M, et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci USA. 2007;104:6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratton MA, Schulte BA, Smythe NM. Quantification of the stria vascularis and strial capillary areas in quiet-reared young and aged gerbils. Hear Res. 1997;114:1–9. doi: 10.1016/s0378-5955(97)00025-7. [DOI] [PubMed] [Google Scholar]
- 9.Hukee MJ, Duvall AJ., III Cochlear vessel permeability to horseradish peroxidase in the normal and acoustically traumatized chinchilla: A reevaluation. Ann Otol Rhinol Laryngol. 1985;94:297–303. [PubMed] [Google Scholar]
- 10.Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol. 2009;174:1692–1704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakagami M, Matsunaga T, Hashimoto PH. Fine structure and permeability of capillaries in the stria vascularis and spiral ligament of the inner ear of the guinea pig. Cell Tissue Res. 1982;226:511–522. doi: 10.1007/BF00214780. [DOI] [PubMed] [Google Scholar]
- 12.Dai M, et al. Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier. Am J Pathol. 2010;177:3089–3099. doi: 10.2353/ajpath.2010.100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- 14.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 15.Pottiez G, Flahaut C, Cecchelli R, Karamanos Y. Understanding the blood-brain barrier using gene and protein expression profiling technologies. Brain Res Brain Res Rev. 2009;62:83–98. doi: 10.1016/j.brainresrev.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 17.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochweller K, Striegler J, Hämmerling GJ, Garbi N. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur J Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 19.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Guelte A, Gavard J. Role of endothelial cell-cell junctions in endothelial permeability. Methods Mol Biol. 2011;763:265–279. doi: 10.1007/978-1-61779-191-8_18. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Yamagishi SI, Sata M. Structure-function relationships of PEDF. Curr Mol Med. 2010;10:302–311. doi: 10.2174/156652410791065255. [DOI] [PubMed] [Google Scholar]
- 22.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitajiri SI, et al. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res. 2004;187:25–34. doi: 10.1016/s0378-5955(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, et al. Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS ONE. 2011;6:e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleich O, Piña AL. Protein expression of pigment-epithelium-derived factor in rat cochlea. Cell Tissue Res. 2008;332:565–571. doi: 10.1007/s00441-008-0608-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, et al. Identification of the antivasopermeability effect of pigment epithelium-derived factor and its active site. Proc Natl Acad Sci USA. 2004;101:6605–6610. doi: 10.1073/pnas.0308342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda S, Yamagishi SI, Okuda S. Anti-vasopermeability effects of PEDF in retinal-renal disorders. Curr Mol Med. 2010;10:279–283. doi: 10.2174/156652410791065291. [DOI] [PubMed] [Google Scholar]
- 28.Hibino H, et al. An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4. 1), in cochlear stria vascularis of inner ear: Its specific subcellular localization and correlation with the formation of endocochlear potential. J Neurosci. 1997;17:4711–4721. doi: 10.1523/JNEUROSCI.17-12-04711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cable J, Steel KP. Identification of two types of melanocyte within the stria vascularis of the mouse inner ear. Pigment Cell Res. 1991;4:87–101. doi: 10.1111/j.1600-0749.1991.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 30.Conlee JW, Parks TN, Schwartz IR, Creel DJ. Comparative anatomy of melanin pigment in the stria vascularis. Evidence for a distinction between melanocytes and intermediate cells in the cat. Acta Otolaryngol. 1989;107:48–58. doi: 10.3109/00016488909127478. [DOI] [PubMed] [Google Scholar]
- 31.Steel KP, Barkway C. Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–463. doi: 10.1242/dev.107.3.453. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana M. Cochlear melanocytes and MITF signaling. J Investig Dermatol Symp Proc. 2001;6:95–98. doi: 10.1046/j.0022-202x.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 33.Cai J, et al. γ-Secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J Biol Chem. 2011;286:42514–42523. doi: 10.1074/jbc.M111.296590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheikpranbabu S, Haribalaganesh R, Lee KJ, Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end products-induced retinal vascular permeability. Biochimie. 2010;92:1040–1051. doi: 10.1016/j.biochi.2010.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.