Abstract
Global climate carbon-cycle models predict acceleration of soil organic carbon losses to the atmosphere with warming, but the size of this feedback is poorly known. The temperature sensitivity of soil carbon decomposition is commonly determined by measuring changes in the rate of carbon dioxide (CO2) production under controlled laboratory conditions. We added measurements of carbon isotopes in respired CO2 to constrain the age of carbon substrates contributing to the temperature response of decomposition for surface soils from two temperate forest sites with very different overall rates of carbon cycling. Roughly one-third of the carbon respired at any temperature was fixed from the atmosphere more than 10 y ago, and the mean age of respired carbon reflected a mixture of substrates of varying ages. Consistent with global ecosystem model predictions, the temperature sensitivity of the carbon fixed more than a decade ago was the same as the temperature sensitivity for carbon fixed less than 10 y ago. However, we also observed an overall increase in the mean age of carbon respired at higher temperatures, even correcting for potential substrate limitation effects. The combination of several age constraints from carbon isotopes showed that warming had a similar effect on respiration of decades-old and younger (<10 y) carbon but a greater effect on decomposition of substrates of intermediate (between 7 and 13 y) age. Our results highlight the vulnerability of soil carbon to warming that is years-to-decades old, which makes up a large fraction of total soil carbon in forest soils globally.
Keywords: climate feedback, soil organic matter, soil respiration, radiocarbon, soil incubation
The potential for carbon stored on land to become a source of carbon dioxide (CO2) to the atmosphere in the 21st century is a key uncertainty in predictions of future climate. Global warming increases the rate of decomposition of soil organic carbon (C), a major loss pathway of C from the land surface to the atmosphere, thus contributing to the increase in atmospheric CO2 and hence, global temperatures. However, how much of the estimated 3,000 Pg C (1) stored in soils globally is vulnerable to enhanced decomposition with warming is highly uncertain and difficult to assess (2). In particular, the temperature sensitivity of C cycling on decadal timescales is a key uncertainty controlling the size of potential soil C responses to warming (3). Although there are no global estimates of decadal-aged C, it makes up the majority of C in mineral soils in temperate forests (4). We took advantage of a decade-long, whole-ecosystem C-isotope label to isolate the effect of warming on decomposition of decades-old C in a laboratory incubation experiment.
The temperature sensitivity of decades-old C is difficult to observe using traditional approaches, such as response of CO2 flux to experimental warming, because respiration is dominated by soil C cycling on fast timescales of 1 y or less. Previous studies using C isotopes to identify older C and assess its temperature sensitivity do not provide consistent results (recently reviewed in refs. 5 and 6). Most of these studies used a change in vegetation type (e.g., from C3 to C4 photosynthetic vegetation) as a means to distinguish old and young C. However, such vegetation shifts also change the amount and quality of C inputs to soil, affecting the decomposition process and potentially confounding measurements of temperature sensitivity. In addition, most studies took place in agricultural soils and may not be representative of less managed systems. Other methods to determine the temperature sensitivity of slower-cycling C also have significant drawbacks. Extended incubation periods to deplete the soil of fast-cycling C pools can change the decomposition process through substrate limitation (7). The response of slow-cycling soil C to warming is difficult to detect on the timescales of manipulative experiments, and it may be affected by covarying factors along natural temperature gradients (6). Model-derived predictions of temperature sensitivity of C pools cycling on different timescales are highly sensitive to assumptions in underlying model structures, such as which parameters are temperature-sensitive (8). Moreover, any inference of temperature sensitivity from bulk CO2 fluxes alone is difficult to relate to soil C destabilization processes, because respiration integrates across C pools stabilized by multiple interacting controls. Specifically, the mean residence time of different soil C pools is affected by both biology and physicochemical conditions, which are both likely to be temperature-sensitive (9). As a result, the effect of warming on the stability of soil C stocks is a topic of intense debate.
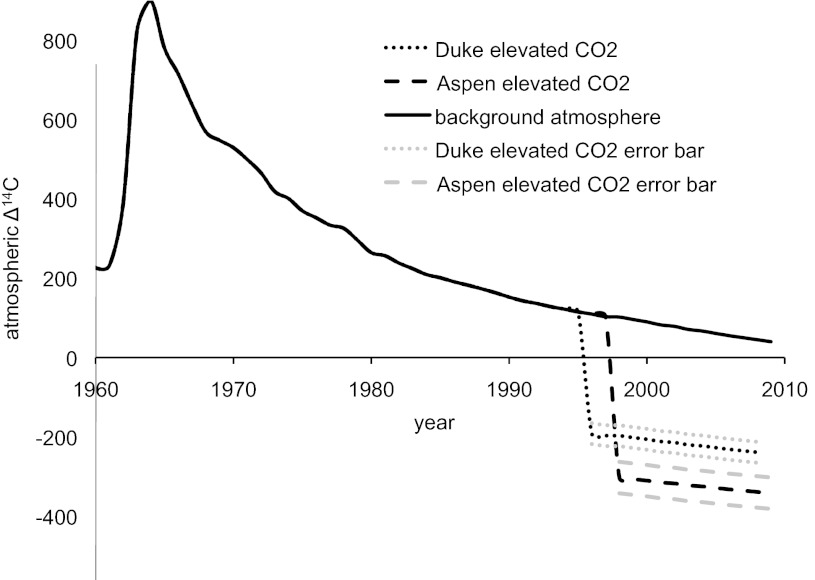
We investigated the temperature sensitivity of decades-old C by taking advantage of a whole-ecosystem C-isotope label in two temperate forest sites. Both sites had free air CO2 enrichment (FACE) experiments, where atmospheric CO2 concentrations in treatment plots are raised by fumigating with fossil-derived CO2 that has a distinct C-isotope signature in 14C and 13C compared with background air [fumigation gas Δ14C value of −1,000‰ compared with 50–100‰ for background air (10) and δ13C value of −43‰ compared with about −8‰ in background air (11, 12)]. Thus, C fixed by photosynthesis and incorporated into plant material and soil C under elevated CO2 is isotopically distinguishable from previously existing soil C (FACE label). CO2 enrichment began at both sites more than a decade before we sampled soils (Table 1), and therefore, the C-isotope label allows us to distinguish the contribution of decades-old C (pre-FACE C > 10 y) from the contribution of more recent C (FACE C < 10 y) to heterotrophic respiration during incubation using standard isotopic mixing models (13).
Table 1.
Site information
| Site | Location | MAT (°C) | Mean annual precipitation (mm) | Planted (y) | +CO2 (y) | Vegetation | Soil type | Soil texture |
| Aspen | 45°N 89°W | 4.9 | 810 | 1997 | 1998–2009 | Populous tremuloides | Ultic Hapludalf | Sandy loam |
| Duke | 35°N 79°W | 15.5 | 1,140 | 1983 | 1996–2010 | Pinus taeda | Alfic Haplorthod | Clay loam |
Although elevated CO2 soils provide us with a unique opportunity to constrain how much decades-old C contributes to respiration by using the large difference in 14C and 13C between C fixed before and after FACE (Fig. 1), measurements of background levels of 14C in the nonenriched ambient CO2 plots provide an additional age constraint. In ambient CO2 plots, the 14C content of soil-respired CO2 reflects the relative contribution of 14C fixed by photosynthesis into ecosystem C pools since aboveground nuclear weapons testing in the 1960s (Fig. 1). The atmospheric 14CO2 signature has been declining by ∼5‰/y in recent years (14), and therefore, the mean age of respired C—the mean time elapsed since respired C was fixed from the atmosphere—can be determined using a time-dependent, steady-state model and the atmospheric history of 14C (15). With the bomb-derived 14C label, we can detect differences between C fixed from the atmosphere from 1 y to several decades before the date of sampling.
Fig. 1.
Atmospheric 14C content by year. The solid line represents background atmosphere Δ14C value since 1960, and the dashed and dotted lines represent atmosphere with FACE in elevated CO2 plots at Aspen and Duke, respectively. Light gray lines represent the potential variability in 14C signature of the elevated CO2 treatments based on the SD of CO2 concentrations measured in elevated CO2 plots (SI Methods).
We sampled soils from both ambient and elevated CO2 treatment plots at two FACE sites (Aspen FACE, Rhinelander, WI; Duke FACE, Durham, NC) after they had been exposed to elevated CO2 for 11 y. Both sites are temperate forest plantations on old agricultural soils, but they differ with respect to species, lifeform, and stand age (Table 1). At Aspen FACE, deciduous aspen clones were planted in monoculture in 1997, and CO2 enrichment was initiated the next growing season. At Duke FACE, evergreen loblolly pines were planted in 1983, and CO2 enrichment began when the trees were already 13 y old. We incubated surface mineral soils (0- to 15-cm depth) at their site mean annual temperatures (MATs; 5 °C and 15 °C, respectively) and under two warming treatments (+10 °C and +20 °C). Respired CO2 was collected for determination of flux rates and 14C and 13C content of respiration.
The two isotope constraints allowed us to distinguish the contribution of soil C cycling on three different timescales—years, decades, and intermediate between the two time periods—to CO2 fluxes across incubation temperatures. Specifically, we tested a common assumption of global ecosystem models that all ages of soil C have similar temperature sensitivity. We can expect one of four possible outcomes.
i) If the temperature sensitivity of C up to several decades old is greater than the temperature sensitivity of C of younger age, we would expect more enriched C-isotope values of respiration under higher temperature in both FACE and ambient CO2 treatments, with (i) a gradual increase in Δ14C of respiration from ambient CO2 soils, reflecting greater decomposition of C fixed since 1960, and (ii) a rapid increase in Δ14C of respiration from elevated CO2 soils, reflecting increased contribution of isotopically distinct, decades-old C fixed before CO2 enrichment.
ii) If the temperature sensitivity of C around a decade old is greater than the temperature sensitivity of the younger and older age classes, we would expect an increase in Δ14C of respiration with warming from both FACE and ambient CO2 treatments at a similar rate, reflecting relatively faster decomposition of 10-y-old C fixed during the CO2 enrichment period, with slightly higher 14C content than the youngest C because of the gradual decline in atmospheric 14C in both CO2 treatments over this time period.
iii) If the temperature sensitivity of the youngest C is greater than the temperature sensitivity of the two older age classes, we would expect a decrease in Δ14C of respiration with warming from elevated CO2 soils and ambient CO2 soils.
iv) If the temperature sensitivity of all ages of C is similar, we would expect Δ14C of respiration from elevated CO2 and ambient CO2 soils to remain constant across temperature treatments.
Results
Respiration Sensitivity to Warming.
Warming consistently increased respiration rates from incubated surface soils for both CO2 levels at the two sites (P < 0.0001 for temperature effect). Although respiration rates dropped with time (Fig. S1) in the Duke soils, the effect of temperature on respiration rate was consistent over the many months (up to 12 mo for Duke soils) of the experiment. The increase in respiration rates corresponded to a Q10 of 1.5–1.9 for Duke and 2.9–3.1 for Aspen. The elevated atmospheric CO2 treatment also significantly increased fluxes (P = 0.006) and interacted with the temperature effect (P = 0.044) at the Aspen site, but it had no statistically significant effect on fluxes at Duke.
Temperature Dependence of Decades-Old C (Pre-FACE C).
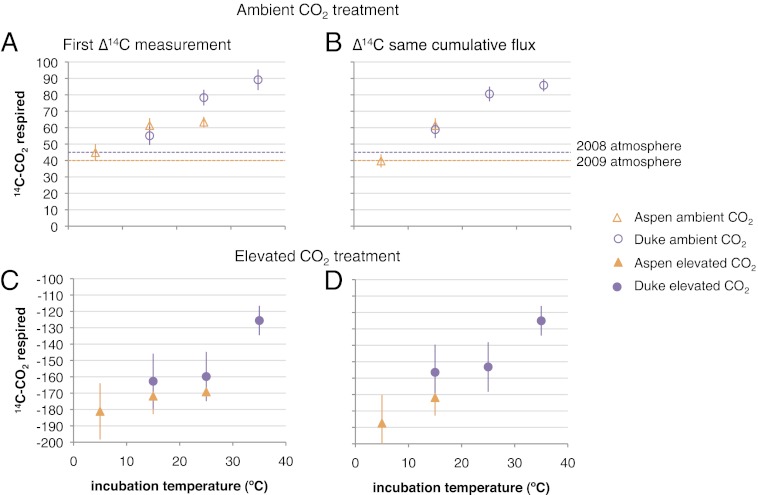
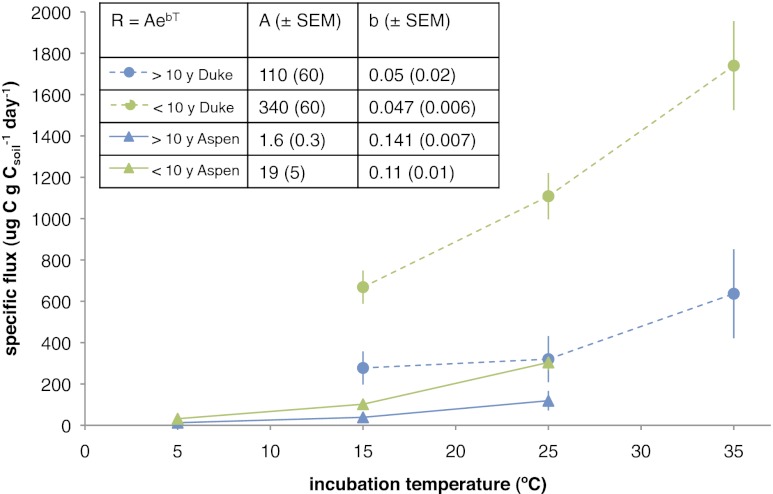
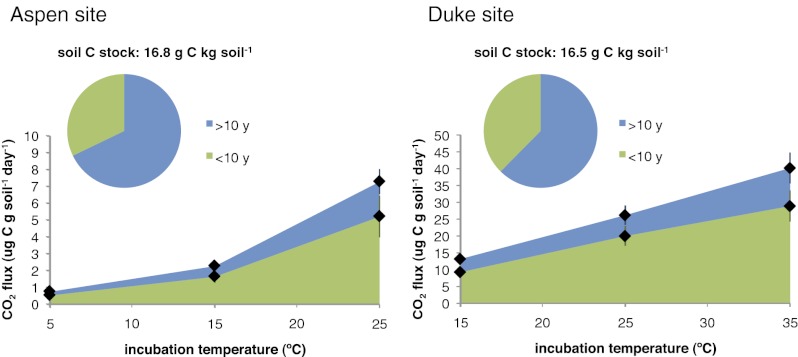
Isotopic signatures of the CO2 respired in the incubations reflect the large influence of the isotopically depleted C fixed in the FACE treatments (Fig. 2). We used an isotopic mixing model to partition fluxes from the elevated CO2 treatment into FACE-derived (<10 y) and pre-FACE (>10 y) pools using the FACE 14C label (SI Methods). In the FACE soils, roughly one-third of the C respired was fixed before the FACE experiment, regardless of temperature (Table 2). Warming increased the rate of losses from both pools, showing that decades-old C (>10 y) is vulnerable to immediate, enhanced losses on warming and has similar temperature sensitivity as younger FACE-derived (<10 y) C (Fig. 3). Isotopic partitioning using the δ13C label also supports the conclusion that both FACE and pre-FACE C are equally sensitive (Table 2).
Fig. 2.
Average Δ14C values of CO2 respired by incubated soils from Aspen (n = 3) and Duke (n = 4) FACE sites by incubation temperature (error bars are SEM). (A and B) Ambient CO. (C and D) Elevated CO2. (A and C) Δ14C–CO2 respired at the first flux measurement period. (B and D) Cumulative Δ14C–CO2 respired to a given loss of initial soil carbon (0.11% for Aspen and 2.68% for Duke). Dashed lines indicate atmospheric Δ14C values in the years of sampling at Aspen (2009) and Duke (2008) sites.
Table 2.
Fraction of soil C stock (0- to 15-cm mineral soil) and respiration flux (first sampling) coming from pre-FACE (>10 y) C (±SEM) identified with 14C and 13C mixing models
| Respiration |
||||
| Stock (f>10 y by 13C) | Temperature (°C) | f>10 y by 14C | f>10 y by 13C | |
| Duke | ||||
| CO2 ambient | 15 | 0.33 (0.10) | ||
| CO2 ambient | 25 | 0.31 (0.15) | ||
| CO2 ambient | 35 | 0.27 (0.12) | ||
| CO2 elevated | 0.62 (0.03) | 15 | 0.29 (0.16) | 0.38 (0.15) |
| CO2 elevated | 25 | 0.24 (0.16) | 0.28 (0.07) | |
| CO2 elevated | 35 | 0.28 (0.22) | 0.42 (0.06) | |
| Aspen | ||||
| CO2 ambient | 5 | 0.40 (0.31) | ||
| CO2 ambient | 15 | 0.34 (0.13) | ||
| CO2 ambient | 25 | 0.35 (0.12) | ||
| CO2 elevated | 0.68 (0.07) | 5 | 0.28 (0.12) | 0.43 (0.21) |
| CO2 elevated | 15 | 0.27 (0.08) | 0.44 (0.09) | |
| CO2 elevated | 25 | 0.28 (0.10) | 0.37 (0.11) | |
Fig. 3.
Respired CO2 (micrograms C grams Csoil−1 day−1) partitioned into >10 y C (pre-FACE) and <10 y C (FACE) using the 14C mixing model for Aspen (n = 3) and Duke (n = 4) elevated CO2 soils. Average fluxes from each pool are shown, and error bars are SEMs calculated from propagated error in the model. Inset shows parameters (±SEM) from regression of respiration flux on temperature by R = AebT.
We quantified the temperature effect on partitioned fluxes with an exponential model, where the temperature sensitivity coefficient b defines the increase in flux per change in temperature and A is a constant that represents the basal reaction rate (8). Within each site, we observed no significant differences in b for C pools of different ages (Fig. 3 Inset). The flux of pre-FACE (>10 y) C was slightly more temperature-sensitive (but not statistically different) than the flux of FACE (<10 y) C. Values of A were always higher for FACE C than pre-FACE C, confirming that the model separated pools with different overall cycling rates.
Increase in Substrate Availability with Temperature.
Along with increased fluxes, we observed an immediate shift in the Δ14C signature of respired CO2 from warmed soils relative to the site MAT control soils (Fig. 2 A and C). Warming increased the mean age of respired C, which was shown by the significant increase in Δ14C of respiration with incubation temperature from the ambient CO2 treatment at both Aspen (P = 0.0454) and Duke (P = 0.0058). For the elevated CO2 soils, Δ14C of respiration also tended to increase with warming, although this difference was not statistically significant because of greater variability in Δ14C of respiration among replicates (Table 3).
Table 3.
Mean CO2 flux and isotope values (±SEM) from first sampling
| Temperature (°C) |  |
Δ14C–CO2 | δ13C–CO2 | |
| Duke | ||||
| Ambient CO2 | 15 | 775 (31) | 55.1 (5.6) | −26.56 (0.2) |
| Ambient CO2 | 25 | 1,399 (76) | 78.3 (4.8) | −26.68 (0.4) |
| Ambient CO2 | 35 | 2,682 (63) | 89.2 (6.3) | −26.80 (0.2) |
| Elevated CO2 | 15 | 946 (89) | −162.7 (17.8) | −34.30 (0.9) |
| Elevated CO2 | 25 | 1,429 (72) | −159.8 (16.1) | −35.60 (0.4) |
| Elevated CO2 | 35 | 2,377 (188) | −125.6 (8.8) | −33.92 (0.3) |
| Aspen | ||||
| Ambient CO2 | 5 | 38 (7) | 44.9 (5.2) | −26.29 (0.7) |
| Ambient CO2 | 15 | 116 (6) | 61.3 (4.4) | −27.47 (0.3) |
| Ambient CO2 | 25 | 338 (16) | 63.4 (3.2) | −27.45 (0.3) |
| Elevated CO2 | 5 | 45 (3) | −181.1 (17.0) | −34.08 (1.7) |
| Elevated CO2 | 15 | 141 (8) | −171.8 (11.0) | −34.50 (0.6) |
| Elevated CO2 | 25 | 423 (28) | −169.2 (16.3) | −35.33 (0.7) |
To test whether this pattern was caused by rapid depletion of fast-cycling C substrates, we normalized the isotopes of CO2 flux data by amount of C lost (rather than by time). This normalization allows us to compare the sources of the equivalent amount of C respired across temperatures (Fig. S2) (16). If the same substrates were used at all temperatures but more rapidly depleted under warming, we would expect the same C-isotope content for the equivalent amount of initial soil C respired. Instead, we observed a shift to higher Δ14C values under warming (Fig. 2 B and D), which was similar in magnitude to the shift observed by comparing isotopes of flux at the same time in the incubation (Fig. 2 A and C). With this adjustment, Δ14C respired under warming from ambient CO2 soils was still significantly higher than from the MAT treatments, indicating that an isotopically distinct soil C source was used at higher temperatures.
The hypothesis of new substrate becoming available under warming is also supported by the observed decrease in flux rates over the incubation period. We modeled the change in CO2 fluxes over time of incubation with a two-pool exponential equation to resolve an active pool (Ca), a slow pool (Cs), and their respective turnover rates (17). If substrate depletion was occurring in warmed soils, we would expect a more rapid initial decline of flux rates relative to the MAT treatment represented by an increase in the decay constant of the active pool (ka). However, we found that increased respiration under warming in the early part of incubation is best modeled with an increase in the size of the active pool (Ca) with warming, whereas the decay constant (ka) for that pool stays relatively constant (Table S1) in the Duke soils (flux data from Aspen soils did not fit the model). This pattern has been found in many studies, and it has resulted in an ongoing debate about whether temperature dependence can be in both the pool size terms and the rate constant (18–20). These results suggest that increased substrate availability may be the key to the initial stages of the warming response.
Age of Respired C Substrates.
From the ambient CO2 treatment soils incubated at the site MAT, bomb 14C modeling estimates of the age of C respired were 2 y for Aspen (<1–5 y, 95% confidence interval) and 3 y for Duke (<1–6 y). Warming increased the mean age of C respired by 3–5 y at both sites relative to the MAT treatment (MAT + 10°C: +3 y at Aspen, +4 y at Duke; MAT + 20°C: +3.5 y at Aspen, +5 y at Duke). The young age of respired C agrees with the expectation that C with the fastest turnover time is metabolized early in incubation and that the youngest C dominates the heterotrophic respiration signal.
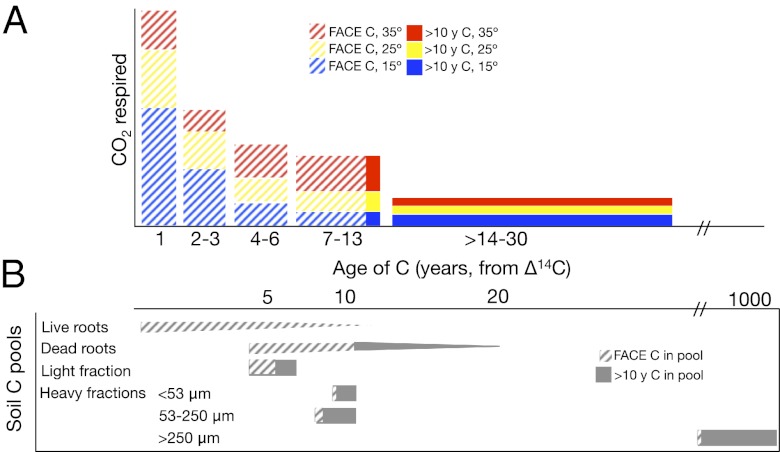
To confirm that additional substrate made available by warming was less than a decade old, we modified our original mixing model to include a warming-induced pool defined by the change in flux and Δ14C–CO2 respired from warmed soils over the MAT control soils (Fig. 4). Using data for the same cumulative C loss across temperatures, we assumed an equal contribution of <1-y C to fluxes in ambient CO2 soils and the same Δ14C end members at all temperatures, which enabled us to solve for the Δ14C value of the warming-induced substrates. We estimate this pool to have a mean age of 7–13 y in Aspen and 9–12 y in Duke.
Fig. 4.
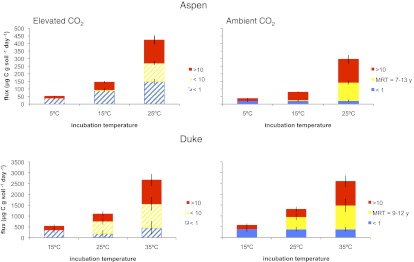
Cumulative respired CO2 (micrograms C grams soil−1 day−1) partitioned by modified isotopic mixing model. Solid bars represent soil CO2 fluxes with the 14C signature of the atmosphere, and hatched bars represent soil CO2 fluxes with the 14C signature of the FACE label. Colors represent the age of C: red, >10 y; yellow, intermediate-aged and warming-induced C; blue, <10 y for elevated CO2 soils and <1 y for ambient CO2 soils. Error bars represent SEMs propagated through the mixing model.
Discussion
Vulnerability of Decades-Old Soil C to Warming.
In these two temperate forest soils, warming increased respiration of soil C more than a decade old fixed before the FACE treatment. Such decades-old C is a major component of organic matter in these soils and temperate forests more broadly (4), implying that a large portion of soil organic C is vulnerable to increased decomposition with global warming. C more than a decade old made up 70% of mineral soil C in the 0- to 15-cm depth that we incubated, but ∼30% of the CO2 respired from it. The difference implies that there is some component of the soil C stock, often referred to as passive or inert C, that is not contributing detectably to respiration (21, 22). We estimate that this passive pool makes up 6–47% of the C in the Aspen and <17% of the C at Duke (SI Methods). Hence, at least 53–94% at Aspen and >83% at Duke of the top 15 cm mineral soil C is vulnerable to increased decomposition with warming or 1,850–4,700 and 1,750–2,160 g C m−2, respectively (SI Methods).
Temperature Sensitivity of Decades-Old Soil C Decomposition Is Robust Across Sites.
Warming increased decomposition rates of decades-old C at both sites, despite large differences in overall soil cycling rates (Fig. 3 and Table 3). Aspen had much slower respiration rates than Duke (Table 3) (23) and less contribution of decades-old C to the soil C stock, which was indicated by bomb-derived 14C in bulk ambient CO2 soils (Δ14C values of 51‰ at Aspen and 73‰ at Duke). The total amount of soil C and its distribution among physical fractions also differed greatly between the sites (Table S2) (24, 25). Specifically, there was a greater proportion of mineral stabilized C at Aspen FACE relative to Duke FACE. Along with the Δ14C of bulk soils, this finding suggests a larger proportion of prebomb C and a larger overall pool of passive C not contributing to soil respiration at Aspen FACE than Duke FACE.
Nevertheless, the proportion of pre-FACE C in respiration and its response to warming was similar at both sites. For elevated CO2 soils from Aspen and Duke, decades-old C comprised a surprisingly large 30% of respired CO2 across all incubation temperatures. Based on the Δ14C of heterotrophically respired CO2 from the ambient CO2 soils, the estimated mean age of respired C was 2–3 y; however, additional information from the FACE-labeled soils indicates that this finding is averaging of very young C with almost one-third that is more than a decade old. Other studies with in situ isotope labels from agroecosystems show that up to 66% of respiration derived from decades-old C [>45 y old (26); other studies: 0–21%>40 y (27); 52%>14 y old (28); 45%>26 y old (26)].
The age distribution of respired C at both sites for the control temperature and warming treatments was consistent for both types of C-isotope tracers. Specifically, the offset between Δ14C–CO2 respired in incubation and the Δ14C value of the atmosphere in the year of sampling was nearly the same at both sites, indicating similar residence time of respired C. Thus, respirable C was more uniform between soils than overall C stocks. Importantly, the similar response to warming—measured by the effect of warming on both proportional contribution of decade-old C to respiration fluxes and the increase in Δ14C respired—suggests that the sites have similar age distributions of C sources contributing to respiration and perhaps, similar mechanisms of temperature response.
What Is Decadal-Aged C at These Sites?
We used the C-isotope signatures of soil C components (e.g., roots, microbes, and physical soil fractions) to identify sources of respired CO2 and particularly, decades-old CO2 (Fig. 5). C that was recently deposited by roots, either as exudate or litter, is probably the source of most C respired from these soils, although visible roots were removed before incubation. Roots are increasingly recognized as the primary source of C to microbes in A horizon soils (29). The age of roots coincides with Δ14C values of respired C at these sites; at Duke FACE, the mean age of roots was 4–6 y, with some roots >18 y old (30), and at Aspen FACE, the mean age of roots was 1–3 y old for <2-mm roots and 3–5 y old for >2-mm roots. Given these values, the time spent by C in structural root tissue was sufficient to give the respired CO2 age without significant additional time in soil C; the inferred age of respired C may be a function of time spent in structural root tissue rather than soil C pools.
Fig. 5.
Age distribution of C in Duke FACE respiration (A) and soil C pools (B). A gives our best estimate of the contributions of different age classes to respiration at the three incubation temperatures (i.e., blue bar shows respiration at 15 °C, yellow bar shows additional respiration at 25 °C above the 15 °C respiration rate, and red bar + yellow bar shows additional respiration at 35 °C). Hatched areas represent FACE-derived C, and solid bars represent C predating the FACE experiment (>10 y C). Temperature sensitivity is similar between FACE and >10 y C, but it is higher for C aged 7–13 y, which is indicated by the relatively larger red bar, increasing the average age of respiration in warmer soils. B shows the 14C-derived mean age of soil C pools. The hatched gray area represents FACE-derived C, and solid gray is C predating the FACE experiment in each physically separated soil C fractions.
Although decay of root tissues may be a major component of respired CO2, it is not the primary source of decades-old C to respiration. Although root tissue containing pre-FACE C may have been present at Duke FACE, roots at Aspen FACE are composed only of FACE-derived C, because those trees experienced an enriched CO2 atmosphere for their whole lives. The source of decades-old C to respiration is more likely to be C that is associated with minerals <250 μm in size, which were ∼20% and ∼30% of soil C at Aspen and Duke, respectively (24, 25). Temperature sensitivity of this fraction is consistent with the increase in respiration of both FACE-derived and pre-FACE C, because this fraction contains significant portions of both age classes of C and is the only soil C pool with enough bomb-derived 14C to have caused the increase in Δ14C of respiration with warming. The larger-size fraction (>250 μm) of mineral-associated C at Duke FACE has very low 14C values in both elevated and ambient CO2 treatments, suggesting very slow turnover and negligible contribution to respiration. Although we do not have Δ14C measurements for physical soil fractions at Aspen FACE, incorporation of 13C-depleted C from the FACE label in mineral-associated size fractions suggests similar C turnover patterns as observed in the Duke FACE soils (24).
Microbial biomass has a similar C isotopic signature to its C sources and respiration (29), but it is too small of a C pool in itself to be solely responsible for the observed respiration flux. Up to 9% of total soil C at Duke was respired as active-pool C in the warmest soils compared with living microbial biomass that is, at most, 4–5% of total C in these soils (31).
Another explanation for the large release of decades-old C is a disturbance effect; however, this reason is unlikely to be a full explanation in our experiment. A major criticism of the incubation method is that preincubation sample preparation may change soil C decomposition rates. Particularly if soils are sieved, previously protected, decades-old soil C may be exposed to microbial attack and vulnerable to degradation. However, potential disturbance effects are unlikely to yield similar results for both sites, because Aspen soils were sieved, whereas Duke soils were not. In addition, soil aggregation is probably not the mechanism of soil C protection in Duke FACE soils (13).
Increased Vulnerability of Intermediate-Aged (7–13 y) C with Warming.
The central question of our experiment was whether decades-old C had different temperature sensitivity than faster-cycling C, because the feedback between soil respiration and climate warming in earth system models is particularly sensitive to this premise (32, 33). The FACE C-isotope label (both 13C and 14C) allowed us to unequivocally determine that decades-old C fixed before FACE had similar temperature sensitivity to C fixed during the last 10 y. However, integrating the bomb-derived 14C label into the analysis, we identified a subtle difference in the age of C respired with increasing temperature. Specifically, we observed a parallel increase in Δ14C of CO2 respired with warming in both ambient and elevated CO2 soils that was not caused by exhaustion of fast-cycling C. The similar increase in Δ14C in both CO2 treatments suggests that warming increased the contribution of C fixed earlier in the decade since the FACE treatment began, reflecting the ∼60‰ decline in atmospheric Δ14C over the period of the FACE experiment in ambient CO2 plots and an ∼40‰ decline under enriched CO2, where the decline in background atmosphere Δ14C was diluted by addition of FACE label C during CO2 enrichment.
Our study is not unique in the finding that the 14C content of respiration increased with warming. Two other incubations of forest soils with background levels of 14C inferred higher temperature sensitivity of soil C with a similar age as the age in our study. In boreal forest soils, increased 14C of respiration with warming corresponded to higher temperature sensitivity of decadally cycling C compared with annually and centennially cycling C pools (34). In a temperate forest soil, 25 °C of warming increased the 14C-derived mean residence time of respiration by up to 4 y from 7.9 to 11.9 y (35). These findings show that warming increases the respiration of C up to several decades old (fixed during the postbomb period; i.e., post-1960).
With the additional time constraint of the FACE label, we can eliminate the possibility that C much older than 10 y was more temperature-sensitive than other ages of C. If this finding were the case, we would expect to observe a much more rapid increase in 14C respired by elevated CO2 soils than ambient CO2 soils. From the similar rate of change in both types of isotope labels, we conclude that some portion of soil organic C aged 7–13 y responded disproportionately to warming. Different temperature sensitivity of this age of C does not contradict our finding of similar temperature sensitivity of decades-old and younger C; in fact, C of this age would be partitioned into both the FACE and pre-FACE C pools in the mixing model.
Higher temperature sensitivity of intermediate-aged C provides a potential explanation for inconsistencies between conclusions of previous studies that used C-isotope labels in soil to infer temperature sensitivities of different ages of C. Isotope label studies differ in the length of the labeling period before sampling, resulting in varying definitions of older C. If C with a similarly disproportionate temperature sensitivity and age is present in the soils of these studies, then inconsistent conclusions for temperature sensitivity of older C may depend on whether this C was included as part of the older or younger C pool. In shorter experiments, temperature sensitivity of this intermediate C is likely to be categorized as older C, and therefore, its disproportionate response to warming gives the appearance that older C is more temperature-sensitive [e.g., soils sampled after 5 y of label (36) or 14 y of label (29)]. In contrast, studies with a longer labeling period [e.g., labels of 26 and 45 y (26) or 33 y (16)] find equal temperature sensitivity between age classes. This finding suggests that, when this intermediate-aged C with higher temperature sensitivity is categorized as younger C, its response cannot be resolved from the temperature response of the majority of respiratory C substrate, resulting in equal apparent temperature sensitivity of the two age classes. The finding of equal sensitivity with longer label times suggests that the contribution of disproportionately temperature-sensitive C to the total flux is relatively minor.
The combination of the FACE isotope label and the bomb 14C signal allowed us to identify the effects of warming on three different timescales of C cycling and avoid some confounding factors present in previous studies. The age constraint of the FACE label improved age estimates over those estimates from bomb-derived 14C alone. Also, measurement of 14C has advantages over the 13C label used in most studies. Although Δ14C data reported here are corrected for mass-dependent fractionation, the 13C isotope may be affected by temperature-dependent kinetic fractionation by microbial respiration (37) or preferential use of 13C-depleted substrate (38). Other confounding factors include differential substrate depletion between temperature treatments (39), differences in substrate conditions because of seasonal effects (40), and differences in C cycling under C3 and C4 vegetation. In FACE experiments, manipulation of CO2 concentrations may have altered decomposition rates, resulting in differences between CO2 treatments at these sites (41, 42); however, this manipulation is unlikely to affect our results. Although the Δ14C mixing model assumes similar decomposition rates of pre-FACE C between CO2 treatments, model results are not sensitive to this term. In addition, the δ13C mixing model gave similar estimates of the fraction of pre-FACE C and does not require the assumption that C cycling rates are similar between the two treatments.
What Potential Mechanisms Underlie the Observed Temperature Response?
The similarity in temperature sensitivity of the two broad age classes suggests a common suite of mechanisms of soil C response to warming. This finding is consistent with the conceptual framework emerging from recent synthesis efforts (6, 9), which emphasizes different temperature controls over microbial respiration and supply of soil C to microbial respiration. In the short term, the temperature sensitivity of microbial respiration is the primary control of the temperature dependence of soil respiration. The constant proportion of decades-old and younger C respired across temperatures is likely determined by their fractional contributions to microbially assimilable C. Hence, the warming response could simply reflect faster respiration of assimilable C by microbes. Alternatively, it could mean that the availability of younger and older C sources was controlled by the same process or that their respective controls were similarly temperature-sensitive.
Although the temperature sensitivity of microbial respiration has been well-established, much less is known about temperature dependence of substrate supply to microbial respiration, which controls C availability in the long term. Multiple lines of evidence suggest that warming increased the supply of C of both age classes to microbes, including consistently higher flux rates over the whole incubation period, larger pools of actively cycling C, and increase in the mean age of respiration substrate at higher temperature. Previous incubation and litter decomposition studies also suggest that warming increases the fraction of soil C that is assimilable by microbes (43, 19, respectively).
Various potentially temperature-sensitive processes could influence substrate supply or cause an apparent change in supply in an incubation, such as shift in microbial community composition (44), change in microbial efficiency (45), increased turnover of microbial biomass (46), change in biochemical composition of soil C substrates respired (47), increased desorption of mineral-adsorbed organic C (6), and increased diffusion. In our study, increased substrate availability coincided with an increase in respiration of soil organic C with a mean age of 7–13 y, suggesting that a greater proportion of C of this age became available with warming. Some of these processes can be ruled out, because they would increase assimilation of substrates without a change in substrate age, such as increased diffusion, change in microbial efficiency, or increased turnover of microbial biomass.
Other mechanisms may be consistent with a change in the age of respired CO2. A warming-induced shift in microbial community or enzyme production could change the use C of different ages (48); however, it is unlikely that such a shift would happen within the relatively short time period over which we collected CO2 from these soils (7). Chemical kinetic theory, also known as the carbon quality temperature hypothesis (8, 49), provides a potential explanation for an increased contribution of slower turnover compounds because of higher temperature sensitivity of compounds with greater total bond strength, which is often associated with compounds that are more structurally complex (i.e., more chemical bonds) (47). If substrates with greater complexity are also retained in soils longer (i.e., become older) and warming disproportionately promotes their decomposition, we would expect to see an increase in the mean age of respired CO2 with increased temperature. However, the radiocarbon age of soil C is not necessarily indicative of biochemical stability (48), and we do not know the extent to which biochemical stability or activation energy of compounds per se controls C decomposability in mineral soils (50).
It is difficult to tease apart mechanisms in incubations such as this incubation or field respiration studies; heterotrophic respiration integrates over multiple C sources and reflects overlapping mechanisms of soil C stabilization. In addition, extended incubation periods have been criticized for their departure from in situ conditions (51, 52). Incubation isolates soils from sources of C input and results in rapid onset of substrate limitation to decomposers, which can modify the apparent response of respiration to warming (53). Substrate depletion was eventually observed in the incubation of Duke FACE soils at all temperatures, suggesting that the increase in amount of assimilable C under warming may not be sustained over time. It remains an open question whether increased substrate availability observed with warming in incubations would be sustained in a field setting or is the product of a finite, exhaustible pool as some studies suggest (54).
Modeling the Temperature Response of Soil C Decomposition.
In many soil C models, temperature sensitivity is expressed exclusively in the rate constants of linear, donor-controlled soil C pools (55). When we modeled our data with this model structure, increased respiration was best simulated with an increase in the size of the active pool rather than a change in the rate constants. Indeed, including the effect of warming on substrate availability in current model structures would require a highly temperature-sensitive pool to rapidly transfer previously slow-cycling C to the fast pool. If this new warming-induced supply is rapidly depleted, then the flux from this highly temperature-sensitive pool may be transitory—a case we cannot determine with an incubation, because substrate limitation is observed at all temperatures. In that case, however, inferring the changes in respiration rates using only a temperature-sensitive rate constant may overstate the warming effect on the soil C stock.
If chemical bond strength (activation energy) were the fundamental limit to decomposition rates, then the Arrhenius equation of chemical kinetic theory (8) can be used to quantify increases in respiration substrate availability with warming. However, recent attempts to model this effect either explicitly (16) or implicitly (49) assumed that respiratory substrate stays constant under warming by parameterizing temperature sensitivity in the rate constants that directly control respiration rates. This approach predicts a more rapid loss of active pool C in warmed soils than soils at the MAT control temperature, which is counter to our findings. In contrast, our data suggest that the change in respiration rate with warming is more strongly controlled by substrate availability than temperature. As a result, caution must be taken in deriving parameter estimates in models from measurements of warming on respiration in incubations or field studies.
Earth system models are designed to predict future climate, but they still lack a predictive understanding of how much soil C is vulnerable on timescales of the next century. In this timeframe, the most important C response will come from C cycling on decadal timescales. Older pools (centuries to millennia) are also an important component of global soil C stocks (15), but their very long turnover times (and correspondingly slow decomposition rates) indicate that they will not have much effect on feedbacks in the 21st century and cannot be measured in incubation experiments in any case (56).
Our results indicate that large amounts of C (1,750–4,700 kg m−2 in the top 15 cm of mineral soils at these two temperate forest sites) were vulnerable to increased decomposition losses with warming. The fact that we saw similar results at the two sites, despite differences in soil C stabilization therein, suggests that the pattern we observed may apply more broadly. The importance of decadal-aged C to the large amount of C in forest soils globally suggests that soil C could become a source of atmospheric CO2 under global warming.
A continuing challenge for models is to understand the unresolved mechanisms where C of different ages and stability can have the same temperature sensitivity. Although more research is needed to better incorporate soil C decomposition processes into models, our results suggest that we need models and experiments that explicitly separate the temperature sensitivity of microbial metabolism and the temperature sensitivity of substrate supply rather than parameterizing the temperature sensitivity of any particular compound or fraction.
Methods
We sampled the top 0–15 cm mineral soil from the Duke and Aspen FACE sites, which have been documented extensively elsewhere (57). These FACE experiments have a similar design, consisting of replicate 30-m diameter forested plots, one-half of which receive CO2 fumigation (elevated CO2 plots; +200 ppm above ambient) and one-half of which served as CO2 fumigation control (ambient CO2 plots). The evergreen plantation at Duke already had a closed canopy when FACE CO2 enrichment began, whereas deciduous aspens were planted just as CO2 enrichment began at Aspen FACE (Table 1).
We treated each plot as the level of replication for our laboratory incubation experiment (Duke n = 4, Aspen n = 3). Three soil cores per plot were sampled from Duke FACE in July of 2008, with each core assigned one of three temperature treatments (15 °C, 25 °C, or 35 °C) and incubated separately. Five cores per plot were sampled from Aspen FACE in July of 2009 and subsequently composited in the laboratory, with a subsample (∼140 g) from each plot assigned to each of three temperatures (5 °C, 15 °C, and 25 °C). Before incubation, visible roots and rocks were removed (both sites) and sieved to 4 mm (Aspen only).
Field-moist samples were placed in glass jars with airtight lids fitted with a sampling port. The jar headspace was purged with CO2 free air and then incubated continuously at one of three temperatures (site MAT, +10 °C, or +20 °C). Rates of CO2 increase were measured on 2-mL aliquots headspace air by a LiCor 6252 infrared gas analyzer. Fluxes reported here are calculated as the total amount of CO2 evolved by the soil on the time of isotope sampling. δ13C–CO2 was measured by isotope ratio MS (Thermo Finnigan Gas Bench coupled to continuous flow Delta Plus) on a subsample of headspace air injected into He-filled vials to a target concentration of 3,500 ppm CO2. When CO2 concentrations were high enough for a ≥0.5 mg C subsample (>3,000 ppm), we collected headspace air by attaching 0.5-L stainless steel evacuated canisters to the lid sampling port. CO2 was extracted from the canisters on a vacuum line, graphitized for 14C, and measured at University of California at Irvine’s W. M. Keck Carbon Cycle Accelerator Mass Spectrometer (58).
To compare the same amount of respired C between temperature treatments, we chose a target amount of C equal to the total C respired at 35 °C for Duke (2.68% of initial C respired) and at 15 °C for Aspen (0.11% of initial C respired) at the first sampling period. We summed fluxes from the lower temperatures until reaching the target C loss (Fig. S2), solving for the amount of time to respire the same amount of C at each temperature (16). C-isotope values for equivalent amounts of C were computed by linearly interpolating between isotopes measurements over this time period.
We estimated the age of respired C for ambient CO2 soils by using a time-dependent, steady-state model of soil C that assumes that inputs to the soil have the same 14C signature as the atmosphere that year (15). The mean age of respired C is equivalent to the average time spent in the soil plus the length of time that C spent in plant tissue before it was deposited to the soil. In 2008 and 2009 (the years of sampling), the difference between pools with turnover times of 1 and 3 y is detectable—we would expect a 10‰ difference between respiration from these pools, which is greater than the combined errors from 14C analysis and spatial variability as determined from replicate samples of incubated ambient CO2 soils (Table 3). To partition the contribution of FACE C vs. pre-FACE C to respired CO2 flux, we used an isotopic mixing model independently at each temperature. We divided fluxes into pre-FACE (>10 y) flux and FACE-derived (<10 y) flux by assuming that flux of pre-FACE C and its 14C content was the same for both CO2 treatments and applying isotopic end members, Δ14C of the FACE atmosphere for FACE-derived C in elevated CO2 soils, and Δ14C of background atmosphere for recent C in ambient CO2 soils. We applied a similar mixing model to 13C of CO2 fluxes from elevated CO2 soils alone to confirm these results. More details and equations can be found in SI Methods.
The temperature sensitivities of these two pools were quantified by assuming an exponential relationship between flux rates (R) and temperature (T) of the form R = AebT, where A and b are parameters found by fitting the model to partitioned fluxes by incubation temperature for each site. The temperature dependence of R can be written 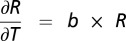 , where b is the temperature sensitivity coefficient. Similarly, the temperature sensitivity parameter Q10 is the factor by which respiration rate increases with 10 °C of warming (e.g., R = Ae10b).
, where b is the temperature sensitivity coefficient. Similarly, the temperature sensitivity parameter Q10 is the factor by which respiration rate increases with 10 °C of warming (e.g., R = Ae10b).
Another way to define C pools with different turnover times and determine their temperature sensitivity is by modeling the change in flux rate over time at different temperatures (27). We fit a two-pool, first-order decay model of the form 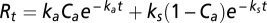 to flux rates over time (17) for the Duke soils (in Aspen soils, there was no statistically detectable change in fluxes). We found the best-fit parameters ka (decay rate of active pool), ks (decay rate of slow pool), and Ca (proportional size of active pool) separately for each temperature treatment (59).
to flux rates over time (17) for the Duke soils (in Aspen soils, there was no statistically detectable change in fluxes). We found the best-fit parameters ka (decay rate of active pool), ks (decay rate of slow pool), and Ca (proportional size of active pool) separately for each temperature treatment (59).
We report error as the SEM of experimental replicates or by propagating the error in isotope calculations (60). Reported P values are from comparisons of treatment means in ANOVA done using PROC GLM (unless t test was indicated), and exponential fits to data are done by PROC NLIN in SAS 9.2.
Supplementary Material
Acknowledgments
We thank Xiaomei Xu and the staff of the W. M. Keck Carbon Cycle Accelerator Mass Spectrometer, University of California at Irvine for radiocarbon analyses. We thank Tim Filley and Sara Top of Purdue University for collection of soils from Aspen FACE and John Lichter at Bowdoin College for providing density and size fractions of soil from Duke FACE. We also thank to the principle investigators and staff of the Duke and Aspen FACE experiments for site access, Carlos Sierra for discussion, and Claudia Czimczik and two anonymous reviewers for insightful comments on the manuscript. The Duke FACE experiment was funded by US Department of Energy’s Office of Science (DOE-BER) Grant DE-FG02-95ER62083. The Aspen FACE experiment was funded by DOE-BER, with additional support from the US Forest Service (USFS) Global Change Program, Michigan Technological University, the Canadian Forest Service, and the USFS Northern Research Station. F.M.H. was supported by a National Science Foundation Graduate Research Fellowship, an Achievement Rewards for College Scientists Foundation Scholarship, and a Ralph and Carol Cicerone Graduate Fellowship. M.S.T. was supported by DOE-BER Contract DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 10152 (volume 109, number 26).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120603109/-/DCSupplemental.
References
- 1.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009 23, GB2023. [Google Scholar]
- 2.Denman KL, et al. In: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 3.Trumbore SE, Czimczik CI. Geology - An uncertain future for soil carbon. Science. 2008;321:1455–1456. doi: 10.1126/science.1160232. [DOI] [PubMed] [Google Scholar]
- 4.Hahn V, Buchmann N. A new model for soil organic carbon turnover using bomb carbon. Global Biogeochem Cycles. 2004 18, GB1019. [Google Scholar]
- 5.von Lützow M, Kögel-Knabner I. Temperature sensitivity of soil organic matter decomposition—what do we know? Biol Fertil Soils. 2009;46:1–15. [Google Scholar]
- 6.Conant RT, et al. Temperature and soil carbon decomposition – synthesis of current knowledge and a way forward. Glob Change Biol. 2011;17:3392–3404. [Google Scholar]
- 7.Bradford MA, Watts BW, Davies CA. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob Change Biol. 2010;16:1576–1588. [Google Scholar]
- 8.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MWI, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 10.Pataki DE, et al. Tracing changes in ecosystem function under elevated carbon dioxide conditions. Bioscience. 2003;53:805–818. [Google Scholar]
- 11.Schlesinger WH, Lichter J. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature. 2001;411:466–469. doi: 10.1038/35078060. [DOI] [PubMed] [Google Scholar]
- 12.Pregitzer K, Loya W, Kubiske M, Zak D. Soil respiration in northern forests exposed to elevated atmospheric carbon dioxide and ozone. Oecologia. 2006;148:503–516. doi: 10.1007/s00442-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 13.Taneva L, Gonzalez-Meler MA. Decomposition kinetics of soil carbon of different age from a forest exposed to 8 years of elevated atmospheric CO2 concentration. Soil Biol Biochem. 2008;40:2670–2677. [Google Scholar]
- 14.Levin I, et al. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO(2) Tellus B Chem Phys Meteorol. 2010;62:26–46. [Google Scholar]
- 15.Trumbore S. Age of soil organic matter and soil respiration: Radiocarbon constraints on belowground C dynamics. Ecol Appl. 2000;10:399–411. [Google Scholar]
- 16.Conant RT, et al. Sensitivity of organic matter decomposition to warming varies with its quality. Glob Change Biol. 2008;14:868–877. [Google Scholar]
- 17.Paul EA, Morris SJ, Böhm S. In: Assessment Methods for Soil Carbon. Lal R, Kimble JM, Follett RF, Stewart BA, editors. Boca Raton, FL: Lewis; 2001. pp. 193–206. [Google Scholar]
- 18.Dalias P, Anderson JM, Bottner P, Couteaux MM. Long-term effects of temperature on carbon mineralisation processes. Soil Biol Biochem. 2001;33:1049–1057. [Google Scholar]
- 19.Couteaux MM, Sarmiento L, Bottner P, Acevedo D, Thiery JM. Decomposition of standard plant material along an altitudinal transect (65-3968 m) in the tropical Andes. Soil Biol Biochem. 2002;34:69–78. [Google Scholar]
- 20.Braakhekke WG, de Bruijn AMG. Modelling decomposition of standard plant material along an altitudinal gradient: A re-analysis of data of Couteaux et al. (2002) Soil Biol Biochem. 2002;39:99–105. [Google Scholar]
- 21.Trumbore SE, Bonani G, Wölfli W. In: Soils and the Greenhouse Effect. Bouwman AF, editor. New York: Wiley; 1989. pp. 407–414. [Google Scholar]
- 22.Townsend AR, Vitousek PM, Trumbore SE. Soil organic matter dynamics along gradients in temperature and land use on the island of Hawaii. Ecology. 1995;76:721–733. [Google Scholar]
- 23.Lukac M, et al. Forest soil carbon cycle under elevated CO2—a case of increased throughput? Forestry. 2009;82:75–86. [Google Scholar]
- 24.Hofmockel KS, Zak DR, Moran KK, Jastrow JD. Changes in forest soil organic matter pools after a decade of elevated CO2 and O3. Soil Biol Biochem. 2011;43:1518–1527. [Google Scholar]
- 25.Lichter J, et al. Soil carbon sequestration and turnover in a pine forest after six years of atmospheric CO2 enrichment. Ecology. 2005;86:1835–1847. [Google Scholar]
- 26.Conen F, Leifeld J, Seth B, Alewell C. Warming mineralizes young and old soil carbon equally. Biogeosciences. 2006;3:515–519. [Google Scholar]
- 27.Townsend AR, Vitousek PM, Desmarais DJ, Tharpe A. Soil carbon pool structure and temperature sensitivity inferred using CO2 and 13CO2 incubation fluxes from five Hawaiian soils. Biogeochemistry. 1997;38:1–17. [Google Scholar]
- 28.Waldrop MP, Firestone MK. Altered utilization patterns of young and old soil C by microorganisms caused by temperature shifts and N additions. Biogeochemistry. 2004;67:235–348. [Google Scholar]
- 29.Kramer C, et al. Recent (< 4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem. 2010;42:1028–1037. [Google Scholar]
- 30.Matamala R, Gonzàlez-Meler MA, Jastrow JD, Norby RJ, Schlesinger WH. Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science. 2003;302:1385–1387. doi: 10.1126/science.1089543. [DOI] [PubMed] [Google Scholar]
- 31.Phillips RP, Finzi AC, Bernhardt ES. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett. 2011;14:187–194. doi: 10.1111/j.1461-0248.2010.01570.x. [DOI] [PubMed] [Google Scholar]
- 32.Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408:184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 33.Friedlingstein P, et al. Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. J Clim. 2006;19:3337–3353. [Google Scholar]
- 34.Karhu K, et al. Temperature sensitivity of soil carbon fractions in boreal forest soil. Ecology. 2010;91:370–376. doi: 10.1890/09-0478.1. [DOI] [PubMed] [Google Scholar]
- 35.Bol R, Bolger T, Cully R, Little D. Recalcitrant soil organic materials mineralize more efficiently at higher temperatures. J Plant Nutr. 2003;166:300–307. [Google Scholar]
- 36.Vanhala P, et al. Old soil carbon is more temperature sensitive than the youn in an agricultural field. Soil Biol Biochem. 2007;39:2867–2970. [Google Scholar]
- 37.Czimczik CI, Trumbore SE. Short-term controls on the age of microbial carbon sources in boreal forest soils. J Geophys Res. 2007;112:G03001. [Google Scholar]
- 38.Andrews JA, Matamala R, Westover KM, Schlesinger WH. Temperature effects on the diversity of soil heterotrophs and the δ13C of soil-respired CO2. Soil Biol Biochem. 2000;32:699–706. [Google Scholar]
- 39.Conant RT, Haddix M, Paustian K. Partitioning soil carbon responses to warming: Model-derived guidance for data interpretation. Soil Biol Biochem. 2010;42:2034–2036. [Google Scholar]
- 40.Conen F, et al. Temperature sensitivity of young and old soil carbon—Same soil, slight differences in 13C natural abundance method, inconsistent results. Soil Biol Biochem. 2008;40:2703–2705. [Google Scholar]
- 41.Talhelm AF, Pregitzer KS, Zak DR. Species-specific responses to atmospheric carbon dioxide and tropospheric ozone mediate changes in soil carbon. Ecol Lett. 2009;12:1219–1228. doi: 10.1111/j.1461-0248.2009.01380.x. [DOI] [PubMed] [Google Scholar]
- 42.Drake JE, et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett. 2011;14:349–357. doi: 10.1111/j.1461-0248.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald NW, Zak DR, Pregitzer KS. Temperature effects on kinetics of microbial respiration and net nitrogen and sulfur mineralization. Soil Sci Soc Am J. 1995;59:233–240. [Google Scholar]
- 44.Zogg GP, et al. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J. 1997;61:475–481. [Google Scholar]
- 45.Steinweg JM, Plante AF, Conant RT, Paul EA, Tanaka DL. Patterns of substrate utilization during long-term incubations at different temperatures. Soil Biol Biochem. 2008;40:2722–2728. [Google Scholar]
- 46.Blagodatsky SA, Heinemeyer O, Richter J. Estimating the active and total soil microbial biomass by kinetic respiration analysis. Biol Fertil Soils. 2000;32:73–81. [Google Scholar]
- 47.Fierer N, Craine JM, McLauchlan K, Schimel JP. Litter quality and the temperature sensitivity of decomposition. Ecology. 2005;86:320–326. [Google Scholar]
- 48.Rethemeyer J. Complexity of soil organic matter: AMS 14C analysis of soil lipid fractions and individual compounds. Radiocarbon. 2004;46:465–473. [Google Scholar]
- 49.Craine JM, Fierer N, McLauchlan KK. Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat Geosci. 2010;3:854–857. [Google Scholar]
- 50.Marschner B, et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J Plant Nutr Soil Sci. 2008;171:91–110. [Google Scholar]
- 51.Reichstein M, et al. Temperature sensitivity of decomposition in relation to soil organic matter pools: Critique and outlook. Biogeosciences. 2005;2:317–321. [Google Scholar]
- 52.Janssens IA, Vicca S. Soil carbon breakdown. Nat Geosci. 2010;3:823–824. [Google Scholar]
- 53.Gershenson A, Bader NE, Cheng W. Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Glob Change Biol. 2009;15:176–183. [Google Scholar]
- 54.Melillo JM, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 55.Todd-Brown KEO, Hopkins FM, Kivlin SN, Talbot JM, Allison SD. A framework for representing microbial decomposition in coupled climate models. Biogeochemistry. 2012;109:19–33. [Google Scholar]
- 56.Sierra CA. Temperature sensitivity of organic matter decomposition in the Arrhenius equation: Some theoretical considerations. Biogeochemistry. 2012;108:1–15. [Google Scholar]
- 57.Norby RJ, et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA. 2005;102:18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, et al. Modifying a sealed tube zinc reduction method for preparation of AMS graphite targets: Reducing background and attaining high precision. Nucl Instrum Methods Phys Res B. 2007;259:320–329. [Google Scholar]
- 59.Reichstein M, Beer C. Soil respiration across scales: The importance of a model-data integration framework for data interpretation. J Plant Nutr Soil Sci. 2008;171:344–354. [Google Scholar]
- 60.Phillips DL, Gregg JW. Uncertainty in source partitioning using stable isotopes. Oecologia. 2001;127:171–179. doi: 10.1007/s004420000578. [DOI] [PubMed] [Google Scholar]