Abstract
Fast-growing forests such as tropical secondary forests can accumulate large amounts of carbon (C), and thereby play an important role in the atmospheric CO2 balance. Because nitrogen (N) cycling is inextricably linked with C cycling, the question becomes: Where does the N come from to match high rates of C accumulation? In unique experimental 16-y-old plantations established in abandoned pasture in lowland Costa Rica, we used a mass-balance approach to quantify N accumulation in vegetation, identify sources of N, and evaluate differences among tree species in N cycling. The replicated design contained four broad-leaved evergreen tree species growing under similar environmental conditions. Nitrogen uptake was rapid, reaching 409 (±30) kg⋅ha−1⋅y−1, double the rate reported from a Puerto Rican forest and greater than four times that observed at Hubbard Brook Forest (New Hampshire, USA). Nitrogen amassed in vegetation was 874 (±176) kg⋅ha−1, whereas net losses of soil N (0–100 cm) varied from 217 (±146) to 3,354 (±915) kg⋅ha−1 (P = 0.018) over 16 y. Soil C:N, δ13C values, and N budgets indicated that soil was the main source of biomass N. In Vochysia guatemalensis, however, N fixation contributed >60 kg⋅ha−1⋅y−1. All species apparently promoted soil N turnover, such that the soil N mean residence time was 32–54 y, an order of magnitude lower than the global mean. High rates of N uptake were associated with substantial N losses in three of the species, in which an average of 1.6 g N was lost for every gram of N accumulated in biomass.
Keywords: carbon sequestration, forest regrowth, nitrogen cycle, soil organic nitrogen, species effects
Rates of C accumulation can be rapid in secondary forests, to the extent that forest regrowth following harvest, abandonment of agricultural lands, and deforestation serves as an important sink for atmospheric CO2 (1). The magnitude is on the order of 2.8 Pg C⋅y−1 (2), and thereby mitigates climate change. Moist tropical forests are especially productive: Tropical tree plantations capture 7–10 Mg C⋅ha−1⋅y−1 (3, 4). Abandoned agricultural and pasture land represents roughly half the landscape in the tropics (5); given its high C sequestration potential, if even half of these degraded ecosystems were reforested, it would substantially reduce rates at which CO2 accumulates in the atmosphere. Tropical forests accounted for 55% of the 861 Pg C of the world's forest C stocks in 2007, a decrease from 58% in 1990 (6). From 1990 to 2007, deforestation of 284 Mha of intact tropical forests resulted in a loss of 58 Pg C while tropical forest regrowth on 110 Mha accrued 27 Pg C (6).
Rapid C cycling is integrally linked to N cycling, because N-rich enzymes underlie photosynthesis and high N availability promotes plant productivity. The question becomes: Where does the N come from to match the observed high rates of C accumulation in biomass? Further, do tree species differ in this coupling of C and N cycling? From a conceptual framework, species-specific differences in the capture and utilization of C and N could drive differences in ecosystem-level N cycling because these plant attributes influence the quantity and chemistry of detrital inputs to the soil, which in turn influence decomposers and soil N dynamics.
Our objectives were to quantify whether N accumulation in biomass did in fact keep pace with C accrual, to examine the sources of this N, and to explore the extent to which tree species differed in their effects on ecosystem N budgets. We made use of an exceptional experimental setting at La Selva Biological Station in lowland Costa Rica. In truly replicated forest plantations, state factors sensu Amundson and Jenny (7) were all similar except for the planted species. That is, all plots had the same parent material, soil type, climate, land-use history, and topography. In this site, 16-y-old monodominant plantations of four tree species were situated on an Oxisol, a soil order that accounts for 23% of all tropical soils (8). The site was deforested in the 1950s, converted to pasture, and grazed until 1987 before the start of the experiment in 1988. Adjacent unplanted abandoned pasture and mature forest plots provided reference sites of previous vegetation. The four tree species planted, all of which are native, included: Hieronyma alchorneoides Allemao; Pentaclethra macroloba (Willd.) Kunth., the only nodulated leguminous species and the dominant tree at La Selva; Virola koschnyi Warb.; and Vochysia guatemalensis Donn. Sm. These species are hereafter referred to by genus. Previous studies demonstrated that C-cycling rates in these plantations were comparable to the nearby mature forest, with aboveground biomass and net primary productivity (NPP) in the plantations averaging 69 Mg C⋅ha−1 and 11.7 Mg C⋅ha−1⋅y−1, respectively. Ecosystem-level C sequestration averaged 5.2 Mg C⋅ha−1⋅y−1 over 17 y (4). High rates of tree growth in the plantations were likely promoted by thinning (9), which effectively minimized competition for light in the fast-growing species.
To address our objectives, we used a mass-balance approach to measure rates of plant N uptake and partitioning among plant components, accumulation of N in biomass, and changes in soil N stocks. Nitrogen uptake is herein defined traditionally, as the amount of nitrogen required to support the production of accumulating biomass (ΔBiomass-N) and short-lived tissues that turn over rapidly, such as fine roots and leaves. This latter component is typically determined by measurements of detritus production (Detrital-N flux). Over any given time period:
where ΔBiomass-N refers to the net accumulation of N in live plant biomass over the time period of interest, and Detrital-N flux refers to the N flux in detritus production (above- and belowground fine and coarse litter production). The plant N that is consumed by herbivores also belongs on the right side of Eq. 1, but is difficult to quantify and is not included. Had we done so, calculated N uptake rates would be greater. We present here mean values collected over a 3-y study, when the trees averaged 16 y old. These N measurements were conducted at the same time as studies of NPP and C accumulation in biomass (4). This allowed for calculation of N use efficiency (NUE; g dry weight produced per g N taken up), a parameter that incorporates N productivity and residence time in a biologically meaningful way (10).
To interpret changes in soil N by independent means, we measured stable C isotope (δ13C) concentrations in soil to evaluate whether older soil organic matter (SOM) had decomposed in the plantations. Carbon isotopic signatures of plants differ as a result of their C uptake, such that the δ13C is less negative in C4 plants (in which CO2 is first fixed as a four-C compound before entering the Calvin cycle) than in C3 plants (in which CO2 is first fixed as a three-C compound). The δ13C (per mil, ‰) equals [(Rsample/Rstd) − 1] × 103, where Rsample = 13C:12C ratio for the sample and Rstd = 13C:12C of the working standard, Pee Dee Belemnite. In our study, the plantation tree species are all C3, but were planted on former pasture that was dominated by C4 grasses, whereas the current abandoned pasture, also situated on former C4 pasture, contains a C4/C3 mixture of species. The mature forest is C3. We also measured δ15N of overstory leaves to evaluate N fixation. The N contributed by biological fixation has a 15N:14N similar to that of air, that is, its δ15N is ∼0‰, whereas N contributed from the soil typically has a higher δ15N (11). The δ15N (per mil, ‰, vs. air) equals [((15N/14N sample)/(15N/14N air) − 1) × 103]. The differences in isotopic fractionation that occur during plant uptake and assimilation processes are reflected in plant tissue δ15N, and thereby provide insight into the sources of N accumulated in biomass, with near-zero values being associated with an atmospheric source, namely N fixation.
Results
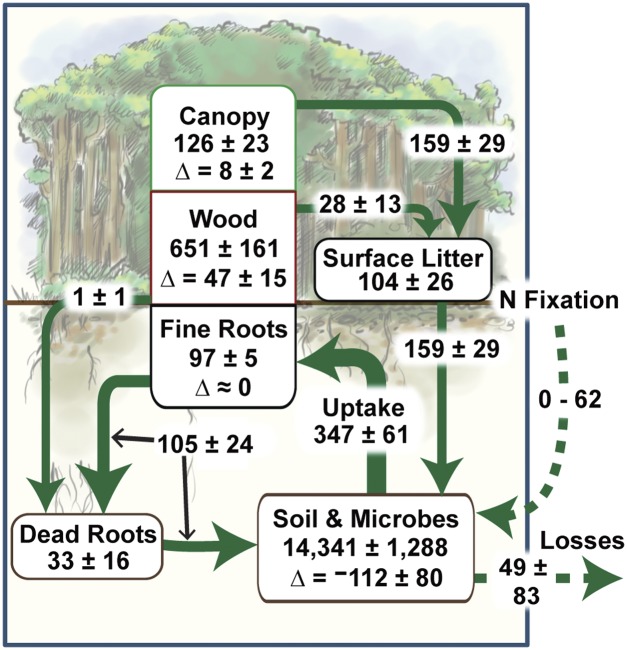
In these plantations, N accrual in biomass was high: 874 (±176) kg⋅ha−1 (mean ± SEM, across species) after 16 y (Fig. 1, Table 1, and Table S1). Differences among species in total biomass N were not significant (P = 0.125). Aboveground detritus, which contained 104 (±26) kg N⋅ha−1, accounted for 15 (±4) % of the total aboveground N (Fig. 1 and Table S1). The majority of N in the system was in the soil, which contained 14,341 (± 1,288) kg⋅ha−1 (0–100 cm depth) (Fig. 1, Table 1, and Table S2).
Fig. 1.
Nitrogen cycling in experimental plantations in lowland Costa Rica. Units for fluxes (over arrows) are kg N⋅ha−1⋅y−1; stocks (in boxes) are in kg N⋅ha−1. Means (±SEM) are across four tree species. Total N uptake is partitioned among plant components as in Table S3. Sources of plant-available N include: (i) canopy, fine-root (surface only, 0–15 cm), and woody detritus; (ii) soil and microbes; and (iii) N fixation. Annual net rates of accumulation in biomass and soil are referred to as “Δ”; “wood” biomass includes all roots >2 mm in diameter and stumps; “surface litter” includes coarse woody debris. Data are from Table 1 and Tables S1–S3.
Table 1.
Nitrogen stocks and dynamics in plantations in lowland Costa Rica after 16 y of forest development
| Species |
|||||||
| Stock or flux | Units | Hieronyma | Pentaclethra | Virola | Vochysia | Mean | P > F |
| N in plant biomass | kg⋅ha−1 | 674 ± 53 | 967 ± 152 | 790 ± 127 | 1,065 ± 81 | 874 ± 176 | 0.125 |
| Total N in detritus | kg⋅ha−1 | 142 ± 4 | 147 ± 15 | 114 ± 8 | 144 ± 14 | 137 ± 15 | 0.264 |
| Total N in soil | kg⋅ha−1 | 14,498 ± 481AB | 12,777 ± 915B | 14,175 ± 498B | 15,914 ± 146A | 14,341 ± 1,288 | 0.034 |
| Detrital N production | kg⋅ha−1⋅y−1 | ||||||
| Canopy | 155 ± 10AB | 197 ± 18A | 126 ± 3B | 157 ± 11AB | 159 ± 29 | 0.010 | |
| Fine roots | 127 ± 9A | 94 ± 10AB | 75 ± 9B | 122 ± 22AB | 105 ± 24 | 0.038 | |
| Woody tissues | 25 ± 3BC | 44 ± 9A | 13 ± 2C | 34 ± 3AB | 29 ± 13 | 0.004 | |
| ΔBiomass-N | kg⋅ha−1⋅y−1 | 35 ± 4B | 75 ± 11A | 51 ± 9AB | 57 ± 1AB | 54 ± 17 | 0.055 |
| Total plant N uptake | kg⋅ha−1⋅y−1 | 341 ± 11AB | 409 ± 35A | 265 ± 18B | 371 ± 21A | 347 ± 61 | 0.011 |
| N use efficiency | g DW⋅g N−1 | 98 ± 6A | 74 ± 4B | 88 ± 1AB | 94 ± 2A | 88 ± 11 | 0.008 |
| ΔSoil N stocks* | kg⋅ha−1⋅16 y | −1,633 ± 481AB | −3,354 ± 915B | −1,956 ± 498AB | −217 ± 146A | −1,790 ± 1,288 | 0.035 |
| ΔN in plantations† | kg⋅ha−1⋅16 y | −817 ± 524AB | −2,240 ± 1,032B | −1,053 ± 398AB | +992 ± 230A | −779 ± 1,335 | 0.039 |
| Soil N MRT‡ | y | 43 ± 3AB | 32 ± 5B | 54 ± 5A | 43 ± 2AB | 43 ± 9 | 0.027 |
Means (±SE) within a row followed by the same superscript letter do not differ significantly among species (Tukey’s HSD, P = 0.05). Statistical test results are reported in Table S4. DW, dry weight.
*Mineral soil to 1-m depth.
†All N in vegetation, detritus, and soil to 1-m depth.
‡Mean residence time of N in soil, calculated as the quotient of soil N stocks (0–1 m) and total plant N uptake.
Plant N uptake averaged 347 (±61) kg⋅ha−1⋅y−1 across species, with 76% of uptake invested in fast-turnover plant components, namely the canopy and fine roots, 159 (±29) and 105 (±24) kg⋅ha−1⋅y−1, respectively (Fig. 1). A further 54 (±17) kg⋅ha−1⋅y−1 accumulated in biomass (ΔBiomass-N; Eq. 1) (Fig. 1 and Table S3). Calculation of the long-term rate of N accrual in biomass, made by dividing total biomass N by age (16 y), supports the magnitude of the annual net accumulation of N by vegetation: N accumulated in biomass at an average annual rate of 55 (±11) kg⋅ha−1 over the lifespan of the plantations (Table S3). The magnitude of plant N uptake and its high allocation to relatively short-lived, nonwoody, nitrogen-rich, resource-capturing tissues such as leaves and fine roots suggest rapid recycling of detrital nitrogen. However, even if these components were recycled efficiently, it does not explain the original source of that plant-available N.
Soil N loss averaged 1,790 (±1,288) kg⋅ha−1 or 112 (±80) kg⋅ha−1⋅y−1 (Fig. 1 and Table 1). Thus, mobilization of N from soil, that is, mineralization of SOM, could have released enough N to have supplied all N that accrued in biomass. Two other independent datasets, soil δ13C and soil C:N, also indicated that substantial mineralization of SOM occurred under all four tree species. Soil δ13C values in all of the plantations were intermediate relative to the abandoned pasture and mature forest (Fig. 2), indicating that older, more C4-rich SOM produced during the pasture phase was mineralized and replaced with C3 organic matter produced by the plantations. Although one consequence of mineralization of SOM was N loss from the soil, there was not a concomitant net loss of soil C. Indeed, soil C stocks in the surface soil increased significantly during the first 16 y (11). Consistent with δ13C data, soil C:N was higher in the plantations than in the mature forest and abandoned pasture (Table S2), signaling a less-decomposed state of more recent OM inputs from the plantations.
Fig. 2.
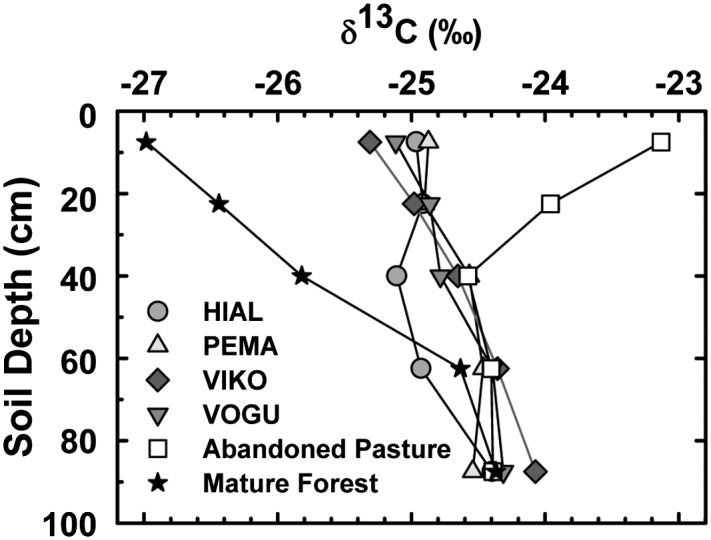
Soil δ13C by depth in experimental plantations and reference sites in lowland Costa Rica. Plantations are identified by four-letter acronyms of the dominant tree species: HIAL, H. alchorneoides; PEMA, P. macroloba; VIKO, V. koschnyi; VOGU, V. guatemalensis.
Whereas site-level N budgets indicated that SOM could have been the sole source of plant N (Fig. 1), species-level N budgets tell a different story. That is, the quantity of soil N loss over 16 y was greater than the quantity accrued in vegetation in only three of the species (Table 1). In Vochysia, however, soil N loss of 217 (±146) was substantially less than the 1,065 (±81) kg⋅ha−1 N that accrued in biomass. Vochysia (Family Vochysiaceae) does not have root nodules. Nevertheless, N fixation was the most likely other source of N taken up in this species. By mass balance, the rate of N supply from N fixation was at least 62 kg⋅ha−1⋅y−1. Stable isotopic trends, although not significant due to high variability, were consistent with this conclusion of substantial N fixation in Vochysia plantations. Foliar δ15N was relatively low, 1.51 (±0.23) ‰, and on par with values of 1.37 (±0.37) ‰ in Pentaclethra, a legume with nodulated roots. The other two species had higher values: 2.21 (±0.59) and 2.01 (±0.23) ‰ in Hieronyma and Virola, respectively (Tables S4 and S5). Thus, for Vochysia, we conclude that mobilization of N from soil and fixation both contributed to biomass N.
These results also highlight surprising differences among species in their mobilization of soil N. Despite significantly higher N uptake (P = 0.011) in Pentaclethra, soil N loss was so much higher than in other species (P = 0.035) that it translated into significantly greater N loss from the system (P = 0.039) (Table 1). Thus, soil N stocks were lowest beneath the single nodulated legume species in our study (Table 1 and Table S2). With soil N losses exceeding N accumulation in biomass in three of the species, average net N loss from the plantation systems was 49 ± 83 kg⋅ha−1⋅y−1 across species (Fig. 1).
Discussion
Nitrogen accumulation in biomass was high in these plantations, with aboveground biomass N ranging from 482 (±45) in Hieronyma to 841 (±64) kg⋅ha−1 in Vochysia (Table S1). Similarly aged, fertilized stands in Hawaii had aboveground biomass N stocks of 134 kg⋅ha−1 in Eucalyptus saligna and 323 in Albizia falcataria, a legume (3). Nitrogen fluxes in leaf litterfall in our plantations and the mature forest at La Selva were similar, however, at 129 (±20) (this study) and 126 kg⋅ha−1⋅y−1 (12), respectively. From a global perspective, these fluxes are high but consistent with other lowland tropical forests and plantations (13). Nitrogen uptake was rapid in our study, reaching 409 (±35) kg⋅ha−1⋅y−1 in Pentaclethra, more than four times that measured at Hubbard Brook Forest (New Hampshire, USA) (14) and double the rate for a late-successional rainforest in Puerto Rico (15). Thus, the previously measured high rates of C accumulation in biomass in the plantations of this study (4) were matched by high N accrual (Table 1). This supports the concepts that N cycling recovers rapidly following disturbance in the tropics (16) and that it keeps pace with C cycling (17).
Soil was the major source of N accumulated in biomass in all four species, as evidenced by mass-balance data in conjunction with soil C:N and δ13C values (Table 1, Fig. 2, and Table S2). After 16 y, SOM with a C:N of 11.9 (± 0.3) (Table S2) continued to be mineralized and incorporated into vegetation with a C:N of 97 ± 13. These results challenge the concept that N transfer from mineral soil is a short-term response, and also that N is relatively well sequestered in SOM (18). These findings support the model that ecosystem-level C sequestration is promoted by this redistribution of N from SOM with a lower C:N into vegetation with a higher C:N (18, 19).
Soil N stocks in this site were high, but were not atypical for moist and wet tropical and subtropical forests (20). Indeed, only 1.7 Mha or 36% of the tropics is dominated by soils with low nutrient reserves, with the majority being of intermediate or high fertility (8). Other studies demonstrate that the phenomenon of redistribution of N from soil into trees is widespread. Working in highly weathered, low-fertility soils, Richter et al. (21) found that soil N stocks were depleted by 820 kg N⋅ha−1 over 35 y of pine forest development, whereas 1,100 kg N⋅ha−1 accumulated in the vegetation and forest floor. Our results demonstrate the remarkable magnitude of soil N mobilization that can occur in some cases. Terrestrial soils contain 95–150 Pg of N (20), or 23 times the amount present in vegetation (22). Thus, soil N stocks represent a very large pool from which plants can potentially acquire N. Our results show that the degree to which soil N is mobilized to support plant growth is dependent, at least in part, on the plant species present.
Our data further indicate that N turnover within forest systems can be very fast: Mean residence time (MRT) of soil N across species in our site was 43 (±9) y, calculated as the quotient of soil N mass (to 1-m depth, kg⋅ha−1) and plant N uptake (kg⋅ha−1⋅y−1) (Table 1). This soil N MRT is similar to the average global soil C MRT of 32 y (23), a completely independent calculation that lends confidence in our mass-balance estimates. The soil at this study site has a relatively high specific surface area (24, 25), and most of the SOM is physically protected by clay minerals rather than being chemically resistant to oxidation (24–26). The current conceptual framework indicates that this type of organo-mineral complex would have a much longer MRT than SOM stabilized biogeochemically (27); this would explain the high storage capacity for soil C in this site. It does not explain the lability of SOM observed in the plantations, however. Current perceptions of SOM dynamics also highlight that physicochemical factors may be trumped by biological effects (28). We hypothesize that the trees in these plantations destabilized mineral–SOM interactions. In other studies, fine roots have been shown to stimulate or “prime” microbial activity, and thereby promote faster decomposition of older organic matter (29, 30).
External inputs via total biological N fixation are generally reported to contribute between 14.7 and 36.1 kg N⋅ha−1⋅y−1 to tropical evergreen forests (31). In our study, although all species mobilized N from soil, biological N fixation also contributed N to accruing biomass in at least two of the species, as indicated by their low δ15N values. Indeed, all four species had foliar δ15N values that were <3‰, whereas soil had a mean δ15N of 5.8 (±0.7) across species (Table S5). Whereas ecosystem N stocks are generally expected to increase under species with a root-nodule symbiosis (32), soil N stocks were significantly lower under Pentaclethra, such that there was a net N loss of 2,240 (±1,032) kg⋅ha−1 (P = 0.039) (Table 1) in that species. In contrast, Vochysia (not a legume, actinorhizal, or nodulated) also presumably facilitated N fixation, at a minimum estimated rate of 62 kg⋅ha−1⋅y−1, but with a different outcome. Soil N stocks were significantly higher, such that Vochysia had a net N gain to the system of 992 (±230) kg⋅ha−1 (Table 1). With mineralization of SOM apparently stimulated in the plantations and a mean annual rainfall of 4 m in this site, labile N was clearly subject to loss from the system via leaching and gaseous losses. Whereas leaching losses alone in mature forest at La Selva are estimated to be 9 kg N⋅ha−1⋅y−1 (33), similar to inputs of N from precipitation of 9.6 kg N⋅ha−1⋅y−1 (34), total net N losses in the plantations averaged 49 (±83) kg⋅ha−1⋅y−1 across species (Fig. 1).
Tree species influenced the magnitude of N losses, however, and these differences among species were associated with various plant attributes related to the capture and utilization of C and N. The species differed primarily in: growth-rate trajectories, and thus accumulation of biomass C (4); partitioning of N uptake to fine-root growth, hereafter referred to as partitioning to fine roots (Tables S3 and S4); tissue N concentrations (Tables S4 and S6); decay of nonwoody litter and fine roots (35, 36); NUE (Table 1 and Table S4); and soil N MRT (Table 1). Specifically, Pentaclethra was characterized by consistently low rates of tree growth throughout this experiment and thus lower biomass C, lower partitioning to fine roots, higher N concentrations in all tissues, lower NUE, faster decay of nonwoody litter, and faster turnover of soil N. In contrast, Vochysia was distinguished by its consistently higher growth rate and biomass C, greater NUE, slower decay of nonwoody litter, and faster decay of fine roots. Although Hieronyma had greater NUE and higher partitioning to fine roots, it also had the lowest tissue N concentrations. Virola, a mature-forest species, had slow growth rates, low partitioning to fine roots, very slow fine-root decay, and low tissue N concentrations. Any of these attributes, either singly or in combination, could have influenced the amount of soil N loss from the plantations, but one attribute stands out: Biomass C accumulation over the 16-y period was significantly negatively correlated with soil N loss (r = −0.59, P = 0.016, n = 16). We suggest that accumulation of biomass was an important driver of variation in N retention among these plantations.
In their own distinct ways, however, all four species did the same thing: They promoted an abundance of plant-available N that supported high rates of productivity, substantial biomass accumulation (4) (Tables S1 and S2), and extremely rapid N cycling (Fig. 1). Even Virola had N uptake rates in excess of 250 kg⋅ha−1⋅y−1 (Table 1), despite relatively low growth rates and tissue N concentrations. All four tree species apparently stimulated SOM decomposition that liberated plant-available N. On a global basis, soils contain 375 times as much N as is taken up by plants annually (22). At our site, however, soil N turned over every 32–54 y, with tree species differing significantly in their effect on soil N MRT (P = 0.027) (Table 1). Rapid N cycling had a cost: For every gram of N accumulated in biomass, 0.2–3.5 g N was lost from the soil and 0–2.3 g N from the plantations (Table 1). Given the magnitude of soil N turnover in the plantations, it is important to identify the processes by which these young trees promoted soil N lability and captured this source of N.
Materials and Methods
Site Description.
The study plantations were situated at La Selva Biological Station, in the Atlantic lowlands of Costa Rica (10° 26′ N, 83° 59′ W). In this lowland evergreen broad-leaved rainforest, mean annual rainfall is 3,960 mm and mean annual temperature is 25.8 °C (37). Soils were classified as Mixed Haplic Haploperox (38). This soil is acidic, highly leached, low in base saturation, and relatively high in organic matter. In the 12-ha experimental site, the mature forest was cleared in 1955, slash was burned, and a pasture of C4 grasses, Panicum maximum L. and Melinis minutiflora Pal., was established, with grazing continuous until abandonment in 1987. Established in 1988, the randomized complete block design consisted of four blocks of 12 0.25-ha (50 m × 50 m) plots, containing 11 tree species in monoculture (39). This study focused on the four species listed above—all native—that have survived. In addition, we established four replicate plots in each of two types of reference vegetation, abandoned pasture and mature forest, which were situated adjacent to the plantations and thus shared the same soil type, climate, and relief (11). The abandoned pasture reference plots were dense swards of P. maxiumum, with an aboveground biomass ∼3% that of Vochysia (4).
Field Sampling and Calculations.
Nitrogen in plant biomass and N uptake were sampled at the same time as C in biomass and NPP, as described in Russell et al. (4) and summarized as follows. Biomass N was determined at the plot level in each of 3 y for overstory (planted) trees ≥10 cm dbh (diameter at breast height); understory trees and saplings ≥2.5 cm dbh; and understory herbs and shrubs. The first two categories of measurements were based on annual inventories of all trees within plots for trees ≥10 cm dbh, and in subplots for saplings. To convert field measurements of trees and saplings to biomass, we harvested eight trees of each species and obtained separate weights for each of five aboveground components: leaves, flowers, and fruits; twigs (diameters ≤1 cm); branches (diameters 1–10 cm); large branches (diameters ≥10 cm); and boles (diameters ≥10 cm). We developed linear regressions for estimating the dry mass of each component and total aboveground tree biomass from (dbh)2 × h (R2 > 0.94 for each of the species) (4). For nonwoody understory vegetation, we conducted direct harvests within subplots and separated the biomass by the same components as for trees. Belowground biomass was measured for three size categories, 0–2 mm, >2–10 mm, and >10 mm, based on direct measurements of the stump plus crown-root biomass of three individuals per species and soil coring. The biomass of each component was multiplied by its measured N content to determine N mass on a per-tree basis. Plant N uptake was measured over the 3-y time frame of this study by summing N increments in plant biomass and N fluxes in detritus production for the same categories as for biomass: overstory and understory canopy and branches; and bolewood. To determine total aboveground N uptake at the plot level, each individual component of aboveground NPP was multiplied by the N concentrations of the corresponding tissues and summed across components:
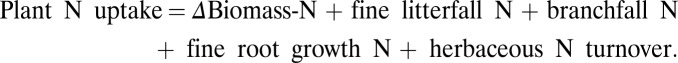 |
ΔBiomass-N was calculated as the product of tree growth (by plant component, i.e., leaves, branches, wood ≥10 cm in diameter, coarse roots, and fine roots) and N concentration of the corresponding plant component. Tree growth by tree and plant component was determined from annual tree inventories of overstory and understory trees, using site- and species-specific biomass regressions (4). Growth increments of individual trees were within plots over 2 y. Herbaceous vegetation was assumed to replace itself annually, so biomass was assumed equal to annual net production. Belowground NPP was determined for woody belowground components by measuring stumps and stump-root growth allometrically over 2 y, and estimating between-tree root growth as previously described (4). We measured fine-root ingrowth over 4-mo-long intervals for 1 y using 15-cm-tall plastic-mesh cylinders that were filled with root-free soil and inserted into the soil as described by Valverde-Barrantes et al. (40). Nitrogen accumulation rate was calculated as the biomass N divided by 16 (the age of the trees). Turnover time was calculated as the quotient of soil N mass (to 1-m depth, kg⋅ha−1) and N uptake (kg⋅ha−1⋅y−1). Soil total N concentrations were measured in year 15 of the experiment for two depth intervals, 0–15 (surface soil) and 15–30 cm, in 2003, and for 30–100 cm depth intervals in 2005, as described by Russell et al. (11). Without initial soil data to 1-m depth, we compared the 16-y-old plantations with adjacent reference plots to estimate depletion of soil N stocks; mature forest was the reference because it contained all topographic positions in this hilly landscape.
Chemical Analyses.
Total C and N in plants and soil were analyzed by dry combustion using a Thermo-Finnigan EA Flash (Series 1112; CE Elantech). Leaves of the overstory planted trees were also analyzed for N isotope ratios, expressed as δ15N values by the Colorado Plateau Stable Isotope Laboratory at Northern Arizona University; δ13C of plant tissues and soil was analyzed by the Iowa State University Stable Isotope Laboratory of German Mora.
Statistical Analyses.
Species differences among plantations were evaluated using the appropriate randomized complete block model in the SAS System’s GLM procedure, with Tukey–Kramer honestly significant difference (HSD) post hoc tests (experiment-wise error rate of α = 0.05) (41). The SAS System MIXED procedure was used to evaluate whether adjustments for spatial correlations were warranted for the soil data (42). We report only the results from the nonspatial models, as conclusions did not differ when spatial correlations were included in the model. In evaluations among all vegetation types, a randomized incomplete block design was used. Comparisons among the four plantation treatments are more precise than comparisons with abandoned pasture or mature forest, because the former are within-block comparisons but the latter are between-block comparisons. Computations were done using the SAS MIXED procedure with the Satterthwaite adjustment for degrees of freedom.
Supplementary Material
Acknowledgments
We thank Peter Vitousek and two anonymous reviewers for insightful comments during a previous version of this manuscript. We thank Ricardo Bedoya, Flor Cascante, Dennis Chavarría, Bernal Paniagua, Eduardo Paniagua, Marlon Hernandez, Nathan O’Leary, and Oscar Valverde-Barrantes for assistance in sample collection and processing; Bruce Hungate for the δ15N analyses; German Mora for the δ13C analyses; Philip Dixon for statistical advice; Tim Read and Bill Shoemaker for artistry in Fig. 1; and Knute Nadelhoffer and Samantha Weintraub for insightful comments on an earlier draft of this manuscript. This material is based on work supported by US National Science Foundation Grants DEB 0236502 and 0703561.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204157109/-/DCSupplemental.
References
- 1.Houghton RA, Hall F, Goetz SJ. Importance of biomass in the global carbon cycle. J Geophys Res. 2009;114:G00E03. [Google Scholar]
- 2.Canadell JG, et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci USA. 2007;104:10288–10293. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binkley D, Ryan MG. Net primary production and nutrient cycling in replicated stands of Eucalyptus saligna and Albizia facaltaria. For Ecol Manage. 1998;112(1):79–85. [Google Scholar]
- 4.Russell AE, Raich JW, Arrieta RB, Valverde-Barrantes OJ, González E. Impacts of individual tree species on carbon dynamics in a moist tropical forest environment. Ecol Appl. 2010;20:1087–1100. doi: 10.1890/09-0635.1. [DOI] [PubMed] [Google Scholar]
- 5.Silver WL, Kueppers LM, Lugo AE, Ostertag R, Matzek V. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecol Appl. 2004;14:1115–1127. [Google Scholar]
- 6.Pan Y, et al. A large and persistent carbon sink in the world's forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 7.Amundson R, Jenny H. On a state factor model of ecosystems. Bioscience. 1997;47:536–543. [Google Scholar]
- 8.Sanchez PA, Logan TJ. Myths and science about the chemistry and fertility of soils in the tropics. 1992 Myths and Science of Soils of the Tropics, Special Publication, eds Lal R, Sanchez PA. Soil Sci Soc Am 29:35–46. [Google Scholar]
- 9.Haggar J, Wightman K, Fisher R. The potential of plantations to foster woody regeneration within a deforested landscape in lowland Costa Rica. For Ecol Manage. 1997;99(1–2):55–64. [Google Scholar]
- 10.Berendse F, Aerts R. Nitrogen-use-efficiency: A biologically meaningful definition? Funct Ecol. 1987;1:293–296. [Google Scholar]
- 11.Russell AE, Raich JW, Valverde-Barrantes OJ, Fisher RF. Tree species effects on soil properties in experimental plantations in tropical moist forest. Soil Sci Soc Am J. 2007;71:1389–1397. [Google Scholar]
- 12.Wood TE, Lawrence D, Clark DA. Determinants of leaf litter nutrient cycling in a tropical rain forest: Soil fertility versus topography. Ecosystems (N Y) 2006;9:700–706. [Google Scholar]
- 13.Vitousek PM, Sanford RL., Jr Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17:137–167. [Google Scholar]
- 14.Likens GE, Bormann FH. Biogeochemistry of a Forested Ecosystem. New York: Springer; 1995. [Google Scholar]
- 15.Chestnut TJ, Zarin DJ, McDowell WH, Keller M. A nitrogen budget for late-successional hillslope tabonuco forest, Puerto Rico. Biogeochemistry. 1999;46(1–3):85–108. [Google Scholar]
- 16.Davidson EA, et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature. 2007;447:995–998. doi: 10.1038/nature05900. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Luo Y, Finzi AC. Carbon and nitrogen dynamics during forest stand development: A global synthesis. New Phytol. 2011;190:977–989. doi: 10.1111/j.1469-8137.2011.03645.x. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience. 2004;54:731–739. [Google Scholar]
- 19.Rastetter EB, Agren GI, Shaver GR. Responses of N-limited ecosystems to increased CO2: A balanced-nutrition, coupled-element-cycles model. Ecol Appl. 1997;7:444–460. [Google Scholar]
- 20.Post WM, Pastor J, Zinke J, Stangenberger A. Global patterns of soil nitrogen storage. Nature. 1985;317:613–616. [Google Scholar]
- 21.Richter DD, et al. Legacies of agriculture and forest regrowth in the nitrogen of old-field soils. For Ecol Manage. 2000;138(1–3):233–248. [Google Scholar]
- 22.Chapin F, III, Matson P, Vitousek P. Principles of Terrestrial Ecosystem Ecology. 2nd Ed. New York: Springer; 2011. [Google Scholar]
- 23.Raich JW, Schlesinger WH. The global carbon dioxide flux in soil respiration and its relationship to climate and vegetation. Tellus B. 1992;44(2):81–99. [Google Scholar]
- 24.Kleber M, Mikutta R, Torn MS, Jahn R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur J Soil Sci. 2005;56:717–725. [Google Scholar]
- 25.Siregar A, Kleber M, Mikutta R, Jahn R. Sodium hypochlorite oxidation reduces soil organic matter concentrations without affecting inorganic soil constituents. Eur J Soil Sci. 2005;56:481–490. [Google Scholar]
- 26.Mikutta R, Kleber M, Torn M, Jahn R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry. 2006;77(1):25–56. [Google Scholar]
- 27.von Lützow M, et al. Stabilization mechanisms of organic matter in four temperate soils: Development and application of a conceptual model. J Plant Nutr Soil Sci. 2008;171(1):111–124. [Google Scholar]
- 28.Schmidt MWI, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478(7367):49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 29.Fontaine S, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007;450:277–280. doi: 10.1038/nature06275. [DOI] [PubMed] [Google Scholar]
- 30.Kuzyakov Y. Factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci. 2002;165:382–396. [Google Scholar]
- 31.Cleveland CC, et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem Cycles. 1999;13:623–645. [Google Scholar]
- 32.Binkley D, Giardina C. Why do tree species affect soils? The warp and woof of tree-soil interactions. Biogeochemistry. 1998;42(1–2):89–106. [Google Scholar]
- 33.Schwendenmann L, Veldkamp E. The role of dissolved organic carbon, dissolved organic nitrogen, and dissolved inorganic nitrogen in a tropical wet forest ecosystem. Ecosystems (N Y) 2005;8:339–351. [Google Scholar]
- 34.Eklund TJ, McDowell WH, Pringle CM. Seasonal variation of tropical precipitation chemistry: La Selva, Costa Rica. Atmos Environ. 1997;31:3903–3910. [Google Scholar]
- 35.Raich JW, Russell AE, Bedoya-Arrieta R. Lignin and enhanced litter turnover in tree plantations of lowland Costa Rica. For Ecol Manage. 2007;239(1–3):128–135. [Google Scholar]
- 36.Raich JW, Russell AE, Valverde-Barrantes OJ. Fine root decay rates vary widely among lowland tropical tree species. Oecologia. 2009;161:325–330. doi: 10.1007/s00442-009-1379-9. [DOI] [PubMed] [Google Scholar]
- 37.McDade LA, Bawa K, Hespenheide H, Hartshorn G. La Selva: Ecology and Natural History of a Neotropical Rain Forest. Chicago: Univ of Chicago Press; 1994. [Google Scholar]
- 38.Kleber M, Schwendenmann L, Veldkamp E, Rößner J, Jahn R. Halloysite versus gibbsite: Silicon cycling as a pedogenetic process in two lowland neotropical rain forest soils of La Selva, Costa Rica. Geoderma. 2007;138(1–2):1–11. [Google Scholar]
- 39.Fisher RF. Amelioration of degraded rain forest soils by plantations of native trees. Soil Sci Soc Am J. 1995;59:544–549. [Google Scholar]
- 40.Valverde-Barrantes OJ, Raich JW, Russell AE. Fine-root mass, growth and nitrogen content for six tropical tree species. Plant Soil. 2007;290:357–370. [Google Scholar]
- 41.Littell R, Freund R, Spector P. SAS System for Linear Models. Cary, NC: SAS Inst; 1991. [Google Scholar]
- 42.Littell RCMG, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Inst; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.