Abstract
Integrated physiological systems, such as the cardiac and the respiratory system, exhibit complex dynamics that are further influenced by intrinsic feedback mechanisms controlling their interaction. To probe how the cardiac and the respiratory system adjust their rhythms, despite continuous fluctuations in their dynamics, we study the phase synchronization of heartbeat intervals and respiratory cycles. The nature of this interaction, its physiological and clinical relevance, and its relation to mechanisms of neural control is not well understood. We investigate whether and how cardiorespiratory phase synchronization (CRPS) responds to changes in physiological states and conditions. We find that the degree of CRPS in healthy subjects dramatically changes with sleep-stage transitions and exhibits a pronounced stratification pattern with a 400% increase from rapid eye movement sleep and wake, to light and deep sleep, indicating that sympatho-vagal balance strongly influences CRPS. For elderly subjects, we find that the overall degree of CRPS is reduced by approximately 40%, which has important clinical implications. However, the sleep-stage stratification pattern we uncover in CRPS does not break down with advanced age, and surprisingly, remains stable across subjects. Our results show that the difference in CRPS between sleep stages exceeds the difference between young and elderly, suggesting that sleep regulation has a significantly stronger effect on cardiorespiratory coupling than healthy aging. We demonstrate that CRPS and the traditionally studied respiratory sinus arrhythmia represent different aspects of the cardiorespiratory interaction, and that key physiologic variables, related to regulatory mechanisms of the cardiac and respiratory systems, which influence respiratory sinus arrhythmia, do not affect CRPS.
Keywords: physiologic transitions, scaling, heart rhythm, breathing, nonlinear dynamics
It is an open problem to adequately determine interactions between complex systems where their coupling is not known a priori, and where the only available information is contained in the output signals of the systems. For integrated physiological systems, this problem is further complicated by transient nonlinear characteristics and continuous fluctuations in their dynamics. In physiological studies, the interaction between two key physiological systems under neural regulation, the cardiac and the respiratory system, is traditionally identified through the respiratory sinus arrhythmia (RSA), which accounts for the periodic variation of the heart rate within a breathing cycle (1, 2). Recent advances in the field of nonlinear dynamics and statistical physics have led to the development of advanced phase-synchronization approaches (3, 4) that have identified a previously unknown form of cardiorespiratory coupling (5). In the context of cardiorespiratory coupling, phase-synchronization is defined as a consistent occurrence of heartbeats at the same relative phases within consecutive breathing cycles (Fig. 1). Earlier studies have focused on short data segments to identify certain degrees of cardiorespiratory phase synchronization (CRPS) in healthy young subjects (5, 6) and in newborn infants (7). A more recent study has identified an intriguing effect of maternal-fetal heartrate synchronization (8). However, both cardiac and respiratory dynamics exhibit long-term transient changes associated with different physiologic states and conditions; how CRPS responds to these changes in relation to underlying mechanisms of physiologic control remains not understood. Moreover, whether RSA and CRPS represent different aspects of the cardiorespiratory coupling, and how these two measures are influenced by the same physiologic variables, is not known.
Fig. 1.
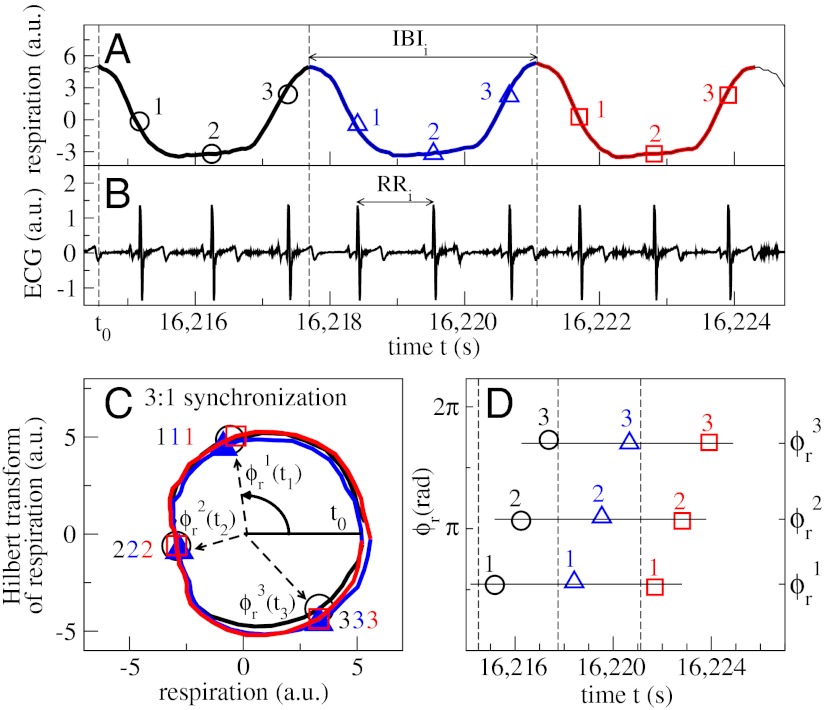
Cardiorespiratory phase synchronization (CRPS) and synchrogram method. (A) Three consecutive breathing cycles (in black, blue, and red), and (B) a simultaneously recorded ECG signal. Horizontal arrows indicate an interbreath interval (IBI) and a RR beat-to-beat interval. (C) Demonstration of phase synchronization between the heartbeats and respiratory cycles shown in A and B. The respiratory signal is plotted versus its Hilbert Transform (HT) (3), and each breathing cycle appears as a close-to-circular trajectory in the same color as in A. The instantaneous phase ϕr(t) is defined as the angle of the respiratory signal and its HT relative to the beginning of each breathing cycle. For each breathing cycle, the first heartbeat occurs at the same respiratory phase 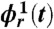 , and the second and third heartbeats within each cycle occur at
, and the second and third heartbeats within each cycle occur at  and
and 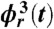 , respectively (symbols collapse), indicating robust CRPS. (D) Cardiorespiratory synchrogram method: Each heartbeat in the ECG signal (B) is shown with its phase ϕr(t) relative to the beginning of the breathing cycle in which it occurs. Different symbols represent heartbeats in different breathing cycles as in A and C; vertical dashed lines show the beginning of each breathing cycle. Three horizontal parallel lines formed respectively by the first, second, and third heartbeats in the three consecutive breathing cycles indicate 3∶1 phase synchronization. In our analysis, we consider a broad range of CRPS with different n∶m ratios, where n is the number of heartbeats synchronized with m breathing cycles.
, respectively (symbols collapse), indicating robust CRPS. (D) Cardiorespiratory synchrogram method: Each heartbeat in the ECG signal (B) is shown with its phase ϕr(t) relative to the beginning of the breathing cycle in which it occurs. Different symbols represent heartbeats in different breathing cycles as in A and C; vertical dashed lines show the beginning of each breathing cycle. Three horizontal parallel lines formed respectively by the first, second, and third heartbeats in the three consecutive breathing cycles indicate 3∶1 phase synchronization. In our analysis, we consider a broad range of CRPS with different n∶m ratios, where n is the number of heartbeats synchronized with m breathing cycles.
To gain insight into the mechanism of cardiorespiratory coupling, we investigate CRPS during (i) transitions from one physiological state to another, and (ii) under different physiologic conditions.
Empirical studies have reported a strong variation in linear and nonlinear characteristics in both cardiac and respiratory dynamics with the sleep–wake cycle (9), across circadian phases (10–12) and sleep-stage transitions (13–16), indicating significant changes in the regulatory mechanisms with transitions across physiological states. Thus, we hypothesize that cardiorespiratory coupling may undergo phase transitions with transitions across physiological states. Because sleep stages are well-defined physiologic states and are associated with distinct mechanisms of autonomic control (15, 16), we also hypothesize that cardiorespiratory coupling will be characterized by a specific degree of CRPS during each sleep stage.
Further, to test the influence of different physiologic conditions on cardiorespiratory coupling, we investigate the effect of aging. Aging is traditionally associated with the process of decline of physiologic function and reduction of physiologic complexity (17–19) that result from changes in the underlying control mechanisms—regulatory networks of neural and metabolic pathways that interact through coupled cascades of nonlinear feedback loops over a range of timescales (20). Although healthy aging is accompanied by a reduction in heart rate and respiratory variability (21) leading to decreased responsiveness and loss of sensitivity to external and internal stimuli (22), recent studies have shown that certain scaling and nonlinear properties remain intact (15, 16, 20). These observations indicate that, although the coupled feedback loops controlling physiologic dynamics across different timescales may still be present in healthy elderly subjects (hence preserving the scaling features), the reduction in physiologic responsiveness to external and internal stimuli suggests a reduced coupling strength of these feedback interactions with advanced age. Thus, we hypothesize that the strength of CRPS as a measure of cardiorespiratory coupling decreases with aging.
Results
Nonlinear oscillatory systems characterized by nonidentical eigenfrequencies and highly irregular signal output can synchronize even when their coupling is weak—i.e., their respective frequencies and phases “lock” at a particular ratio (3, 4) (Fig. 1). Despite the significant difference in the periodicity of the cardiac and respiratory rhythms represented by the heartbeat and interbreath intervals, and the complex variability in these rhythms (Fig. 2 A, B, D, and E), episodes of regular heartbeat-respiration phase relationship emerge.
Fig. 2.
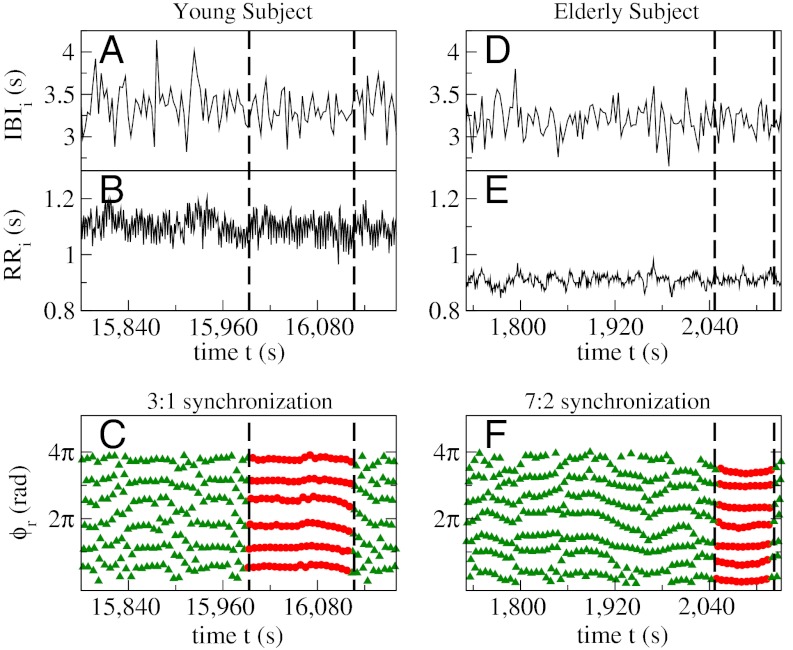
Complex fluctuations in interbreath and heartbeat intervals, and corresponding cardiorespiratory synchrograms of a healthy young (Left) and a healthy elderly (Right) subject. Simultaneously recorded interbreath (IBI) and heartbeat (RR) intervals over a period of 400 s show higher variability for the young subject (A and B) and reduced variability for the elderly (D and E) subject (20, 21). Cardiorespiratory synchrogram for the young subject (C) obtained from the data in A and B, and for the elderly subject (F) corresponding to the data in D and E. Vertical dashed lines in C and F indicate segments of continuous phase synchronization (parallel, almost horizontal lines of red filled circles) between heartbeats and breathing cycles in the time intervals marked by vertical dashed lines in A and B, and D and E, respectively. An episode of 3∶1 synchronization is shown for the young subject (n = 6 heartbeats within m = 2 breathing cycles are consistently placed at the same respiratory phases ϕr over many consecutive breathing cycles), and a segment of 7∶2 synchronization (n = 7 heartbeats are synchronized with each m = 2 breathing cycles) for the elderly subject. Note that the young subject exhibits a longer period of CRPS despite significantly higher interbreath and heartbeat variability compared to the elderly subject.
The key feature of this phase relation is that sequences of heartbeats consistently occur at the same respiratory phases for consecutive breathing cycles (Fig. 1C)—segments of horizontal lines in the phase synchrogram (Figs. 1D and 2 C and F). Such phase synchronization of heartbeats and respiration is a manifestation of the temporal organization and adjustment of the cardiac and respiratory rhythms due to their underlying coupling. Because the variability as well as the scale-invariant and nonlinear features of the cardiac and respiratory systems change with physiological state (e.g., wake/sleep and different sleep stages; refs. 9, 13, 14, 16, and 23) and with physiologic conditions (e.g., disease and age; refs. 16 and 24), quantifying CRPS across physiologic states and conditions can provide insight into how physiologic regulation affects cardiorespiratory coupling.
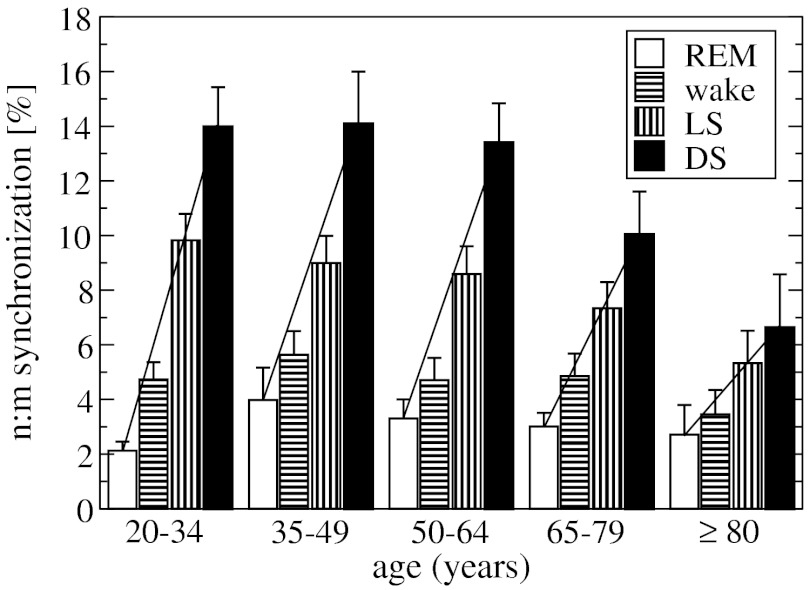
Our analysis of data obtained from healthy subjects across all age groups during sleep shows a change in the degree of CRPS during different sleep stages. We find a relatively low phase synchronization of (3.1 ± 0.3)% (group average ± standard error) during rapid eye movement (REM) sleep and (4.8 ± 0.4)% during wake state, higher synchronization with (8.5 ± 0.5)% during light sleep (LS) and highest (12.8 ± 0.8)% during deep sleep (DS), indicating an approximately 400% increase in the degree of cardiorespiratory coupling with transition from REM to DS (Fig. 3). To test for significance in these differences, we examine for each subject the changes in CRPS between the sleep stages by applying to all subjects in the database a one-way ANOVA with repeated measures (comparisons between all sleep stages), and we obtain a p value of p < 10-3 (all pairwise multiple comparison procedures by Tukey’s test yield p < 0.05). These results show a remarkable sensitivity of the cardiorespiratory coupling in response to sleep-stage transitions.
Fig. 3.
Phase synchronization as a measure of cardiorespiratory coupling across sleep stages. A significant increase in CRPS is observed during deep sleep (DS) and light sleep (LS) compared to REM sleep and quiet wake, indicating a significant modulation in cardiorespiratory coupling due to sleep regulation. Left bars represent the group mean n∶m phase synchronization obtained by averaging the percent of synchronization for each subject in the group. Error bars indicate the standard error. The analysis is based on data from 189 healthy subjects during 8 h of sleep by exploring all n∶m ratios, for n > 1 and m ≤ 3 (where n is the number of heartbeats occurring in m adjacent respiratory cycles). Statistical significance of the results for each sleep stage is demonstrated by a comparison to a surrogate test (right bars) performed on the same group of subjects, where the Fourier phases of the respiratory data from each subject were randomized (36) prior to phase-synchronization analysis. A Mann–Whitney rank sum test comparing the synchronization obtained from the real data to the synchronization of the surrogate data yields p < 10-3 for each sleep stage. See Materials and Methods for details on the surrogate data and test. Dashed line (not a fitting line) highlights the sleep-stage stratification pattern in CRPS.
The cardiac and respiratory rhythms can synchronize in different n∶m ratios. Because modulation in sympatho-vagal balance associated with sleep-stage transitions leads to change in the frequency and variability of the cardiac and respiratory rhythms during different sleep stages (15, 16, 21, 25), which in turn may affect cardiorespiratory coupling, we ask whether distinct n∶m phase-synchronization patterns are present during different sleep stages. We find that n∶1 synchronization dominates in all sleep stages, with a significant reduction in the degree of n∶2 and n∶3 type synchronization (see SI Text). However, for each synchronization ratio n∶1, n∶2, and n∶3, we consistently observe a pronounced sleep-stage dependence as shown in Fig. 3—low degree of synchronization during REM and wake, higher during LS, and most pronounced cardiorespiratory synchronization during DS (Fig. S1). Such robust sleep-stage stratification pattern in CRPS for different n∶m ratios indicates a strong influence of sleep regulation on the neural mechanism controlling cardiorespiratory coupling.
Important aspects of cardiac and respiratory dynamics change with advanced age (26). Thus, we next investigate whether changes in physiologic condition under healthy aging affect cardiorespiratory synchronization. Our analyses of subjects from different age groups show a gradual and significant decrease in the degree of CRPS across age groups (Fig. 4). Considering all n∶m synchronization ratios, we find an approximately 40% decline in synchronization when comparing the youngest and the oldest groups (Mann–Whitney rank sum test: p = 0.021). Our analyses show that the decline in synchronization is not monotonous with age—note the weak decline for the young and middle-age groups and the large drop in synchronization between the 65- to 79-y age group and the ≥80-y age group. These results indicate that the strength of cardiorespiratory coupling is significantly affected by aging. The age-dependence of CRPS for different n∶m ratios is shown in Fig. S2. Details of the analyses and statistical tests related to different n∶m ratios across age groups are presented in the SI Text.
Fig. 4.
Significant reduction of approximately 40% in CRPS with age indicates decreased cardiorespiratory coupling in elderly subjects. The n∶m synchronization is obtained from each subject during 8 h of sleep without differentiating between sleep stages and is averaged over all subjects in each age group (error bars represent standard error). The same data as in Fig. 3 are analyzed (see Materials and Methods). Right bars show a surrogate test for the subjects in each age group based on synchronization between heartbeats and Fourier phase-randomized respiratory data, indicating statistical significance of the results for all age groups (Mann–Whitney rank sum test for all age groups, p < 10-3). Dashed line (not a fit) highlights the decrease in synchronization with age.
To understand the interrelation between the separate mechanisms of sleep regulation and aging, and their respective influences on the dynamics of cardiorespiratory coupling, it is important to dissociate the effects of sleep-stage transitions and aging on CRPS. One hypothesis is that, with age, synchronization decreases proportionally across all sleep stages. Alternatively, the observed significant decline in the total n∶m phase synchronization (Fig. 4), and in particular synchronization ratios (Fig. S2), may be due to a predominant loss of synchronization during specific sleep stages. To this end, we analyze CRPS across all sleep stages, separately for each age group in our database. We find that the decrease in synchronization with age is due to a significant reduction during DS and LS—an approximately 50% drop comparing the youngest to the oldest group (Fig. 5). In contrast, the n∶m synchronization during REM and wake does not significantly change with age. These intriguing results indicate that the decline in CRPS with age is primarily mediated through the neuroautonomic mechanisms regulating DS and LS.
Fig. 5.
Sleep-stage stratification pattern in CRPS for different age groups. All age groups exhibit the same stratification pattern with lowest percent synchronization during REM, higher during wake and LS, and highest during DS. This sleep-stage difference significantly decreases from a factor of 7 (comparing REM to DS) for the youngest subjects to a factor of 2 for the oldest age group. Error bars in all panels show the standard error. For different n∶m ratios see SI Text and Fig. S3.
This reduction in synchronization with age (Fig. 5) cannot be attributed to changes in sleep architecture that are traditionally associated with marked reduction of DS in elderly (16, 26) because our analyses are based on the percentage of synchronization within a given sleep stage, irrespective of age-related changes in sleep-stage total duration throughout the night. Because total LS duration increases with aging (16, 26), our observation of decreased synchronization in elderly during LS (Fig. 5) indicates that the impact of aging on cardiorespiratory coupling is not a consequence of age-related changes in sleep architecture. Finally, our findings demonstrate that, despite the significant reduction in CRPS with age (Figs. 4 and 5), the consistent difference in the degree of synchronization across sleep stages is preserved for all age groups (Fig. 5). This sleep-stage stratification pattern in CRPS persists for different n∶m ratios (Fig. S3).
Discussion
Our results demonstrate that changes in physiologic regulatory mechanisms with different physiologic states and conditions strongly affect the coupling between physiological systems. In the context of physiological states, we find that CRPS, a measure of complex nonlinear physiologic coupling, changes significantly with sleep-stage transitions and exhibits a pronounced pattern across sleep stages. This sleep-stage stratification pattern is characterized by a consistently higher degree of synchronization during DS, lower during LS, and lowest during REM and wake (Fig. 3), supporting earlier observations of different cardiorespiratory synchronization during non-REM and REM sleep (27). Considering the degree of phase synchronization as an order parameter characterizing the system, our observations of a continuous decline of CRPS from DS and LS to REM and wake, which is also paralleled by an increase in the long-range power-law correlation exponents of the cardiac and respiratory dynamics (9, 14–16), is reminiscent of a second-order phase transition in physical systems.
Comparing different physiologic conditions, we show that cardiorespiratory coupling changes in the process of healthy aging with a significant reduction in the degree of CRPS in the elderly (Fig. 4). However, a remarkably similar sleep-stage stratification pattern is observed across all age groups (Fig. 5), indicating a surprisingly robust effect of sleep-stage transitions on cardiorespiratory coupling, despite significant reduction in physiologic responsiveness, and cardiac and respiratory variability with age (26). These results indicate that the influence of sleep regulation on the interaction between the cardiac and the respiratory system does not break down with progressive aging, and that the effect of sleep on cardiorespiratory coupling is stronger than the effect of healthy aging.
Sleep stages are associated with regulatory mechanisms characterized by different neuroautonomic tone and levels of sympatho-vagal balance (28). Our finding of a high degree of CRPS during LS and DS (Fig. 3 and Fig. S1), when sympathetic activity is reduced, and weak synchronization during REM and wake, when the sympathetic tone is dominant, suggests that cardiorespiratory coupling is strongly influenced by neuroautonomic regulation. Further, this relation between synchronization and sympathetic activity is in agreement with our observation of a significant approximately 40% loss of CRPS with advanced age (Fig. 4 and Fig. S2), where neuroautonomic regulation is characterized by suppressed parasympathetic tone and dominant sympathetic nerve activity (26). Further, because our analyses are based on the percent synchronization within each sleep stage and do not depend on changes in sleep architecture with aging (e.g., shorter duration of DS and increased LS), our findings of reduced synchronization in LS and DS in elderly subjects indicate that aging directly affects the mechanism of cardiorespiratory coupling independently of parallel changes in sleep architecture.
Changes in sympatho-vagal balance affect physiologic variability. For example, bursts of sympathetic tone and parasympathetic withdrawal lead to increased nonstationarity of the heartbeat RR interval time series characterized by higher values of the standard deviation σRR (SDNN) during wake and REM. With gradual decrease of sympathetic tone during LS and DS, the degree of nonstationarity also decreases, and thus σRR is reduced (15). Because this trend is observed for both young and old subjects (15), although with lower values of σRR for all sleep stages due to reduced parasympathetic tone in the elderly (17–19, 21), σRR reflects both sympathetic and parasympathetic activity. In contrast, the standard deviation σΔRR (RMSSD) of the increments ΔRR in consecutive heartbeat intervals is sensitive only to changes in parasympathetic activity, and thus changes only with age but not across sleep stages (15). Our results show that the decline in the degree of physiologic variability, as measured by SDNN, from wake and REM to LS and DS, is accompanied by an increase in cardiorespiratory synchronization (Figs. 3 and 5). In contrast, the decrease in RMSSD with aging is accompanied by a decrease in synchronization (Figs. 4 and 5), indicating reduction in certain measures of physiologic complexity with aging (17).
These parallel observations indicate that different aspects of the neuroautonomic control play a role in the modulation of cardiorespiratory coupling across sleep stages and with aging. Notably, the change in the degree of phase synchronization across sleep stages (400% comparing REM vs. DS, Fig. 3) is twice as large as the change in synchronization due to aging (200% comparing oldest to youngest groups, Fig. 4). The same ratio was reported earlier when comparing the difference in SDNN across sleep stages with the difference in RMSSD between age groups (15). These observations suggest that the effect of sleep regulation on cardiorespiratory coupling is stronger compared to the effect of healthy aging.
Modulations in neuroautonomic control across sleep stages affect not only static measures of cardiac and respiratory variability such as SDNN and RMSSD. Scale-invariant and nonlinear features of cardiac and respiratory dynamics over a broad range of scales also change—with strong power-law correlations and a high degree of nonlinearity during wake and REM that gradually decrease during LS, and close to random and linear behavior during DS (13, 14, 16). Further, strong correlations with higher scaling exponents have been associated with dominant sympathetic activity under parasympathetic blockade in healthy subjects (29) and under pathological conditions (30). Our findings of a sleep-stage stratification pattern with lower CRPS during wake and REM when sympathetic tone is dominant, and a significantly higher synchronization during LS and DS when sympathetic tone is low (Figs. 3 and 5), indicate that cardiorespiratory coupling is less effective when the individual cardiac and respiratory dynamics are strongly correlated and nonlinear. In contrast, this coupling is more pronounced when the scaling and nonlinearity of the cardiac and respiratory systems are reduced during LS and DS.
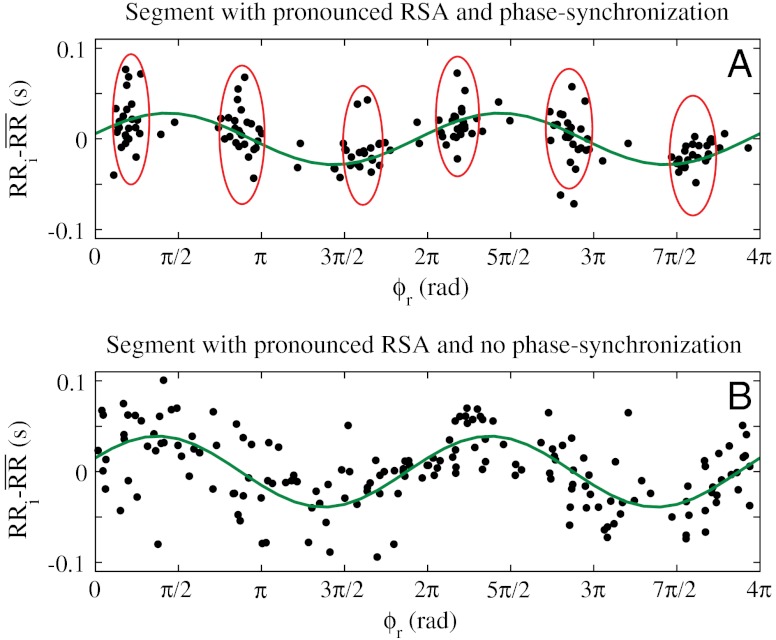
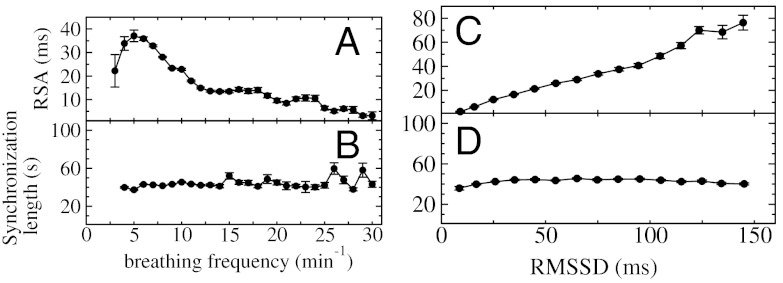
We note that CRPS is very different from the traditionally studied RSA—another aspect of cardiorespiratory interaction also influenced by neuroautonomic regulation (2). Our analyses did not reveal a statistical relation between the degree of CRPS and the strength of RSA. Indeed, segments with pronounced RSA may exhibit strong synchronization or no synchronization at all, even when we analyze segments from the same subject during the same sleep stage (Fig. 6). This difference in behavior is due to the fact that, whereas RSA is a measure of the amplitude of variation of the heartbeat intervals within the breathing cycles, phase synchronization is characterized by the clustering of heartbeats at specific phases in the breathing cycle. This clustering is independent of the amplitude of heart rate modulation (Fig. 6). Further, we find that key physiologic variables influence RSA and CRPS differently. For example, RSA has a strong nonlinear response to changes in breathing frequency—it increases with decreasing breathing frequency and decreases for very low breathing frequencies (1) (Fig. 7A). In contrast, our analysis shows no dependence of phase synchronization on the breathing frequency (Fig. 7B). Moreover, RSA increases with increasing RMSSD (a measure of parasympathetic tone; ref. 25), while CRPS remains unchanged (Fig. 7 C and D). However, we observe that the sensitivity of synchronization in response to sleep-stage transitions is by a factor of 10 higher compared to RSA (Fig. 8).
Fig. 6.
CRPS and RSA represent different aspects of cardiorespiratory coupling. (A) Whereas RSA leads to periodic modulation of the heart rate within each breathing cycle (highlighted by a least-squares-fit sinusoid line to the data points) and is quantified by the amplitude of the heart rate modulation, CRPS leads to clustering of heartbeats at certain phases ϕr of the breathing cycle (highlighted by red ovals). Shown are consecutive heartbeats over a period of 200 s. The x axis indicates the phases ϕr of the breathing cycle where heartbeats occur, and the y axis indicates the deviation of each heartbeat interval RRi from the mean  calculated by averaging all RRi within a given breathing cycle (different cycles are characterized by different values of
calculated by averaging all RRi within a given breathing cycle (different cycles are characterized by different values of  ). Heartbeats are plotted over pairs of consecutive breathing cycles, ϕr∈[0,4π], to better visualize rhythmicity. Data are selected from a subject during DS. (B) For the same subject as in A, heartbeats from another period of 200 s also during DS are plotted over pairs of consecutive breathing cycles. Data show well-pronounced RSA with a similar amplitude as in A, however, heartbeats are homogeneously distributed across all phases of the respiratory cycles, indicating absence of CRPS.
). Heartbeats are plotted over pairs of consecutive breathing cycles, ϕr∈[0,4π], to better visualize rhythmicity. Data are selected from a subject during DS. (B) For the same subject as in A, heartbeats from another period of 200 s also during DS are plotted over pairs of consecutive breathing cycles. Data show well-pronounced RSA with a similar amplitude as in A, however, heartbeats are homogeneously distributed across all phases of the respiratory cycles, indicating absence of CRPS.
Fig. 7.
RSA and phase synchronization involve different mechanisms of cardiorespiratory coupling. (A) RSA gradually increases (> 250%) with decreasing breathing frequency and drops abruptly for very low breathing frequencies (Spearman’s rank order correlation, ρ = -0.42, p < 10-3). (B) In contrast to RSA, the length of phase-synchronization episodes is independent of the breathing frequency (Spearman ρ ≈ 0, p < 10-3). (C) RSA strongly increases with increasing values of RMSSD of heartbeat RR intervals (i.e., σΔRR, a measure of parasympathetic tone; ref. 25) (Spearman ρ = 0.69, p < 10-3). (D) In contrast to RSA, the length of phase-synchronization episodes does not significantly change with RMSSD (Spearman ρ ≈ 0, p < 10-3). Error bars represent the standard error. See Materials and Methods for the procedure used to obtain these dependencies. Data are averaged over all sleep stages and age groups.
Fig. 8.
Change in CRPS, RSA, and average breathing frequency (Bfreq) across sleep stages. Average values for each sleep stage are normalized on the corresponding values during REM sleep. Data are averaged over all age groups. The sensitivity of phase synchronization to sleep-stage transitions is by a factor of 10 higher than RSA, indicating that sleep regulation affects these two aspects of cardiorespiratory coupling differently. Error bars represent the standard error.
Depressed RSA is a predictor of sudden cardiac death among postinfarction patients (31), stressing the importance of cardiorespiratory coupling for prediction of risk. Because the CRPS measure represents a different aspect of the cardiorespiratory interaction compared to RSA, it may yield complementary diagnostic information. Our observations of reduced CRPS under specific physiologic states (sleep stages) and conditions (different age groups) underline the potential of CRPS for clinical application. Because a pronounced decrease of CRPS was found in subjects after myocardial infarcts (32), our findings of a significant reduction in CRPS during REM sleep and in elderly subjects indicate elevated cardiovascular risk. Indeed, the highest occurrence of adverse cardiac events during sleep was found during REM sleep (33), and thus our results indicate that CRPS plays a key role in the mechanisms underlying these adverse events. This study is a necessary first step to understand how change in physiologic function affects CRPS under healthy states and conditions, before one is able to discern the effects of various pathologic perturbations from the influence of distinct physiologic states.
Materials and Methods
Subjects.
We analyzed heartbeat and respiratory data of 189 healthy subjects (90 male and 99 female, ages ranging from 20 to 95 y) recorded at eight sleep laboratories within the European Union project SIESTA (34). The study was approved by the local institutional human subjects review boards of the sleep laboratories involved; all subjects provided written informed consent. General exclusion criteria were a history of drug abuse or habitation (including alcohol), psychoactive medication or other drugs (e.g., β-blockers), or night-shift work. All subjects reported no symptoms of neurological, mental, medical, or cardiovascular disorders. Additional exclusion criteria in ref. 34 for healthy subjects comprised (i) significant medical disorders, (ii) a Mini Mental State Examination score < 25, (iii) a Pittsburgh Sleep Quality Index global score > 5, (iv) a usual bedtime before 10 PM or after 12 AM, (v) a Self-Rating Anxiety Scale raw score ≥33, and (vi) a Self-Rating Depression Scale raw score ≥35.
Measurements.
Heartbeat data (R peaks) were extracted from the ECGs utilizing a semiautomatic peak detector (Raschlab; ref. 35). RR time intervals were calculated between each pair of consecutive R peaks, and a RR interval was labeled as artifact and excluded from the analysis if (i) the interval was shorter than 300 ms or longer than 2,000 ms, or (ii) the interval was more than 30% shorter or more than 60% longer than the preceding RR interval. These exclusion criteria effectively eliminate ectopic heartbeats and artifacts. Respiration was measured by the oronasal airflow through a thermistor and by belts around the chest and abdomen. These three respiratory signals were resampled to 4 Hz (low-pass filter) to eliminate high-frequency fluctuations and to assure that the signal was narrow-banded, and thus its Hilbert transform could be used to calculate the respiratory phase. Sleep stages have been scored in 30-s epochs from full night polysomnographic recordings. The average duration of the recordings is 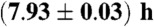 (mean ± standard error). Segments of consecutive normal heartbeat intervals within the same sleep stage shorter than 30 s were discarded.
(mean ± standard error). Segments of consecutive normal heartbeat intervals within the same sleep stage shorter than 30 s were discarded.
Cardiorespiratory Phase Synchronization and Automated Synchrogram Analysis.
Cardiorespiratory synchronization can systematically be studied in an automated way by utilizing an algorithm that evaluates cardiorespiratory synchrograms (27) (Figs. 1D and 2
C and F). The synchrogram is a method in which the phase of a continuous signal [e.g., respiration r(t)] is plotted at incidents tk of a second signal described by a point process (e.g., the occurrence of R peaks in the ECG at times tk). The instantaneous respiratory phase ϕr(t) can be calculated by the analytic signal approach (3) and, in the complex plane, ϕr(t) represents the angle between the respiratory signal r(t) and its Hilbert transform rH(t), which is the imaginary part of the respiratory signal (Fig. 1C). The plot of ϕr(tk) over tk defines the cardiorespiratory synchrogram. Cardiorespiratory phase synchronization exists when n parallel horizontal lines are observed, where n is the number of heartbeats per m breathing cycles (n = 3 and m = 1 in Fig. 1D). In our automated synchrogram algorithm, the times tk of the occurrence of heartbeats are mapped on the cumulative respiratory phase Φr(t), and 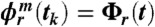 mod 2πm is plotted versus tk. For each m respiratory cycles, where n heartbeats occur at times
mod 2πm is plotted versus tk. For each m respiratory cycles, where n heartbeats occur at times 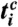 (
(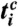 corresponds to the times of i = 1,…,n heartbeats within m respiratory cycles, denoted by c), we replace the phase points
corresponds to the times of i = 1,…,n heartbeats within m respiratory cycles, denoted by c), we replace the phase points 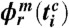 by averages
by averages 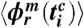 and standard deviations
and standard deviations 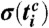 calculated over all phase points in the time window
calculated over all phase points in the time window 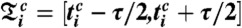 and in the phase interval
and in the phase interval 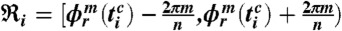 (i.e., we average the phase points along the horizontal lines in Fig. 1D over the time window
(i.e., we average the phase points along the horizontal lines in Fig. 1D over the time window 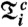 ). Thus, the phase average and standard deviation are defined by
). Thus, the phase average and standard deviation are defined by  and
and 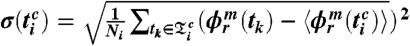 , where Ni is the number of phase points (heartbeats) in the time window
, where Ni is the number of phase points (heartbeats) in the time window 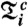 and the phase interval
and the phase interval 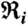 , and where m is the number of respiratory cycles in which n heartbeats occur. Next, for each breathing cycle, we average the standard deviation
, and where m is the number of respiratory cycles in which n heartbeats occur. Next, for each breathing cycle, we average the standard deviation 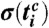 for all i = 1,…,n phase points
for all i = 1,…,n phase points 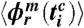 to obtain
to obtain 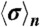 . Only breathing cycles with
. Only breathing cycles with 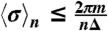 are considered, and synchronization segments are identified only when such consecutive breathing cycles span over time intervals ≥T. Finally, we relate the phase-synchronization segments to the time intervals of the different sleep stages throughout the night, and for each sleep stage we calculate the percent synchronization as the ratio between the time duration of the sum of all synchronization segments and the total time duration of the sleep stage during the night. Segments of data artifacts in both cardiac and respiratory signals are disregarded in these calculations. Because we have three respiratory signals from oronasal airflow, chest and abdomen belts, for each consecutive episode in a given sleep stage, we consider the pair of cardiac and respiratory signals that yields the highest percent synchronization (thus, optimally reducing the influence of breathing artifacts). In our analyses we use Δ = 5 and T = τ = 30 s, corresponding to standard 30-s sleep-stage scoring epochs.
are considered, and synchronization segments are identified only when such consecutive breathing cycles span over time intervals ≥T. Finally, we relate the phase-synchronization segments to the time intervals of the different sleep stages throughout the night, and for each sleep stage we calculate the percent synchronization as the ratio between the time duration of the sum of all synchronization segments and the total time duration of the sleep stage during the night. Segments of data artifacts in both cardiac and respiratory signals are disregarded in these calculations. Because we have three respiratory signals from oronasal airflow, chest and abdomen belts, for each consecutive episode in a given sleep stage, we consider the pair of cardiac and respiratory signals that yields the highest percent synchronization (thus, optimally reducing the influence of breathing artifacts). In our analyses we use Δ = 5 and T = τ = 30 s, corresponding to standard 30-s sleep-stage scoring epochs.
Surrogate Data Analysis.
To test the significance of the results for different sleep stages and age groups, we perform a surrogate test, where for each subject we analyze the phase synchronization between the original heartbeat signal and a surrogate of the respiratory signal, obtained by substituting the Fourier phases in the original respiratory signal with phases randomly drawn from a uniform distribution in the interval [0,2π], while preserving the amplitude of the Fourier coefficients. A bandpass filter in the range [0.16, 0.51 Hz] is applied to this Fourier phase-randomized respiratory signal to remove low-frequency trends and high-frequency noise. Surrogates are obtained from each of the three respiratory signals (airflow, chest, and abdomen), and their synchronization with the original heartbeat signal from the same subject is determined by the automated synchrogram algorithm described above.
Test for Dependency of CRPS and RSA on Breathing Frequency and RMSSD.
To quantify the dependence of RSA on breathing frequency and on RMSSD (Fig. 7 A and C), we consider artifact-free segments of consecutive normal heartbeats with duration ≥300 s, and we determine the RSA amplitude, the breathing frequency, and RMSSD (SD of ΔRR) for each segment. To quantify the dependence of synchronization length on breathing frequency and on RMSSD (Fig. 7 B and D), we consider only the segments of cardiorespiratory phase synchronization with duration T≥30 s, and we determine the breathing frequency and RMSSD for each of these segments. For both RSA and synchronization, we pool the data from all segments across all sleep stages and subjects, and we obtain average values (± standard error) for RSA and synchronization length for each bin of breathing frequency and RMSSD.
Supplementary Material
Acknowledgments.
We thank Pedro Bernaola-Galván for helpful discussions and interpretation of results. We acknowledge support from National Institutes of Health Grant 1R01-HL098437, the US–Israel Binational Science Foundation (BSF Grant 2008137), the Office of Naval Research (ONR Grant 000141010078), the European Community (projects DAPHNet/FP6 IST 018474-2 and SOCIONICAL/FP7 ICT 231288) and the Brigham and Women’s Hospital Biomedical Research Institute Fund. R.P.B. acknowledges support from the German Academic Exchange Service (Deutscher Akademischer Austauschdienst Fellowship).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204568109/-/DCSupplemental.
References
- 1.Angelone A, Coulter NA. Respiratory sinus arrhythmia: A frequency dependent phenomenon. J Appl Physiol. 1964;19:479–482. doi: 10.1152/jappl.1964.19.3.479. [DOI] [PubMed] [Google Scholar]
- 2.Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Appl Psychophysiol Biofeedback. 2003;28:13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996;76:1804–1807. doi: 10.1103/PhysRevLett.76.1804. [DOI] [PubMed] [Google Scholar]
- 4.Pikovsky AS, Rosenblum MG, Kurths J. Synchronization: A Universal Concept in Nonlinear Sciences. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 5.Schäfer C, Rosenblum MG, Kurths J, Abel HH. Heartbeat synchronized with ventilation. Nature. 1998;392:239–240. doi: 10.1038/32567. [DOI] [PubMed] [Google Scholar]
- 6.Prokhorov MD, Ponomarenko VI, Gridnev VI, Bodrov MB, Bespyatov AB. Synchronization between main rhythmic processes in the human cardiovascular system. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:041913. doi: 10.1103/PhysRevE.68.041913. [DOI] [PubMed] [Google Scholar]
- 7.Mrowka R, Patzak A, Rosenblum M. Quantitative analysis of cardiorespiratory synchronization in infants. Int J Bifurcat Chaos. 2000;10:2479–2488. [Google Scholar]
- 8.Leeuwen PV, et al. Influence of paced maternal breathing on fetal-maternal heart rate coordination. Proc Natl Acad Sci USA. 2009;106:13661–13666. doi: 10.1073/pnas.0901049106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov PCh, et al. Sleep-wake differences in scaling behavior of the human heartbeat: Analysis of terrestrial and long-term space flight data. Europhys Lett. 1999;48:594–600. doi: 10.1209/epl/i1999-00525-0. [DOI] [PubMed] [Google Scholar]
- 10.Moelgaard H, Soerensen KE, Bjerregaard P. Circadian variation and influence of risk factors on heart rate variability in healthy subjects. Am J Cardiol. 1991;68:777–784. doi: 10.1016/0002-9149(91)90653-3. [DOI] [PubMed] [Google Scholar]
- 11.Hu K, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov PCh, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci USA. 2007;104:20702–20707. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunde A, et al. Correlated and uncorrelated regions in heart-rate fluctuations during sleep. Phys Rev Lett. 2000;85:3736–3739. doi: 10.1103/PhysRevLett.85.3736. [DOI] [PubMed] [Google Scholar]
- 14.Kantelhardt JW, et al. Breathing during REM and non-REM sleep: Correlated versus uncorrelated behaviour. Physica A. 2003;319:447–457. [Google Scholar]
- 15.Schmitt DT, Stein PK, Ivanov PCh. Stratification pattern of static and scale-invariant dynamic measures of heartbeat fluctuations across sleep stages in young and elderly. IEEE Trans Biomed Eng. 2009;56:1564–1573. doi: 10.1109/TBME.2009.2014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumann AY, Bartsch RP, Penzel T, Ivanov PCh, Kantelhardt JW. Aging effects on cardiac and respiratory dynamics in healthy subjects across sleep stages. Sleep. 2010;33:943–955. doi: 10.1093/sleep/33.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberger AL, Rigney DR, West BJ. Chaos and fractals in human physiology. Sci Am. 1990;262:42–49. doi: 10.1038/scientificamerican0290-42. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan DT, et al. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arking R. Biology of Aging: Observations and Principles. 3rd Ed. New York: Oxford Univ Press; 2006. [Google Scholar]
- 20.Schmitt DT, Ivanov PCh. Fractal scale-invariant and nonlinear properties of cardiac dynamics remain stable with advanced age: A new mechanistic picture of cardiac control in healthy elderly. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1923–R1937. doi: 10.1152/ajpregu.00372.2007. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji H, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 22.Bernaola-Galván P, Ivanov PCh, Amaral LAN, Stanley HE. Scale invariance in the nonstationarity of human heart rate. Phys Rev Lett. 2001;87:168105. doi: 10.1103/PhysRevLett.87.168105. [DOI] [PubMed] [Google Scholar]
- 23.Kantelhardt JW, Havlin S, Ivanov PCh. Modeling transient correlations in heartbeat dynamics during sleep. Europhys Lett. 2003;62:147–153. [Google Scholar]
- 24.Brandenberger G, et al. Age-related changes in cardiac autonomic control during sleep. J Sleep Res. 2003;12:173–180. doi: 10.1046/j.1365-2869.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- 25.Otzenberger H, et al. Dynamic heart rate variability: A tool for exploring sympathovagal balance continuously during sleep in men. Am J Physiol. 1998;275:H946–H950. doi: 10.1152/ajpheart.1998.275.3.H946. [DOI] [PubMed] [Google Scholar]
- 26.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 27.Bartsch R, Kantelhardt JW, Penzel T, Havlin S. Experimental evidence for phase synchronization transitions in the human cardiorespiratory system. Phys Rev Lett. 2007;98:054102. doi: 10.1103/PhysRevLett.98.054102. [DOI] [PubMed] [Google Scholar]
- 28.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 29.Amaral LAN, et al. Behavioral-independent features of complex heartbeat dynamics. Phys Rev Lett. 2001;86:6026–6029. doi: 10.1103/PhysRevLett.86.6026. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov PCh, et al. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- 31.Peltola M, et al. Respiratory sinus arrhythmia as a predictor of sudden cardiac death after myocardial infarction. Ann Med. 2008;40:376–382. doi: 10.1080/07853890701884659. [DOI] [PubMed] [Google Scholar]
- 32.Leder U, et al. Cardiorespiratory desynchronization after acute myocardial infarct. Z Kardiol. 2000;89:630–637. doi: 10.1007/s003920070214. [DOI] [PubMed] [Google Scholar]
- 33.Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: The case for sleep as an autonomic stress test for the heart. Cardiovasc Res. 1996;31:181–211. [PubMed] [Google Scholar]
- 34.Klösch G, et al. The SIESTA project polygraphic and clinical database. IEEE Eng Med Biol Mag. 2001;20:51–57. doi: 10.1109/51.932725. [DOI] [PubMed] [Google Scholar]
- 35.Schneider R. Opensource toolbox for handling cardiologic data. 2005. www.librasch.org.
- 36.Theiler J, Eubank S, Longtin A, Galdrikian B, Garmer DJ. Testing for nonlinearity in time series: The method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.