Abstract
Objective
To compare compliance and infant HIV-1 infection risk at 6 weeks with the Thai-CDC and HIVNET-012 antiretroviral regimens in a field setting.
Design
Randomized clinical trial.
Setting
Tertiary hospital antenatal clinic in Nairobi, Kenya.
Participants
HIV-1 infected women referred from primary care clinics.
Interventions
Thai-CDC zidovudine regimen or HIVNET-012 nevirapine regimen.
Main outcome measures
Women were considered compliant if they used ≥ 80% of the doses. Infants were tested for HIV-1 at 6 weeks.
Results
Seventy women were randomized to Thai-CDC and 69 to HIVNET-012 regimens. More women were compliant with the antenatal (86%) than the intrapartum (44%) Thai-CDC regimen doses (P = 0.001). Ninety-seven per cent took the maternal and 91% gave the infant dose of the HIVNET-012 regimen (P = 0.2). Overall, 41% were compliant with the Thai-CDC regimen and 87% with the HIVNET-012 regimen (P < 0.001). Compliance with the Thai-CDC regimen was associated with partner support of antiretroviral use [odds ratio (OR), 3.0;, 95% confidence interval (CI), 1.0–9.1] and knowledge at recruitment that antiretroviral drugs could prevent infant HIV-1 (OR, 2.9; 95% CI, 1.0–8.1). Compliance with the HIVNET-012 regimen was associated with partner notification (OR, 8.0; 95% CI, 1.5–50) and partner willingness to have HIV-1 testing (OR, 7.5; 95% CI, 1.4–40). There was a trend for a higher risk of transmission with the HIVNET-012 regimen than with the Thai-CDC regimen (22% versus 9%; P = 0.07).
Conclusion
Compliance with the Thai-CDC and HIVNET-012 regimens was comparable to that in efficacy trials. Partner involvement, support and education on perinatal HIV-1 prevention may improve compliance and increase the number of infants protected from HIV-1 infection.
Keywords: antiretroviral therapy, vertical transmission, compliance, prevention of perinatal transmission
Introduction
The Thai-CDC and HIVNET-012 regimens have been shown to decrease effectively perinatal HIV-1 transmission, which is responsible for most of the estimated 1.3 million paediatric HIV-1 infections worldwide [1–4]. The Thai-CDC regimen (zidovudine twice daily from 36 weeks gestation and 3-hourly during labour) reduced the risk of mother-to-child HIV-1 transmission from 19% to 9% in a non-breastfeeding population in Thailand [2]. The same regimen in a breastfeeding population in the Ivory Coast reduced the risk of vertical transmission of HIV-1 from 22% to 12% at 4 weeks [4]. The HIVNET-012 regimen (nevirapine given to the mother at the onset of labour and to the baby within 72 h of delivery) reduced the rate of mother-to-child transmission of HIV-1 from 21% to 12% at 6–8 weeks in Uganda [1].
To date, compliance with short-course antiretroviral regimens to prevent mother-to-child transmission of HIV-1 has mainly been studied in the context of efficacy trials. In Thailand, compliance with the Thai-CDC regimen was high; only 1% of the women missed more than two doses during pregnancy, and 90% of the expected doses were taken during labour [2]. In the Ivory Coast, the median proportion of antenatal doses taken was 88%. Although 81% of the women took zidovudine at the onset of labour, the median adherence with subsequent intrapartum doses was only 33% [4]. In the HIVNET-012 trial, 97% of mothers and 98% of infants received nevirapine [1].
In all of these efficacy trials, women were intensively counselled to ensure compliance and, whenever possible, women delivered at study delivery sites with study personnel administering intrapartum and postpartum drugs. In a field setting, there are no dedicated personnel to administer medications, women deliver at sites of their choice, and intensive counselling is often not feasible. We conducted a randomized clinical trial in a field setting to compare compliance with the HIVNET-012 regimen versus the Thai-CDC regimen, and to determine patient characteristics and attitudes that are associated with non-compliance. We also compared HIV-1 transmission risk at 6 weeks in the two arms of the study.
Materials and methods
Study clinic procedures
HIV-1 infected pregnant women were referred to the study from three Nairobi antenatal clinics. Eligible women were at ≤ 35 weeks gestation, planned to remain in the city until 6 weeks after delivery, and had no contraindication to taking antiretroviral drugs. All women gave written informed consent to participate in the study.
Women were randomized at 36 weeks’ gestation, using block randomization and sealed envelopes, to either the Thai-CDC or HIVNET-012 regimens after which they were interviewed using a standardized questionnaire. Women were seen every 2 weeks until randomization at 36 weeks, after which they were seen weekly until delivery and again at 1 week after delivery. Women delivered at home or at hospitals of their choice. To ensure privacy, but to allow women to disclose results to care providers, women were given a letter indicating that they were receiving antiretroviral agents and had been counselled on infant feeding.
Women randomized to the Thai-CDC regimen were given 20 tablets of zidovudine in an electronic medication bottle (Remind Rx, IBV Technologies, Seattle Washington, USA). The electronic medication bottle recorded the date and time when a button on the bottle was pressed. Each participant was asked to press the button when they took the medicines. At each visit, information from the electronic monitoring vial was downloaded to a computer. Antenatal zidovudine compliance was measured using self-reports, pill-counts, and the electronic medication bottle. Women randomized to the HIVNET-012 regimen were given one 200 mg tablet of nevirapine and 6 mg of nevirapine syrup. HIVNET-012 and intrapartum zidovudine compliance was measured by self-reports.
Infant blood specimens were collected on filter paper at 6 weeks postpartum for HIV-1 testing using DNA PCR.
At completion of the study, women were referred to Maternal Child Health clinics near their homes and the KNH Patient Support Center for follow-up and longterm support.
Focus group discussions
Focus group discussions were held with two groups of women. Four sessions were held with pregnant women who had been tested for HIV-1 but had not yet received their test results, and three sessions were held with postpartum women who had received antiretroviral agents from the Study Clinic. The sessions were tape-recorded and facilitated by the principal investigator.
Data analysis
Compliant women were defined as those who reported using the maternal and infant doses of nevirapine, and those who reported taking ≥ 80% of the antenatal and intrapartum doses of the Thai-CDC regimen. Self-reported compliance with antenatal zidovudine was compared with compliance by pill-counts and electronic medication bottles. Data were analysed using SPSS-PC version 9.0 (SPSS Inc., Chicago, Illinois, USA). Chi-square and Wilcoxon rank sum tests were used to compare categorical and continuous variables, respectively, and paired Wilcoxon signed-rank test was used for paired data.
Results
Recruitment and follow-up
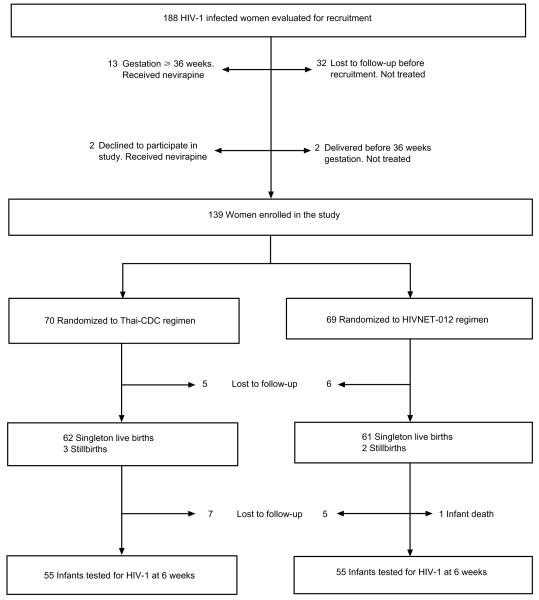
Recruitment began in November 1999 and clinical follow-up was completed in January 2001. Of 188 women seen at the Study Clinic, 139 were randomized(70 to the Thai-CDC and 69 to the HIVNET-012 regimen), 32 were lost to follow-up before recruitment, 13 presented after 35 weeks’ gestation, two declined to participate, and two delivered before 36 weeks’ gestation. One hundred and twenty-eight women (92% of those randomized) were seen 1 week after delivery, and 110 infants (89% of live-born infants) were tested for HIV-1 at 6 weeks (Fig. 1). Among the women randomized, those seen 1 week after delivery were older (median age 25 versus 22 years; P = 0.01), and more likely to be married (81% versus 55%; P = 0.05) than those lost to follow-up. Knowledge and attitudes regarding mother-to-child transmission of HIV-1, partner characteristics, and previous compliance history did not differ between women seen after delivery and those lost to follow-up.
Fig. 1.
Recruitment and follow-up of study subjects.
Study population
The median age was 25 years, 79% were married, and 40% had at least secondary school education. The median monthly house rent (an indirect measure of household income) was Ksh 1300 (US$16) and 60% of the women paid at least Ksh 1000 {US$13; per capita income in Kenya is Ksh 2300 (US$ 29) per month [5]}. At recruitment, 88% knew that HIV-1 infected mothers could transmit the infection to their children at or before delivery, 55% knew that this occurs only some of the time, and 36% reported that medicines could be used to prevent transmission. Seventy-three per cent of the women reported informing their partners of their HIV-1 test results, and 61% said their partners would be willing to have an HIV-1 test. Sixty-nine per cent reported that their partners would support the use of antiretroviral drugs to prevent infant HIV-1 infection.
Information regarding delivery and infant feeding was available for 128 women, 30 (23%) of whom delivered at home, 73 (57%) in public hospitals, and 25 (19%) in private hospitals. The median gestation at delivery was 40 weeks (range 36–44 weeks). At 1 week after delivery, 85 (66%) women were breastfeeding their infants. There were no significant differences in client characteristics, knowledge regarding mother-to-child transmission of HIV-1, labour and delivery characteristics, or infant feeding practices between women receiving nevirapine and those receiving zidovudine (Table 1).
Table 1.
Characteristics of study population.
| Characteristic | Thai-CDC n (%) |
HIVNET-012 n (%) |
P |
|---|---|---|---|
| Client characteristics | n = 70 | n = 69 | |
| Age > 25 years | 37 (52%) | 26 (38%) | 0.1 |
| Married | 53 (76%) | 57 (83%) | 0.3 |
| Secondary level education or more | 23 (33%) | 32 (46%) | 0.1 |
| Employed | 18 (26%) | 18 (26%) | 1.0 |
| Had a previous pregnancy | 54 (77%) | 46 (67%) | 0.2 |
| Pays ≥ KSh 1000 for house rent | 37 (59%) | 40 (61%) | 0.8 |
| Informed partner of HIV-1 test results | 51 (73%) | 50 (74%) | 0.9 |
| Partner willing to have HIV-1 test | 38 (54%) | 47 (69%) | 0.1 |
| Partner will support use of antiretroviral drugs | 45 (64%) | 51 (75%) | 0.2 |
| Knows that MTCT of HIV occurs | 59 (84%) | 63 (91%) | 0.2 |
| Knows that MTCT of HIV occurs only sometimes | 36 (51%) | 41 (59%) | 0.4 |
| Knows that MTCT can be prevented using medicines | 29 (41%) | 21 (30%) | 1.0 |
| Complied with most recently prescribed medications | 52 (75%) | 58 (84%) | 0.2 |
| Characteristics of labour, delivery, and infant feeding | n = 65 | n = 63 | |
| Delivered at home | 14 (22%) | 16 (25%) | 0.6 |
| Premature delivery (< 37 weeks) | 4 (6%) | 2 (3%) | 0.7a |
| Vaginal delivery | 63 (97%) | 57 (91%) | 0.2a |
| Low birth weight (< 2500 g) | 2 (3%) | 1 (2%) | 1.0a |
| Breastfeeding at 1 week after delivery | 43 (66%) | 42 (67%) | 0.8 |
Fisher’s exact test used. MTCT, Mother-to-child transmission.
Compliance
The median duration of the antepartum Thai-CDC regimen treatment was 24 days and 91% of the women received zidovudine for at least 2 weeks. Eighty-six per cent reported taking at least 80% of the antepartum doses. Intrapartum, 75% reported taking the zidovudine at the onset of labour, but only 44% reported taking at least 80% of the expected intrapartum doses. Overall, 41% of the women reported taking at least 80% of the antepartum and 80% of the intrapartum zidovudine doses and were considered compliant with the Thai-CDC regimen (Table 2).
Table 2.
Compliance with Thai-CDC and HIVNET-012 regimens.
| Compliance with Thai-CDC (n = 63) | |
|---|---|
| Duration of antepartum treatment in days [median (range)] |
24 (0–61) |
| Antepartum treatment for ≥ 2 weeks [n (%)] | 53 (91) |
| Proportion of antepartum doses taken [median (range)] | 100 (10–100) |
| Took ≥ 80% of antepartum doses [n (%)] | 55 (86) |
| Took dose at onset of labour [n (%)] | 48 (75) |
| Number of intrapartum doses taken [median (range)] | 2 (0–15) |
| Percentage of intrapartum doses taken [median (range)] | 70 (0–100) |
| Took ≥ 80% of intrapartum doses [n (%)] | 28 (44) |
| Took no intrapartum dose [n (%)] | 14 (22) |
| Took ≥ 80% antepartum and ≥ 80% intrapartum doses [n (%)] | 26 (41) |
| Compliance with HIVNET-012 (n = 61) | |
|
| |
| Took maternal dose [n (%)] | 57 (91) |
| Hours from taking maternal dose to delivery [median (range)] | 6 (0.5–127) |
| Took maternal dose ≥ 4 h before delivery [n (%)] | 49 (76) |
| Gave infant dose [n (%)] | 59 (97) |
| Hours from delivery to giving infant dose [median range)] | 12 (0.1–148) |
| Gave infant dose ≤ 72 h after delivery [n (%)] | 57 (91) |
| Took maternal and gave infant dose [n (%)] | 53 (87) |
Women who were compliant with the Thai-CDC regimen were more likely to have known at enrolment that mother-to-child transmission of HIV-1 can be prevented by antiretroviral agents (OR, 2.9; 95% CI, 1.0–8.1; P = 0.05), and to have partners who supported use of antiretroviral drugs (OR, 3.0; 95% CI, 1.0–9.1; P = 0.05). Women who delivered in private hospitals were more likely to be compliant (OR, 11; 95% CI, 1.8–68; P = 0.01) compared to women who delivered at home and this association remained significant after adjusting for house rent and level of education (P = 0.02) (Table 3).
Table 3.
Correlates of compliancea.
| Thai-CDC regimen (n = 63; compliant 41%) |
HIVNET-012 regimen (n = 61; compliant 86%) |
|||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Client characteristics | ||||
| Age > 25 years | 2.1 (0.8–5.9) | 0.2 | 0.4 (0.1–1.7) | 0.2 |
| Married | 3.1 (0.8–13) | 0.1 | 4.0 (0.8–20 ) | 0.1 |
| Secondary education or higher | 1.5 (0.5–4.4) | 0.4 | 0.4 (0.09–2.0) | 0.4 |
| Had a previous pregnancy | 2.7 (0.7–11) | 0.1 | 1.4 (0.3–6.5) | 0.7 |
| Monthly rent for house ≥ 1000 | 1.2 (0.4–3.6) | 0.7 | 0.9 (0.2–4.3) | 1.0 |
| Knowledge and attitude to MTCT and medications |
||||
| MTCT occurs | 1.0 (0.3–4.1) | 1.0 | 1.7 (0.2–18 ) | 0.5 |
| MTCT preventable with ARV | 2.9 (1.0–8.1) | 0.05 | 1.2 (0.2–65) | 1.0 |
| Feels medicines will be effective | 1.0 (0.2–6.6) | 1.0 | 8.0 (1.0–72 ) | 0.08 |
| Complied with recent medication | 2.4 (0.7–8.6) | 0.2 | 1.4 (0.2–13) | 1.0 |
| Partner characteristics | ||||
| Age > 25 years | 1.5 (0.4–5.7) | 0.6 | 1.0 (0.9–1.1) | 1.0 |
| Secondary education or higher | 1.5 (0.5–4.3) | 0.4 | 0.9 (0.2–5.3) | 1.0 |
| Informed partner of test results | 2.4 (0.8–8.0) | 0.1 | 8.5 (1.5–50) | 0.02 |
| Partner willing to have HIV-1 test | 1.7 (0.6–4.9) | 0.3 | 7.0 (1.2–40) | 0.03 |
| Partner will support use of ARV | 3.0 (1.0–9.1) | 0.05 | 4.1 (0.8–21) | 0.09 |
| Place of delivery | ||||
| Home | 1 | |||
| Public hospital | 2.1 (0.5–9.0) | 0.3 | b | 0.1 |
| Private hospital | 11 (1.8–68) | 0.01 | ||
Compliance defined as ≥ 80% of doses.
Too few non-compliant women to calculate odds ratios. OR, Odds ratio; CI, confidence interval; MTCT, mother-to-child-transmission; ARV, antiretroviral agents.
Ninety-one per cent of the women on the HIVNET-012 regimen reported taking the maternal dose before delivery and 97% reported giving the infant dose. The median interval from taking the maternal dose to delivery was 6 h (range, 0.5–127 h) and from delivery to giving the infant dose the median was 12 h (range, 0.1–148 h). Eighty-seven per cent of the women used both the maternal and infant doses of the HIVNET-012 regimen and were considered compliant (Table 2).
Women compliant with the HIVNET-012 regimen were more likely to have informed their partners of their HIV-1 results (OR, 8.5; 95% CI, 1.5-0–50; P = 0.02) and to report that their partners would be willing to have an HIV-1 test (OR, 7.0; 95% CI, 1.2–40; P = 0.03) (Table 3).
Women were more likely to comply with the antenatal arm of the Thai-CDC regimen than the intrapartum arm (86% versus 44%; P = 0.001). Compliance with the antepartum component of the Thai-CDC and the HIVNET-012 regimen was similar (86% versus 87%; P = 0.9). However, overall more women were compliant with the HIVNET-012 regimen than with the Thai-CDC regimen (87% versus 41%; P < 0.001).
Validation of antenatal zidovudine compliance
Self-reported compliance was highly correlated to compliance measured by pill-counts (r = 0.7; P < 0.01) and electronic medication bottles (r = 0.6; P < 0.01). The number of women who were compliant with antenatal zidovudine by pill-counts and self-reports was similar (88% versus 86%; P = 0.6), but compliance as measured by electronic medication bottles was lower than by self-reports (79% versus 86%; P = 0.02). There were 17 (27%) women by pill-counts and 11 (17%) by electronic medication bottles who apparently took more than the recommended number of doses.
Focus group discussions
Among women who had not yet received their HIV-1 test results, knowledge of mother-to-child HIV-1 transmission was generally good but women were unaware of the use of antiretroviral drugs to prevent mother-to-child transmission of HIV-1 and were sceptical about their efficacy. When asked to rate in order of importance their concerns if they tested HIV-1 positive, infection of the newborn was fifth, after their own health and impending death, concerns about confidentiality, anger of their partner, and childcare for their living children. After delivery, most HIV-1 seropositive women reported that their spouses had been supportive on learning the women’s results although there were instances of separation, quarrels and domestic violence. Women described difficulties with the use of zidovudine during labour because of fear of mistreatment by midwives, uncertainty about the onset of labour, and not having the medications with them in the hospital. Some women reported sharing zidovudine with their partners. Women said they delivered at home due to lack of funds for hospital delivery, lack of transport, and fear of mistreatment by hospital staff particularly midwives who they felt would be reluctant to assist them during delivery if they were known to be HIV infected. Reasons given for poor compliance included forgetting, shock on learning their HIV-1 infection status, lack of privacy particularly with the Thai-CDC regimen, and missing clinic appointments for re-supply of their medicines.
HIV-1 transmission
Seventeen of 110 infants tested at 6 weeks after delivery were infected, a transmission rate of 15% (95% CI, 8–22%). There was a trend for lower transmission in the Thai-CDC arm compared to the HIVNET-012 arm (9% versus 22%; P = 0.07). Women who were compliant with the Thai-CDC regimen tended to have a lower transmission rate than those who were not compliant (0% versus 15%; P = 0.07). Among women who were on HIVNET-012, there was insufficient power to evaluate the effect of compliance on transmission.
Discussion
The field of prevention of mother-to-child transmission of HIV-1 with antiretroviral drugs is evolving rapidly and there are several efficacious regimens of varying complexity. The Thai-CDC and the HIVNET-012 regimens are currently the most commonly used regimens in resource-limited settings such as sub-Saharan Africa. Our findings of high compliance with the HIVNET-012 and the antenatal component of the Thai-CDC regimens suggest that even with limited resources, compliance levels similar to those in efficacy trials can be achieved in the field [1,4]. Although compliance with the intrapartum arm of the Thai-CDC regimen was low, it was higher than in the Ivory Coast trial, which showed good efficacy [4].
An important aim of our study was to identify correlates of compliance that might be modified to improve the effectiveness of these regimens in the field. Our findings show that there is need for greater involvement of partners. Women whose partners supported use of antiretroviral drugs were more likely to comply with the Thai-CDC and those whose partners were willing to have an HIV-1 test were more likely to comply with HIVNET-012 regimen. These findings are consistent with those of previous qualitative studies that support by peers and family members improves compliance [6,7]. Attitudes of health workers also need to be addressed as mistreatment and fear of mistreatment may have reduced compliance particularly with the Thai-CDC regimen. Women who delivered in private hospitals, where patients are generally treated well, were more likely to be compliant with the Thai-CDC regimen than those who delivered at home or in public hospitals. In focus group discussions fear of mistreatment was given as a reason for not complying with intrapartum zidovudine and for opting to deliver at home. Scepticism about the efficacy of drugs to prevent mother-to-child transmission of HIV-1 and the low priority women give prevention of infant HIV-1 on receiving test results may also contribute to poor compliance. To increase compliance, it will be important to disseminate information regarding the availability of antiretroviral drugs for prevention of mother-to-child transmission of HIV-1 and treatment of HIV/AIDS.
Our study was unique in that we validated self-reported compliance by using pill counts and electronic medication bottles. The three methods of measuring compliance were highly correlated and differences in the proportion of women who were compliant when the different methods were used were small, suggesting that the self-reports validly measured actual compliance. We found by pill-counts that some women used more than the prescribed number of pills and in focus group discussions some women reported sharing antiretroviral drugs with family members. Misuse of antiretroviral drugs may be a limitation of multi-dose regimens such as the Thai-CDC regimen in settings in which antiretroviral drugs are not accessible for other indications.
This study had inadequate power to detect a difference in the transmission rate between the two regimens, and the impact of compliance on regimen effectiveness. However, we did observe two interesting trends in our study. First, among women on the Thai-CDC regimen transmission tended to be lower in among those who were compliant (0% versus 15%; P = 0.07) suggesting the importance of ensuring high rates of compliance if this regimen is to be effective in the field. Second, despite a high reported compliance with the HIVNET-012 regimen, there tended to be a higher risk of transmission among women in this arm compared to those on the Thai-CDC regimen (22% versus 9%; P = 0.07). Because HIVNET-012 is a two-dose regimen, we could not use electronic bottles or pill counts to validate whether women actually used nevirapine and when they took the drug. In the PACTG 316 study, in which mothers received 200 mg nevirapine during labour, cord blood nevirapine concentrations < 100 ng/ml (potentially subtherapeutic) were found in 16% of all infants and 60% of infants whose mothers received nevirapine less than 2 h before delivery [8]. Unlike the HIVNET-012 and the PACTG 316 trials that administered nevirapine to women in hospital, women in our study were given nevirapine to take wherever they delivered, an approach that is currently being used in many programs. It will be important to determine the effectiveness of this approach in ensuring adequate nevirapine levels in infants at the time of delivery.
A limitation of our study is that we were not able to collect any information directly from the partners of these women; thus, information regarding partner support and willingness to have HIV-1 testing was surrogate information provided by the women. However, women’s perceptions of the attitudes of their partners may be more important in determining medication compliance than actual attitudes. Our study may overestimate compliance, by selecting more motivated women who came to the study clinic after referral and excluding those lost to follow-up before recruitment. Finally, although we observed some intriguing trends in differences in transmission risk to infants, the study did not have sufficient power to draw definitive conclusions regarding infant HIV-1 infection rates.
In the context of prevention of mother-to-child HIV-1 transmission, this is the first detailed study of antiretroviral compliance in a field setting in Africa. We have identified partner involvement and support, knowledge regarding use of antiretroviral drugs to prevent mother-to-child transmission of HIV-1, and attitudes of care providers as potentially modifiable correlates of non-compliance.
Acknowledgements
Sponsorship: J. N. Kiarie was a scholar in the International AIDS Research and Training Program, supported by the Fogarty International Center, National Institutes of Health (D43-TW00007).
References
- 1.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. on behalf of the Bangkok Collaborative Perinatal HIV Transmission Study Group Short-course zidovudine for perinatal HIV-1 transmission in Bangok, Thailand: a randomized controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. WHO Report on the global HIV/AIDS epidemic: Global HIV/AIDS and STD Surveillance. 2000 http://www.unaids.org/epidemic_update/report/index.html.
- 4.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire: a randomized trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 5.Statistics Division of the United Nations Secretariat and International Labour Office Indicators of income and economic activity. 1999 http://www.un.org/depts/unstd/social/inc-eco.htm.
- 6.Siegel K, Gorey E. HIV-infected women: barriers to AZT use. Soc Sci Med. 1997;45:15–22. doi: 10.1016/s0277-9536(96)00303-6. [DOI] [PubMed] [Google Scholar]
- 7.Misener T, Sowell R. HIV-infected women’s decisions to take antiretrovirals. West J Nurs Res. 1998;20:431–437. doi: 10.1177/019394599802000403. [DOI] [PubMed] [Google Scholar]
- 8.Microchnick M, Dorenbaum A, Blanchard S, Philip V, Coleen C, Richard G, et al. Pre-dose infant nevirapine (NVP) concentrations with the 2-dose intrapartum-infant NVP regimen [Abstract 101]. The 3rd conference on Global strategies for the prevention of HIV transmission from mothers to infants; Kampala, Uganda. September 2001. [Google Scholar]