Abstract
The purpose of the presented work is to examine the response of engineered cartilage to a transient, 2-week application of anabolic growth factors compared to continuous exposure in in vitro culture. Immature bovine chondrocytes were suspended in agarose hydrogel and cultured for 28 days (Study 1) or 42 days (Study 2) in chondrogenic media with TGF-β1, TGF-β3, or IGF-I either added for only the first 14 days in culture or added to the media for the entire study period. In both studies, there were no statistical differences in tissue mechanical or biochemical properties between the growth factors on day 14. In Study 1, growth factor removal led to a significant and drastic increase in Young’s modulus and GAG content compared to continuously exposed controls on day 28. In Study 2, both TGF-β1 and β3 led to significantly higher mechanical properties and collagen content versus IGF-I on day 42. These results indicate that the rapid rise in tissue properties (previously observed with TGF-β3 only) is not dependent on the type but rather the temporal application of the anabolic growth factor. These findings shed light on possible techniques to rapidly develop engineered cartilage tissue for the future treatment of osteoarthritis.
Keywords: tissue engineering, osteoarthritis, mechanical testing, agarose, chondrocyte
INTRODUCTION
Articular cartilage is the load-bearing material of diarthrodial joints, with excellent friction, lubrication, and wear-resistance characteristics 1. The tissue obtains its ability to resist high compressive loads from the balance between the osmotic swelling pressure of proteoglycans, highly charged macromolecules comprised of glycosaminoglycans (GAGs), and the tension in the collagen fibers that comprise the majority of the tissue matrix 2. Due to its avascular and aneural nature, articular cartilage possesses poor intrinsic healing capability, with localized damage to the tissue eventually worsening to severe damage to the cartilage that is classified as osteoarthritis (OA) 3. The current “gold standard” treatment for end-stage OA is total joint arthroplasty 4, 5 that involves the replacement of the damaged bone and cartilage with a synthetic implant. This procedure is extremely effective in relieving symptoms and restoring patient quality of life, but is usually prescribed for late-aged patients to be conservative with implant durability and lifespan. Therefore, for younger patients with localized cartilage damage that has not progressed to the entire joint, orthopaedic surgeons will employ techniques that aim to generate repair tissue (i.e., microfracture, autologous chondrocyte implantation) or to transplant healthy cartilage to the affected area (i.e., mosaicplasty) 6–8 . Though all of these techniques can minimize pain and restore joint motion in the short-term (≤ 2 years) post-operatively, new studies have discovered deteriorating clinical outcomes at longer follow-up times (>5 years postoperatively) for all techniques 9–11. The limitations of traditional treatment motivate cartilage tissue engineering efforts to create a biological replacement cartilage as a future treatment for osteoarthritis. Such a replacement tissue would grow and remodel with the patient, broadening the age range of eligible patients.
The general paradigm for tissue engineering is to combine a cell source, scaffold, and various stimuli to generate engineered tissue that replicates the in vivo properties of the native tissue 12. The majority of the cartilage engineering research performed in our laboratory utilizes primary chondrocytes (freshly isolated and without passaging) seeded in an agarose hydrogel scaffold. Agarose has been known for its well-documented ability to promote and maintain the chondrocyte phenotype in long-term in vitro cultures 13–15. Recently, however, clinical trials have shown the potential for agarose in cartilage regeneration, with an autologous human chondrocyte-laden agarose-alginate hydrogel in Phase III clinical trials (http://clinicaltrials.gov/ct2/show/NCT00945399) and two year follow-up data showing good clinical outcomes with no gross rejection of the scaffold apparent in the patients 16. To stimulate matrix formation, research has focused on the use of growth factors with serum-free culture media due to the variability of serum 17, 18 and its negative effects on chondrocyte phenotype 19. Three growth factors that have been found to stimulate matrix synthesis in engineered cartilage are transforming growth factor (TGF) isoform β1 and β3, as well as insulin-like growth factor I (IGF-I) 20–23. In addition, the combination of applied mechanical stimuli and the use of these growth factors has shown to synergistically increase matrix synthesis and tissue properties in engineered cartilage constructs 21, 24.
In general, the studies in the literature examining the effects of growth factors on engineered cartilage present the protein to the tissue continuously throughout culture. However, during the rapid matrix formation that occurs during cartilage development and wound healing, different growth factors are up/down-regulated at different times 25–28. Indeed, in our laboratory’s previous research, it was found that short-term, transient supplementation of TGF-β3 resulted in significantly greater matrix synthesis by agarose encapsulated chondrocytes than continuous supplementation in in vitro culture 24. Therefore, we sought to see if this phenomena transfers to other anabolic molecules and to confirm the importance of time in applied growth factor stimulation for cartilage tissue engineering. We hypothesized in this study that a 2-week application of TGF-β1, TGF-β3, or IGF-I followed by culture without any further exposure to any growth factors will lead to significantly greater matrix formation than continuous supplementation of the growth factors. This methodology was based on previous work by our laboratory and collaborators in the literature 23, 24. As tissue engineering is focused on the functional tissue properties and behavior, we evaluated the engineered tissue’s mechanical and biochemical properties over time in culture.
MATERIALS AND METHODS
Experimental Design
Two studies were performed to characterize the response of engineered cartilage to the growth factors TGF-β1, TGF-β3, and IGF-I. In both studies, engineered cartilage constructs were created from 2% weight/volume agarose containing 30 million chondrocytes / mL. These constructs were cultured in a serum-free media known to foster cartilage tissue formation 23. Study 1 examined the effects of continuous versus transient (2 week) growth factor treatment on the engineered cartilage mechanical and biochemical characteristics over 28 days to examine any similarities between the response of engineered cartilage to TGF-β1 and IGF-I to the known response to TGF-β3 23. Non-growth factor supplemented controls were cultured from a separate, multiple animal, mixed chondrocyte isolation (same as the protocol described below) that was later found to be similarly responsive to growth factor treatment (data not shown). Once these results were obtained and analyzed, Study 2 utilized separate, freshly cast constructs and examined the transient application of the growth factors over a 42 day period to study the differences between the growth factors with additional time in culture.
Creation and Culture of Agarose Constructs
Bovine articular chondrocytes were isolated via enzymatic digestion as described previously 29. Briefly, chondrocytes were isolated from calf carpometacarpal joints from an 11 hour digestion of full thickness cartilage slices in 390 u/mL type V collagenase (Sigma Aldrich, St. Louis, MO) using 7.5 mL / g tissue of high glucose DMEM with buffers 30 and 5% fetal bovine serum. Cells were resuspended and mixed with molten type VII agarose (Sigma) in phosphate buffered saline (PBS, Sigma) at 40°C to yield a 2% agarose suspension with 30 × 106 chondrocytes/mL. This suspension was cast between two glass plates and allowed to cool for 20 minutes. Disks were cored out (Ø4.0 × 2.3 mm) and cultured at 37°C and 5% CO2 in 35 mL of chondrogenic media (high glucose DMEM, 1% ITS+, 0.1 μM dexamethasone, 110 μg/mL sodium pyruvate, 50 μg/mL L-proline, 50 μg/mL ascorbate-2-phosphate, sodium bicarbonate, and antibiotics 23 ). For both studies described above in Experimental Design, either 10 ng/mL TGF-β3 23, 10 ng/mL TGF-β1 21, or 100 ng/mL IGF-I 20 (R&D Systems, Minneapolis, MN) was added with each media change. For Study 1, growth factor supplementation was given either continuously or for a 2 week period and then ceased. For Study 2, growth factors were added to the culture media for only the first 2 weeks in culture. For all studies, day 0 mechanical testing was performed prior to any growth factor treatment. Constructs (n=6 per group) were then removed from culture on every 2 weeks for evaluation of mechanical properties and biochemical composition.
Mechanical Testing
Mechanical testing was performed in unconfined compression between two impermeable platens in a custom material testing device as previously described 15. Constructs were first equilibrated under a creep tare load of ~0.02N followed by a stress relaxation test with a ramp displacement of 1 μm/sec to 10% strain (based on the measured post-creep thickness). After equilibrium was reached (2000 sec), a sinusoidal displacement of 40 μm amplitude was applied at 1Hz. Compressive Young’s modulus (EY) was determined from the equilibrium response of the stress relaxation test by dividing the equilibrium stress (minus the tare stress) by the applied strain. Dynamic modulus (G*) at 1Hz was calculated from the ratio of the measured stress amplitude and the applied strain amplitude of the dynamic loading. Following mechanical testing, samples were stored at −20°C for biochemistry or processed for histology (Study 2 only).
Histology
Samples were fixed in acid-ethanol-formalin for 48 hours at 4°C, dehydrated in a graded series of ethanol, cleared, embedded in Tissue Prep embedding media (Fisher Scientific, Pittsburgh, PA), and sectioned at 6 μm. Sections were then either stained with Safranin O (with a Fast Green counterstain) to view GAG distribution or Picrosirius Red to visualize the collagen network.
Biochemical analysis
The samples were thawed, weighed wet, lyophilized, reweighed dry, and digested for 16 h at 56°C with 1 mg/mL proteinase K (EMD Biosciences, San Diego CA) in 50 mM Tris buffered saline containing 1 mM EDTA, 1 mM iodoacetamide and 10 μg/ml pepstatin A (Sigma) 31. These digests were used to determine sample GAG content via the 1,9 dimethylmethylene blue (Sigma) dye-binding assay 32 and for DNA content via the PicoGreen assay (Invitrogen, Carlsbad, CA). The overall collagen content was measured using orthohydroxyproline (OHP) colorimetric assay 33. Collagen content was calculated by assuming a 1:10 OHP-to-collagen mass ratio 34. If necessary, assays were adapted for use in micro-titer plates. The GAG and collagen contents were normalized to the construct wet weight and DNA content.
Statistical Analysis
Statistics were performed using the Statistica (Statsoft, Inc., Tulsa, OK) software package. At least 6 samples were analyzed for each data point, with data reported as the mean and standard deviation. For mechanical and biochemical data, groups were examined using ANOVA with EY, GAG, collagen, and DNA as the dependent variables and culture time and growth factor as the independent variables. Tukey HSD post hoc tests were carried out with statistical significance set at α=0.05
RESULTS
Study 1: Continuous vs. Transient Growth Factor Supplementation
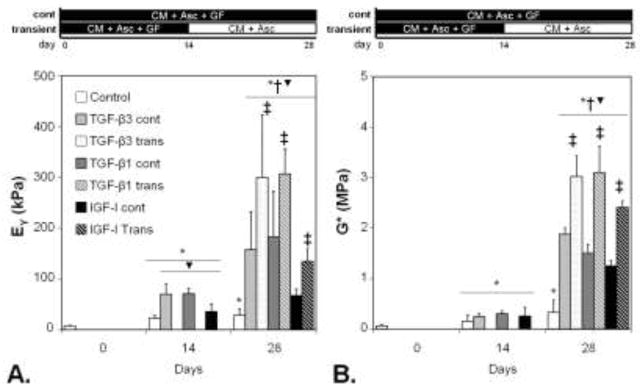
After 14 days in culture, all constructs increased in their compressive Young’s modulus (EY, a measure of a material’s resistance to a static compression at equilibrium) and dynamic modulus (G*, a measure of a material’s resistance to a dynamic, oscillatory compression) (Figure 1). Similar increases in the GAG and collagen content were also observed (Table 1). Constructs cultured in IGF-I possessed a lower average EY than TGF-cultured counterparts, but this was not statistically significant. The day 14 results of this study indicated that the growth factors at the concentrations selected for the study possessed a similar level of potency in spurring functional matrix development. On day 28, control (no growth factor) constructs did not significantly increase in mechanical properties compared to day 14, reaching values of EY = 28.6 ± 12.1 kPa and G* = 0.328 ± 0.247 MPa (Figure 1). However, GAG and collagen content did increase with additional time in culture (Table 1). All growth factor treated groups significantly increased in mechanical properties and measured matrix proteins. However, constructs released from growth factor treatment exhibited significantly greater tissue properties that were up to ~2x those of continuously treated tissues. The highest tissue properties for this study were achieved with transient growth factor treatment (Figure, Table 1, maximum EY ~300 kPa, G* ~3 MPa, GAG ~5.5 %ww, collagen ~2.5 %ww). DNA content for all constructs significantly increased by day 14, but remained steady afterwards (Table 1).
Figure 1.
Young’s modulus (A) and dynamic modulus (B) for engineered cartilage in Study 1, comparing continuous versus 2-week growth factor exposure. In Study 1, engineered cartilage tissues cultured with a 2-week exposure to growth factors exhibited significantly greater compressive Young’s modulus (EY, A) and dynamic modulus (normalized to wet weight, B) compared to continuous exposure groups on day 28. These results are consistent with 23. *p<0.05 vs. day 0, †p<0.05 vs. day 14, ‡p<0.05 vs. continuous treatment.
Table 1.
Biochemical composition of engineered cartilage cultured with continuous vs. transient supplementation of growth factors (Study 1). Transient supplementation of all growth factors resulted in significant increases in GAG and collagen content compared to continuous supplementation.
| Day | Group | GAG %ww | Collagen %ww | DNA %ww |
|---|---|---|---|---|
|
| ||||
| 0 | Control | 0.22%±0.03% | 0.00%±0.00% | 0.026%±0.005% |
|
| ||||
| 14 | Control | 1.06%±0.07%* | 0.19%±0.07%* | 0.045%±0.004%* |
| TGF-β3 cont | 2.02%±0.48%* | 0.87%±0.10%*▼ | 0.055%±0.008%* | |
| TGF-β1 cont | 1.81%±0.56%* | 0.74%±0.12%*▼ | 0.049%±0.005%* | |
| IGF-I cont | 1.16%±0.17%* | 0.75%±0.28%*▼ | 0.051%±0.003%* | |
|
| ||||
| 28 | Control | 3.00%±0.45%*† | 1.65%±0.26%*† | 0.056%±0.010%* |
| TGF-β3 cont | 3.00%±0.40%*† | 2.02%±0.14%*† | 0.045%±0.003%* | |
| TGF-β3 trans | 4.10%±0.90%*†‡ | 2.41%±0.13%*†‡ | 0.047%±0.003%* | |
| TGF-β1 cont | 3.46%±0.23%*† | 1.90%±0.18%*† | 0.056%±0.011%* | |
| TGF-β1 trans | 5.60%±1.30%*†‡▼ | 2.31%±0.21%*†‡ | 0.049%±0.003%* | |
| IGF-I cont | 2.80%±0.19%*† | 1.98%±0.11%*† | 0.049%±0.005%* | |
| IGF-I trans | 4.50%±0.10%*†‡▼ | 2.05%±0.08%*†‡ | 0.051%±0.008%* | |
p<0.05 vs. day 0,
p<0.05 vs. day 14,
p<0.05 vs. continuous treatment
p<0.05 vs. control of the respective time point.
Study 2: Transient Growth Factor Supplementation in Extended Culture
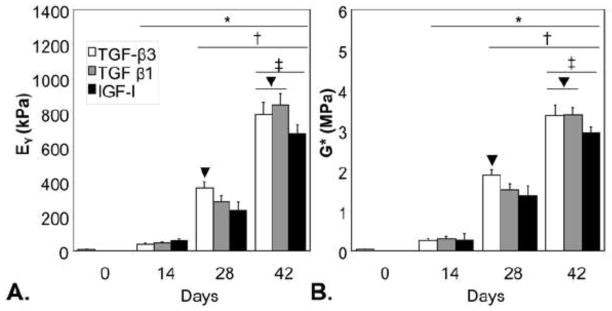
Using a separate batch of chondrocytes isolated from different animals from Study 1, engineered cartilage constructs were created and cultured up to day 42. Compared to day 0 values, constructs after 14 days of growth factor treatment significantly increased in EY (~45 vs. ~8 kPa), G* (~0.15 vs. ~0.03 MPa), GAG (~2.5 vs. 0.1 %ww), and collagen content (~1 vs. ~0.2 %ww), with no significant differences between experimental groups (Figure 2, Table 2; p<0.05). On day 28, constructs exhibited a ~5–10 fold increase in mechanical properties depending on growth factor treatment, with the TGF-β3 group possessing the highest properties of all groups (Figure 2, Table 2; p<0.05). With an additional 2 weeks in culture, both TGF-β treated tissues exhibited significantly higher EY, G*, and collagen content than IGF-I treated tissues (p<0.05) and attained physiologic values for EY and GAG content compared to native cartilage 35 . DNA was found to increase after 14 days in culture ~40% in all culture conditions and held steady afterwards (Table 2).
Figure 2.
The Young’s modulus (EY, A) and dynamic modulus (G*, B) of tissue engineered cartilage culture for Study 2. Constructs treated with TGF-β3 possessed the highest material properties on day 28 (p<0.05). By day 42, tissues treated with IGF-I possessed significantly lower properties than either TGF-β group (p<0.05). *p<0.05 vs. day 0, †p<0.05 vs. day 14, ‡p<0.05 vs. day 28, ▼p<0.05 vs. other groups.
Table 2. The biochemical composition of engineered cartilage in Study 2.
Day 42 constructs cultured with TGF-β possessed significantly greater collagen than IGF-I tissues.
| Day | Group | GAG %ww | Collagen %ww | DNA %ww |
|---|---|---|---|---|
|
| ||||
| 0 | No GF | 0.10%±0.03% | 0.21%±0.27% | 0.029%±0.005% |
|
| ||||
| 14 | TGFB3 | 2.02%±0.48%* | 0.87%±0.10%* | 0.045%±0.006%* |
| TGFB1 | 2.68%±0.91%* | 0.74%±0.12%* | 0.051%±0.006%* | |
| IGF-1 | 3.24%±0.27%* | 0.75%±0.28%* | 0.049%±0.005%* | |
|
| ||||
| 28 | TGFB3 | 4.19%±1.01%*† | 2.02%±0.34%*† | 0.050%±0.010%* |
| TGFB1 | 5.62%±0.84%*† | 1.95%±0.18%*† | 0.050%±0.003%* | |
| IGF-1 | 4.24%±1.31%*† | 2.05%±0.37%*† | 0.047%±0.006%* | |
|
| ||||
| 42 | TGFB3 | 7.92%±1.77%*†‡ | 3.12%±0.42%*†‡▼ | 0.047%±0.006%* |
| TGFB1 | 7.41%±0.81%*†‡ | 3.19%±0.39%*†‡▼ | 0.048%±0.005%* | |
| IGF-1 | 6.92%±0.62%*†‡ | 2.28%±0.38%*†‡ | 0.050%±0.008%* | |
p<0.05 vs. day 0,
p<0.05 vs. day 14,
p<0.05 vs. day 28,
p<0.05 vs. other groups.
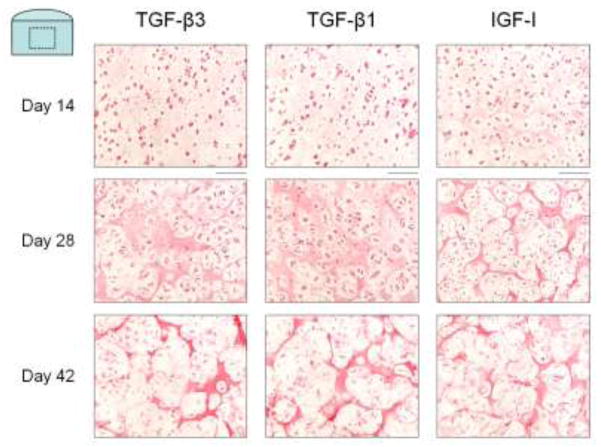
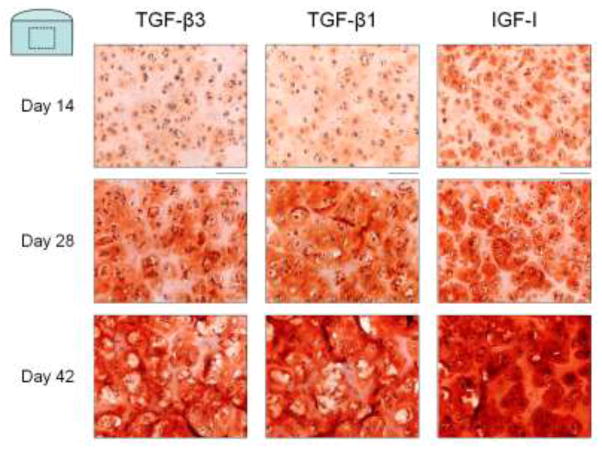
Safranin O histology (Figure 3) revealed intense localization of proteoglycans to the pericellular region for constructs cultured with IGF-I at day 14 through to day 42. Constructs cultured with either TGF-β isoform exhibited more diffuse proteoglycan distribution throughout time in culture. Picrosirius Red histology (Figure 4) of constructs cultured with IGF-I showed formation of a collagen matrix with a sharper, more defined border between chondrocyte clusters at day 14 and day 28. At these time points, constructs cultured with either TGF-β1 or TGF-β3 exhibited qualitatively more diffuse collagen staining. By day 42, however, no qualitative differences were noted in the distribution or structure of collagen staining between the growth factor groups.
Figure 3.
Representative images of Safranin O histology for Study 2. IGF-I treated constructs (right) had greater pericellular proteoglycan deposition throughout the culture duration compared to TGF-β3 (left) and TGF-β1 (middle) treated constructs. Scale bar = 100 μm.
Figure 4.
Representative images of Picrosirius Red histology for Study 2. The collagen deposition of IGF-I treated constructs (right) appeared to form more defined borders around the chondrocytes on days 14 and 28 than TGF-β3 (left) and TGF-β1 (middle) treated constructs. No qualitative differences in organization or distribution were noted on day 42. Scale bar = 100 μm.
DISCUSSION
The primary purpose of this study was to analyze the effects of a transient vs. prolonged exposure of anabolic growth factors on tissue engineered cartilage with the goal to trigger the initial steps of rapid tissue remodeling that occurs during development and wound healing. In well-defined, serum-free chondrogenic media, all three growth factors stimulated cartilage tissue formation by day 28 superior to previously attained properties with serum-based media 30. When the growth factor application was ceased, all of the tissue constructs responded with a rapid increase in tissue properties resulting in significantly higher tissue properties than continuously exposed controls, affirming our hypothesis. For the 2-week exposure, the similar trends between the three growth factors tested implies that the rapid increase in tissue properties is not dependent on the anabolic growth factors used in this study, but rather the temporal application itself. This may be related to the transient profile of growth factors observed in vivo during wound healing 25–27 or during fetal development 28. This “on-off switch” mechanism for rapid matrix synthesis is not well studied in the literature and opens a new avenue for further research.
In our data, we observed that similar increases in the gross composition of GAG and collagen across the TGF-β and IGF-I groups did not necessarily correlate to similar increases in mechanical properties. Though the effect of the transient exposure was much greater than the effect of the different growth factors, this is explained by findings in the literature that IGF-I and TGF-β isoforms stimulate functional matrix formation in chondrocytes/cartilage differently and result in differing changes in the mechanical properties 36, 37. The use of TGF-β1 or -β3 led to tissues with significantly greater equilibrium and dynamic compressive properties and collagen content compared to those cultured with IGF-I after 42 days in culture (Study 2). This difference can be explained by the well-known increase in collagen synthesis and collagen cross-link formation that leads to increased cartilage tensile properties (which play a role in the dynamic modulus 38) that occurs with administration of TGF-β isoforms but not with IGF-I 36, 37. On a morphological scale, IGF-I has been shown in the literature to increase pericellular matrix formation whereas TGF-β was found to increase extracellular matrix formation 39. This was apparent in the proteoglycan staining of Study 2 constructs (Figure 3). Therefore, to explain our data, it would appear that changes in the type, size, structure, and/or spatial location of the matrix components are responsible for the disparity between the gross biochemical composition and the mechanical properties in our studies. Overall, the results of our studies confirm the differences in the stimulation of chondrocytes with exposure to TGF-β isoforms and IGF-I, but show that the action of the growth factors can be further modulated by the timing of their exposure.
Comparing the two TGF-β isoforms, TGF-β3 induced higher mechanical properties than TGF-β1 on day 28 in Study 2, but no differences were observed in the mechanical properties in Study 1, the histology of Study 2, or in the biochemical content in either study. In addition, day 42 results for both TGF-β isoforms were statistically similar. Though little literature exists for chondrocyte/cartilage models, TGF-β3 can reduce scar tissue and induce more natural tissue regeneration in dermal wound healing models as compared to TGF-β1 40. It is likely that similar, differential matrix formation may be occurring within the engineered cartilage in response to the TGF isoforms as well. Further studies are needed to qualify the exact differences in the response of chondrocytes between TGF β1 and β3. Likely there are structural changes and changes in synthesis of other important cartilage proteins such as link protein and cartilage oligomeric matrix protein (COMP). Interestingly, in other preliminary studies (not shown) it was found that a second phase of TGF-β addition and removal did not re-stimulate matrix synthesis by the chondrocytes. This may be due to previously observed modulation of TGF-β signals by the presence of elaborated pericellular matrix 41.
The results of this study strongly indicate that a transient application of anabolic growth factors elicits greater matrix formation over prolonged supplementation. As tissue engineering progresses towards a clinical application, this rapid tissue growth with only 2 weeks of growth factors can lead to quicker tissue production with the added benefit of reduced production costs. Clearly, the rapid tissue growth in this study will not occur with growth factors or cytokines that elicit a response other than matrix formation (e.g., FGF-2, PDGF 42, 43). Our laboratory has administered IL-1α, which initiates a catabolic response from chondrocytes, to engineered cartilage and found that the cellular response depended heavily on when the cytokine was added during the culture period 44. In contrast to our results presented in this manuscript, Kalpackci, et al. found no beneficial effect of intermittent TGF-β1 supplementation on the tissue properties of engineered fibrocartilage constructs 45, implying a tissue-specific, temporal effect of growth factors. The age of the cells may also play a role as experiments in our laboratory with mature bovine and canine chondrocytes found no benefit of a transient growth factor treatment 46–48.
It is clear that the macro-scale measurements utilized in the present work, though insightful, are not sufficient to fully elucidate the differences occurring within the cells and tissues with exposure to TGF-β1, TGF-β3, and IGF-I. Molecular biology techniques are needed to understand the signal transduction underlying the observed results. In addition, determination of specific matrix molecules, size of matrix molecules, and imaging techniques to observe structure (e.g., electron microscopy to view matrix fiber structure, proteoglycan-collagen interactions) and quantitative matrix distribution (e.g., FT-IRIS 49) would allow better understanding of the differences in the tissue formed between the different growth factors used. This need is evident in our study as the significant increases in proteoglycan and collagen in the control specimens over time did not lead to increased mechanical properties in contrast to growth factor treated tissues. This implies that although the total content of the non-growth factor treated controls is the same, the type, size, and/or structure of the matrix proteins are likely different than those produced in constructs cultured with growth factors.
In conclusion, our results presented in this manuscript indicate that the rapid rise in tissue properties previously observed with the 2-week application of TGF-β3 23, 24 is not dependent on the type but rather the temporal application of the anabolic growth factor. The type of growth factor utilized appears to influence the magnitude of tissue properties and likely the matrix composition and structure. The mechanisms behind these results are likely related to the observed rapid matrix formation during wound healing and fetal development. As cartilage tissue must be mechanically competent to perform its function in vivo, the presented results shed light on possible techniques to rapidly develop a load-bearing engineered cartilage tissue for the future treatment of osteoarthritis.
Acknowledgments
Research funding was provided by the National Institutes of Health (AR46568, AR52871 and AR53530).
References
- 1.Mow VC, Ateshian GA, Ratcliffe A. Anatomic form and biomechanical properties of articular cartilage of the knee joint. In: Finerman GAM, Noyes FR, editors. Biology and Biomechanics of the Traumatized Synovial Joint, The Knee as a Model. AAOS; 1992. pp. 55–81. [Google Scholar]
- 2.Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223–30. doi: 10.1016/0304-4165(91)90270-q. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 4.Scuderi GR, Insall JN. Total knee arthroplasty. Current clinical perspectives. Clin Orthop Relat Res. 1992:26–32. [PubMed] [Google Scholar]
- 5.Slover JD, Rubash HE. Hip resurfacing arthroplasty: time to consider it again? No Instr Course Lect. 2008;57:267–71. [PubMed] [Google Scholar]
- 6.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001:S362–9. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 7.Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 9.Solheim E, Hegna J, Oyen J, Austgulen OK, Harlem T, Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 17:84–7. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Moseley JB, Jr, Anderson AF, Browne JE, Mandelbaum B, Micheli LJ, Fu F, Erggelet C. Long-term durability of autologous chondrocyte implantation : A multicenter, observational study in U.S. patients. Am J Sports Med. 2010;38:238–246. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- 11.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–63. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 12.Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop. 2001:S295–305. [PubMed] [Google Scholar]
- 13.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 14.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–58. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 15.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 16.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 17.Honn KV, Singley JA, Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975;149:344–7. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- 18.Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18:576–84. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- 19.Ng KW, DeFrancis JG, Kugler LE, Kelly TA, Ho MM, O'Conor CJ, Ateshian GA, Hung CT. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008;35:433–8. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909–15. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 21.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 22.Weisser J, Rahfoth B, Timmermann A, Aigner T, Brauer R, von der Mark K. Role of growth factors in rabbit articular cartilage repair by chondrocytes in agarose. Osteoarthritis Cartilage. 2001;9(Suppl A):S48–54. doi: 10.1053/joca.2001.0444. [DOI] [PubMed] [Google Scholar]
- 23.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–34. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on wound healing: immunolocalisation of growth factors and their receptors in a murine incisional model. J Anat. 1997;190(Pt 3):351–65. doi: 10.1046/j.1469-7580.1997.19030351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9:382–9. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 27.Theoret CL, Barber SM, Moyana TN, Gordon JR. Expression of transforming growth factor beta(1), beta(3), and basic fibroblast growth factor in full-thickness skin wounds of equine limbs and thorax. Vet Surg. 2001;30:269–77. doi: 10.1053/jvet.2001.23341. [DOI] [PubMed] [Google Scholar]
- 28.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 29.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134–41. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Riesle J, Hollander AP, Langer R, Freed LE, Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998;71:313–27. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 33.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 34.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–32. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mankin HJ, Mow VC, Buckwalter JA, Iannotti JP, Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. Biology and Biomechanics of the Musculoskeletal System. Rosemont: American Academy of Orthopaedic Surgeons; 2000. pp. 443–470. [Google Scholar]
- 36.Williams GM, Dills KJ, Flores CR, Stender ME, Stewart KM, Nelson LM, Chen AC, Masuda K, Hazelwood SJ, Klisch SM, Sah RL. Differential regulation of immature articular cartilage compressive moduli and Poisson's ratios by in vitro stimulation with IGF-1 and TGF-beta1. J Biomech. 2010;43:2501–7. doi: 10.1016/j.jbiomech.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Regulation of immature cartilage growth by IGF-I, TGF-beta1, BMP-7, and PDGF-AB: role of metabolic balance between fixed charge and collagen network. Biomech Model Mechanobiol. 2008;7:263–76. doi: 10.1007/s10237-007-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84–93. doi: 10.1115/1.1531656. [DOI] [PubMed] [Google Scholar]
- 39.van Osch GJ, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential effects of IGF-1 and TGF beta-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthritis Cartilage. 1998;6:187–95. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 40.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 41.van Osch GJ, van der Veen SW, Buma P, Verwoerd-Verhoef HL. Effect of transforming growth factor-beta on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998;17:413–24. doi: 10.1016/s0945-053x(98)90101-9. [DOI] [PubMed] [Google Scholar]
- 42.Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G, Freed LE. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8:73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 43.Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294:149–54. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 44.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008;14:1721–30. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 45.Kalpakci KN, Kim EJ, Athanasiou KA. Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 2011;7:1710–8. doi: 10.1016/j.actbio.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian LM, Angione SL, Lima EG, Ng KW, Ateshian GA, Hung CT. Tissue-engineered cartilage constructs using mature bovine chondrocytes: Effects of temporal exposure to growth factors and dynamic deformational loading. Trans Orthop Res Soc. 2006:1480. [Google Scholar]
- 47.Ng KW, Lima EG, Bian L, O'Conor CJ, Jayabalan PS, Stoker AM, Kuroki K, Cook CR, Ateshian GA, Cook JL, Hung CT. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–51. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng KW, O'Conor CJ, Lima EG, Lo SB, Ateshian GA, Cook JL, Hung CT. Primed mature canine chondrocytes can develop an engineered cartilage tissue with physiologic properties. Trans Orthop Res Soc. 2008:599. [Google Scholar]
- 49.Kim M, Bi X, Horton WE, Spencer RG, Camacho NP. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: histologic and biochemical correlations. J Biomed Opt. 2005;10:031105. doi: 10.1117/1.1922329. [DOI] [PubMed] [Google Scholar]