Platelet-derived growth factor (PDGF) signaling is implicated in a wide range of diseases, and PDGF drives the pathological responses in many vascular disorders and fibrotic diseases such as atherosclerosis, restenosis, pulmonary arterial hypertension, and pulmonary fibrosis. In vascular smooth muscle cells (VSMC), PDGF is a potent mitogen which promotes proliferation and migration and contributes to pathologic vascular remodeling. Recent studies have demonstrated a role for PDGF in STIM1/Orai1-mediated Ca2+ influx in VSMC1. Regulation cytosolic Ca2+ concentration ([Ca2+]cyt) is critical for many cellular processes. A rise in [Ca2+]cyt is a major trigger for VSMC proliferation and contraction. One of the major routes of Ca2+ entry into cells is through store-operated Ca2+ (SOC) channels. Upon depletion of Ca2+ from the stores (sarcoplasmic or endoplasmic reticulum, SR/ER), a Ca2+ deficiency signal is transmitted to the SOC channels in the plasma membrane via stromal interaction molecule 1 (STIM1). This causes the SOC to open allowing Ca2+ to flow into the cytosol, a process referred to as store-operated Ca2+ entry (SOCE). The cytosolic Ca2+ is then sequestered into the stores by the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA), thus replenishing the stores. Studies have demonstrated a role for Ca2+ release-activated Ca2+ (CRAC) channels in SOCE, with STIM1 and Orai1 as the major components2.
The majority of STIM1 is expressed in the SR/ER membrane where it senses decreased Ca2+ concentration in the SR/ER when inositol 1,4,5-triphosphate (IP3)-mediated activation of IP3receptor induces Ca2+ release. STIM1 then undergoes a conformational change allowing STIM1 to multimerize and translocate to discrete puncta near the plasma membrane where it interacts with and activates Orai1, the pore-forming unit of the CRAC channel3. In this issue, McKeown et. al. investigate the relevance and importance of STIM1/Orai1 redistribution and clustering in PDGF-mediated Ca2+ entry through Orai1 channels. The redistribution and clustering of STIM1 and Orai1 was observed in this study using fluorescently-tagged STIM1 and Orai1. The authors demonstrate that, under normal conditions, Orai1 localizes to intracellular vesicles and has a reasonably uniform distribution in the plasma membrane except in dynamic membrane structures such as membrane ruffles or spiny protrusions, where Orai1 is highly expressed. Additionally, they show that STIM1 can be found in the SR/ER and associated with comet-like structures under non-stimulated conditions. Passive depletion of Ca2+ stores with the SERCA inhibitor thapsigargin (TG) causes significant redistribution and clustering of STIM1 and Orai1 in VSMC, which is clearly demonstrated with the fluorescently-tagged STIM1 and Orai1 used in this study. Interestingly, this study shows that PDGF fails to cause significant STIM1/Orai1 redistribution and that PDGF activates Orai1 channels in the absence of STIM1/Orai1 clustering. The data indicate that PDGF causes significant STIM1/Orai1 redistribution when Orai1 channels are blocked, either by Synta 66 or by two loss-of-function mutations (R91W and E106A). Furthermore, the authors show that under conditions of SR/ER stress (such as acidosis), PDFG-mediated Ca2+ influx is associated with redistribution and clustering of STIM1 and Orai1. Based on these data, the authors conclude that a) PDGF-mediated Ca2+ entry through Orai1 channels is independent of clustering of STIM1 and Orai1, b) non-clustered Orai1 channels serve to maintain Ca2+ in the stores and prevent store depletion, c) redistribution and clustering of STIM1 and Orai1 becomes apparent and important when Orai1 channel activity is compromised by Orai1 channel inhibitors or Orai1 mutations, and d) redistribution and clustering of Orai1 and STIM1 are important for situations where there is SR/ER stress, such as acidosis, and a risk of store depletion.
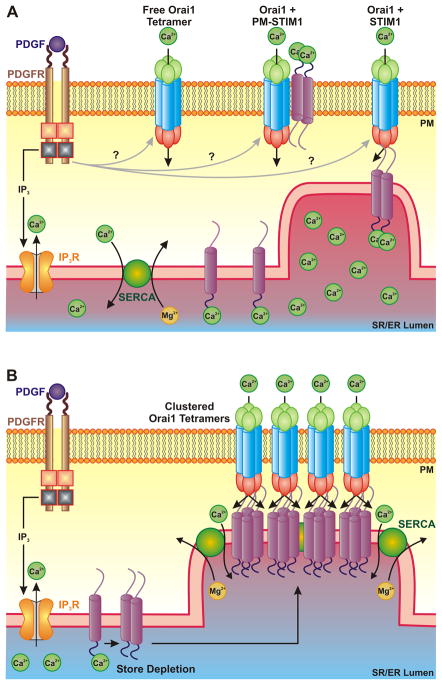
Given that STIM1 is an SR/ER Ca2+ sensor whose response to store depletion is redistribution and clustering, McKeown et. al. suggest that PDGF causes Ca2+ release, but not store depletion, because STIM1 does not cluster in response to PDGF exposure. The authors propose that non-clustering Orai1 channels maintain the stores replete preventing STIM1 clustering. While the exact mechanism by which PDGF activates Orai1 channels without redistribution and clustering is still an unknown, there are several possibilities. It is possible that PDGF binding to the PDGF receptor, a receptor tyrosine kinase, may release a second messenger which activates Orai1 (Figure 1A). This indicates that Orai1 channels may also function as a receptor-operated Ca2+ channel (ROC)-like channel following activation by PDGF. Under conditions which cause store depletion, such as acidosis, inhibition of the SERCA pump, or in the absence of extracellular Ca2+, Orai1 channels cluster with STIM1 and participates in classical SOCE. This suggests that the roles of these voltage-independent Ca2+ channels (e.g., ROC and SOC) may overlap. Indeed, several studies have shown previously that TRPC channels are important for both receptor- and store-operated Ca2+ entry4, 5. That these channels have overlapping functions underlies the critical importance of regulation of Ca2+ to the cell.
Figure 1. PDGF-mediated activation of Orai1 channels.
A. Under normal conditions, PDGF activates Orai1 channels without redistribution or clustering with STIM1 possibly by activating free Orai1 channel, by activating Orai1 channels coupled to plasma membrane localized-STIM1, or by activating constitutively co-assembled STIM1/Orai1 channels. B. Under conditions of store depletion or SR/ER stress, PDGF exposure leads to STIM1/Orai1 clustering and classical SOCE. This may involve co-clustering with SERCA in order for more efficient refilling of the Ca2+ stores. PM, plasma membrane; SR/ER, sarcoplasmic reticulum/endoplasmic reticulum.
Another possibility for the mechanism by which PDGF activates Orai1 in the absence of clustering involves STIM1. McKeown et. al. provide evidence that inhibition of STIM1 with siRNA blocks PDGF-mediated Ca2+ influx suggesting that even in the absence of clustering, activation of Orai1 still requires STIM1. It is possible that non-clustering Orai1 channels interact with STIM1 located on the plasma membrane (Figure 1A). STIM1 has been previously shown to be localized in the plasma membrane where it can interact with TRPC1 and contribute to Ca2+ influx6. The authors suggest it is possible that a fraction of Orai1 and STIM1 proteins are constitutively co-assembled in non-clustered units which do not function until being activated by PDGF (Figure 1A). A recent study identified a splice variant of STIM1 in skeletal muscle which forms permanent clusters with Orai17. PDGF may activate these Orai1/STIM1 units under normal conditions (e.g. 1.5 mM extracellular Ca2+, no SR/ER stress or store depletion) without redistribution or clustering of Orai1 and STIM1 (Figure 1A).
Another interesting concept proposed by McKeown et. al. is the dual response of Orai1 channels to PDGF. Under normal conditions (e.g. 1.5 mM extracellular Ca2+, no SR/ER stress or store depletion), PDGF activates Orai1 channels without clustering. However, PDGF causes STIM1/Orai1 clustering under conditions of store depletion or ER stress. If Orai1 channels are open and allowing Ca2+ entry without clustering in response to PDGF, what advantage does STIM1/Orai1 clustering bring under conditions of ER stress? The authors propose this serves to maximize efficiency of store-filling and minimize store depletion. This suggests that there may be a mechanism by which STIM1/Orai1 clusters allow for direct funneling of Ca2+ to the SERCA pump in order to replenish the Ca2+ stores and prevent an increase in cytosolic Ca2+ (Figure 1B). Release of Ca2+ from the stores has been shown to alter the distribution of SERCA and the IP3 receptor8. STIM1 and Orai1 may also cluster with SERCA in the SR/ER puncta to more efficiently funnel Ca2+ directly into the SR/ER under conditions of store depletion or ER stress (Figure 1B).
In summary, the data from McKeown et. al. provide evidence of PDGF-mediated activation of Orai1 channels without redistribution or clustering with STIM1. Under normal conditions PDGF activates Orai1 channels in the absence of clustering; however, during times of store depletion or ER stress, STIM1/Orai1 clustering occurs. This dynamic regulation of Orai1 activation may be important for efficiently replenishing the Ca2+ stores and demonstrates the importance of regulation of Ca2+ signaling.
References
- 1.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol. 2009;298:C993–1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by STIM-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 5.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. The Journal of Cell Biology. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermassen E, Van Acker K, Annaert WG, Himpens B, Callewaert G, Missiaen L, De Smedt H, Parys JB. Microtubule-dependent redistribution of the type-1 inositol 1,4,5-trisphosphate receptor in A7r5 smooth muscle cells. Journal of Cell Science. 2003;116:1269–1277. doi: 10.1242/jcs.00354. [DOI] [PubMed] [Google Scholar]