SUMMARY
In sub-Saharan Africa, over 22 million people are estimated to be co-infected with both helminths and HIV-1. Several studies have suggested that de-worming individuals with HIV-1 may delay HIV-1 disease progression, and that the benefit of de-worming may vary by individual helminth species. We conducted a systematic review and meta-analysis of the published literature to determine the effect of treatment of individual helminth infections on markers of HIV-1 progression (CD4 count and HIV viral load). There was a trend towards an association between treatment for Schistosoma mansoni and a decrease in HIV viral load (Weighted mean difference (WMD)=−0·10; 95% Confidence interval (CI): −0·24, 0·03), although this association was not seen for Ascaris lumbricoides, hookworm or Trichuris trichiura. Treatment of A. lumbricoides, S. mansoni, hookworm or T. trichiura was not associated with a change in CD4 count. While pooled data from randomized trials suggested clinical benefit of de-worming for individual helminth species, these effects decreased when observational data were included in the pooled analysis. While further trials are needed to confirm the role of anthelmintic treatment in HIV-1 co-infected individuals, providing anthelmintics to individuals with HIV-1 may be a safe, inexpensive and practical intervention to slow progression of HIV-1.
Keywords: Helminth, HIV-1, co-infection, meta-analysis, Kenya
INTRODUCTION
Over 2 billion individuals are infected with one or more helminth species, making them among the most prevalent infections of humans (Hotez et al. 2006; de Silva et al. 2003). In many resource-limited settings, these helminth infections directly contribute to significant morbidity, particularly among young children and pregnant women (WHO, 2010). In addition, growing evidence suggests important immunological interactions between helminths and other diseases, including HIV-1, malaria and tuberculosis (Fincham et al. 2003; Mwangi et al. 2006; Brooker et al. 2007; Elias et al. 2007, 2008; Troye-Blomberg and Berzins, 2008; Labeaud et al. 2009; Moreau and Chauvin, 2010; Roussilhon et al. 2010). In sub-Saharan Africa, where the vast majority of HIV-1 infected individuals reside, over 22 million people are estimated to be co-infected with both helminths and HIV-1 (Fincham et al. 2003; UNAIDS, 2007). Evidence from several studies suggests that de-worming individuals co-infected with HIV-1 may delay HIV-1 disease progression by reducing HIV-1 viral load and/or increasing CD4 counts (Wolday et al. 2002; Brown et al. 2004; Kallestrup et al. 2005; Modjarrad et al. 2005; Nielsen et al. 2007; Walson et al. 2008, 2009). The magnitude of changes in HIV-1 viral load documented in both individual randomized trials and in a pooled analyses of all available data (0·3–0·5 log10 copies/mL) may increase the annual risk of progression to an AIDS-defining illness or death by as much as 25%–44% (Modjarrad et al. 2008; Baggaley et al. 2009). It is plausible that providing anthelmintics to individuals with HIV-1 may be a safe, inexpensive and practical intervention to slow progression of HIV-1.
Variability in the geographical distribution of individual helminth species, as well as species-specific differences in the host response to helminth infection, may influence the relative benefit of de-worming in co-infected individuals (Brooker et al. 2000, 2009; Anthony et al. 2007; Hewitson et al. 2009; Bourke et al. 2010; WHO, 2010). Two randomized trials examining the effect of treating helminths on markers of HIV-1 progression have suggested significant benefit with the treatment of specific helminth species. In a randomized double-blind, placebo-controlled trial conducted in Kenya, significantly higher CD4 counts and a trend towards lower plasma HIV-1 viral load were reported following treatment with albendazole among individuals with documented Ascaris lumbricoides at baseline (Walson et al. 2008). In this study, significant benefit was not observed following the treatment of other species of soil-transmitted helminths. In addition, another randomized trial conducted in Zimbabwe also suggested a significant attenuation of plasma HIV-1 viral load increase following the treatment of Schistosoma mansoni (Kallestrup et al. 2005). It is unclear whether these differences in effect associated with the treatment of individual helminth species represent species-specific differences in the host immune response to de-worming, differences in the effectiveness of treatment regimens used for each species, or a failure to adequately power these studies to detect other important species-specific differences in treatment effects. It is important to examine the effect of eradicating individual helminth species on markers of HIV-1 disease transmission in order to target de-worming strategies to provide maximum benefit to co-infected individuals.
We previously reported data from a systematic review and meta-analysis of the published literature documenting benefit following helminth treatment among HIV-1 co-infected individuals (Walson et al. 2009). Given the possible differences in effect observed with individual helminth species, we have conducted a meta-analysis of these data to determine the effect of treatment of individual helminth infections on markers of HIV-1 progression. In addition, we examined strengths and limitations of available studies and suggest important considerations for the design and methodology of future studies evaluating the impact of de-worming among HIV-1-infected individuals. Determining if the effect of de-worming on markers of HIV-1 progression varies by helminth species may improve our understanding of host immunity and inform the development of effective interventions for HIV-1/helminth co-infected individuals.
MATERIALS AND METHODS
Searching strategy
We conducted a systematic review of the literature using the methods of the Cochrane Collaboration (Higgins and Green, 2009). The full text of the search strategy employed has been published previously (Walson and John-Stewart, 2008). The search was updated in January 2010 using MEDLINE (1966–2010), EMBASE (1980–2010), CENTRAL online (1980–2010) and AIDSearch online (1980–2010) to identify research studies that investigated the association between helminth co-infection and HIV-1 disease progression. The references of all identified articles, as well as review articles were examined to locate additional studies not identified during the computerized search. Studies published in all languages and in all countries were considered.
Selection
Each study was reviewed independently by the authors and included if it met all 5 of the following criteria: (1) study population included HIV-1 sero-positive individuals with documented helminth co-infection; (2) helminth infection was documented by direct stool microscopy, concentration techniques, other microscopic methods (such as Kato-Katz), culture of stool samples, antigen testing methods (e.g. ELISA kits), modified Knott’s concentration methods for microfilaria, or other immunochromatographic testing methods; (3) anthelmintic therapy was defined as any intervention approved for use in the eradication of helminth infection in humans, including benzimidazoles, ivermectin, praziquantel, diethylcarbamazine, bithionol, oxamniquine, pyrantel and nitazoxanide; (4) studies utilized an appropriate control group including comparison to another anthelmintic drug, placebo, no treatment, or helminth uninfected individuals; and (5) study design was limited to either a randomized control trial comparing treatment to placebo among helminth-infected individuals, or a prospective cohort design comparing treatment among helminth-infected individuals to non-treated, non-helminth-infected individuals. The above criteria were selected to increase comparability between the studies.
Quality assessment
Quality assessments of observational and randomized trials have been previously published (Walson and John-Stewart, 2008; Walson et al. 2009). Quality of the studies varied both within study design and between study designs. Randomized studies were of the highest quality of included studies. Quality scores were not assigned.
Data abstraction
Separate standardized data abstraction forms were used for randomized trials and cohort studies. Authors JW and GJS independently extracted the following data elements: author(s), year of publication, year in which study was conducted, study design, study duration, completeness of follow-up for cohort studies, country and location of the study, setting (e.g. urban or rural, hospital or clinic), method(s) of recruitment; number of participants, characteristics of participants (age, gender, socioeconomic status, HIV-1 stage if available), details of intervention (medication, dose, duration, number of treatments), details of outcomes (change in HIV-1 RNA, change in CD4 count, change in rate of clinical HIV-1 disease progression, changes in WHO or CDC staging, mortality), and quality assessment.
Where data were incomplete, attempts were made to contact the original authors for clarification of relevant information. Authors were asked to provide data when outcomes or populations included in this review were not clearly defined in published manuscripts.
Study characteristics
Classification of studies
Study design was classified as either prospective cohort or randomized-controlled trial (RCT). Prospective cohort studies compared HIV-1 and helminth-co-infected individuals who were treated with anthelmintics to an internal comparison group consisting of HIV-1 infected, helminth uninfected individuals who did not receive anthelmintics. Randomized controlled trials were those studies in which assignment to treatment group (active drug or placebo) was determined by random allocation.
Outcome
The primary outcomes of interest were changes in plasma HIV-1 RNA levels and changes in absolute CD4 counts before and after use of anthelminthic therapy. Secondary outcome measures included markers of clinical disease progression, adverse events and mortality.
Quantitative data synthesis
Results from a systematic review and meta-analysis of the data by species are presented for those species evaluated in multiple studies and treated according to a standardized treatment recommendations. This is followed by a summary of results for species evaluated in only one study, or species-specific infections not treated according to the recommended treatment guidelines (Anonymous, 2007). CD4 and viral load outcomes included in this review were reported using similar continuous scales of measurement. CD4 counts were measured in cells/mm3 and viral load was measured as log10 copies/mL. A weighted mean difference (WMD) and 95% confidence interval (CI) were computed as the change in CD4 count and change in log10 HIV-1 RNA after anthelmintic treatment (compared to participants who were helminth uninfected and untreated). WMDs of CD4 count and HIV viral load were evaluated between baseline and 12 weeks post-treatment in treatment and placebo groups in randomized trials (Kallestrup et al. 2005; Nielsen et al. 2007; Walson et al. 2008). The length of time between baseline and post-treatment follow-up used in the calculation of WMDs varied slightly in cohort studies from either 4 months (Elliott et al. 2003; Modjarrad et al. 2005), or 6 months (Brown et al. 2004) and comparisons were between helminth infected/treated and uninfected/untreated (Elliott et al. 2003; Brown et al. 2004; Modjarrad et al. 2005). The meta-analysis used a DerSimonian and Laird random effects model to compute pooled WMDs of CD4 count and HIV viral load. The Cochrane’s chi-squared test for heterogeneity set at a significance of P<0·10 was evaluated. The extent of heterogeneity was measured using tau squared, a measure of between-study variance. Data were analyzed using Stata 11 (Stata Corporation, College Station, TX) and figures were created using Stata version 11.
RESULTS
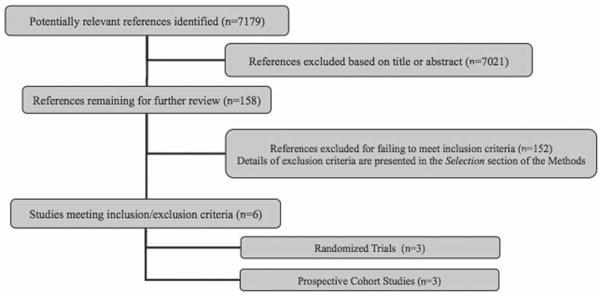
Using the search criteria outlined above, we identified 7,179 published studies of which 6 articles met the pre-specified inclusion criteria (Fig. 1). The study population included HIV-1-infected adults co-infected with at least one helminth species. The number of studies evaluating each helminth species is as follows; (S. mansoni (n=4) (Elliott et al. 2003; Brown et al. 2004; Kallestrup et al. 2005; Modjarrad et al. 2005), Strongyloides stercoralis (n=2) (Brown et al. 2004; Modjarrad et al. 2005), hookworm species (n=4) (Elliott et al. 2003; Brown et al. 2004; Modjarrad et al. 2005; Walson et al. 2008), Trichuris trichiura (n=3) (Elliott et al. 2003; Brown et al. 2004; Walson et al. 2008), A. lumbricoides (n=3) (Elliott et al. 2003; Modjarrad et al. 2005; Walson et al. 2008), Wuchereria bancrofti (n=1) (Nielsen et al. 2007), and Mansonella perstans (n=1)) (Brown et al. 2004). All of the studies included were conducted in sub-Saharan Africa and all were published between 2003 and 2008.
Fig. 1.
Flow diagram of study selection with number of permissive articles.
A total of 3 randomized controlled trials (Kallestrup et al. 2005; Nielsen et al. 2007; Walson et al. 2008) and 3 prospective cohort studies (Elliott et al. 2003; Brown et al. 2004; Modjarrad et al. 2005) were included. Study methodology was similar among randomized trials and prospective cohort studies regardless of the helminth species investigated. Details of the methodology used in each study are listed in Table 1.
Table 1.
Summary of included studies
| Author (Year) | Study design | County: Study Period | Sub-group analysis population | Intervention or treatment group | Comparison group | Time point of evaluation |
|---|---|---|---|---|---|---|
| Kallestrup (2005) | RCT | Zimbabwe: Oct 2001 to June 2003 | HIV-1 (+) adults (18 years and older) co-infected with S. mansoni | HIV/S. mansoni co-infection Praziquantel 40 mg/kg at baseline | HIV/S. mansoni co- infection and placebo | Baseline and 12 weeks |
| Nielsen (2007) | RCT | Tanzania: May to Nov 2002 | HIV-1 (+) adults (18–70 years) co- infected with W. bancrofti | HIV/W. bancrofti: co-infection Diethylcarbamazine 6 mg/kg at baseline | HIV/W. bancrofti co- infection and placebo | Baseline and 12 weeks |
| Walson (2008) | RCT | Kenya: March 2006 to June 2007 | HIV-1 (+) adults co-infected with A. lumbricoides, hookworm, or T. trichiura | HIV/A. lumbricoides co-infection HIV/Hookworm co-infection HIV/T. trichiura co-infection Albendazole 400 mg/day×3 days at enrollment | HIV/any helminth co-infection (A. lumbricoides, hookworm, or T. trichiura) and placebo | Baseline and 12 weeks |
| Brown (2004) | Prospective cohort study | Uganda: Feb 2001 to Mar 2002 | HIV-1 (+) adults co-infected with S. mansoni, hookworm, T. trichiura, S. stercoralis, or M. perstans and HIV-1 (+) adults without co-infection | HIV/Hookworm; HIV/T. trichiura; HIV/M. perstans: Albendazole 400 mg at enrollment; HIV/S. mansoni: Praziquantel 40 mg/kg month 1; HIV/S.stercoralis: Albendazole 800 mg/day×3 days at enrollment | HIV/helminth uninfected and untreated | Baseline and 6 months |
| Elliott (2003) | Prospective cohort study | Uganda: Nov 1999 to Jan 2000 | HIV-1 (+) adults co-infected with S. mansoni, A. lumbricoides, hookworm, T. trichiura and HIV- 1 (+) adults without co-infection | HIV/S. mansoni: Praziquantel, 40 mg/kg at enrollment. HIV/A. lumbricoides, HIV/hookworm, HIV/T. trichiura: Mebendazole, 200 mg/day×3 days at enrollment | HIV/helminth uninfected and untreated | Baseline and 4 months |
| Modjarrad (2005) | Prospective cohort study | Zambia: Mar to Dec 2003 | HIV-1 (+) adults (19–45 years) co- infected with S. mansoni, A. lumbricoides, hookworm, S. stercoralis and HIV-1 (+) adults without co-infection | HIV/A. lumbricoides, HIV/Hookworm, HIV/S. stercoralis: Albendazole 400 mg×1 day and 200 mg×2 days (weeks 1 and 4); HIV/S. mansoni: Praziquantel 40 mg/kg | HIV/helminth uninfected and untreated | Baseline and 4 months |
Treatment courses for nematode infections varied between studies; consisting of either 400 mg of albendazole/day for 3 days (Walson et al. 2008), 400 mg of albendazole on the first day followed by 200 mg per day for 2 days (Modjarrad et al. 2005), a single dose of 400 mg of albendazole (Brown et al. 2004), or 200 mg of mebendazole per day for 3 days (Elliott et al. 2003). One study prescribed 800 mg of albendazole for 3 days for infections with S. stercoralis (Brown et al. 2004). Schistosome infections were evaluated in 4 studies and all used a standard dose of 40 mg/kg of praziquantel for treatment (Elliott et al. 2003; Brown et al. 2004; Kallestrup et al. 2005; Modjarrad et al. 2005).
Pooled analyses were performed on the 4 helminth species in which; (1) results were available from more than 1 study and (2) treatments followed currently recommended guidelines for each of the included species (A. lumbricoides, S. mansoni, Hookworm and T. trichiura - Elliott et al. 2003; Brown et al. 2004; Modjarrad et al. 2005; Walson et al. 2008 – see Table 2). Results of the pooled-estimates evaluating the effect of helminth treatment on CD4 count and HIV viral load are presented below for each species separately. Heterogeneity was minimal (P>0·2) in all of the analyses presented with the exception of change in CD4 count and viral load among individuals with A. lumbricoides (results described below). CD4 results for each species are presented in Table 3, and HIV viral load results are presented in Table 4.
Table 2.
Recommended treatment regimen by helminth species
| Helminth | Treatment regimens used in included studies | Recommended adult treatment1 |
|---|---|---|
| S. mansoni | Praziquantel 40 mg/kg×1d | Praziquantel: 40 mg/kg PO in 2 doses×1d Oxamniquine: 15 mg/kg PO once |
| A. lumbricoides | Albendazole 400 mg×1d Albendazole 400 mg×3d Mebendazole 200 mg×3d |
Albendazole: 400 mg PO once Mebendazole: 100 mg bid PO×3d or 500 mg once Ivermectin: 150–200 mcg/kg PO once |
| Hookworm | Albendazole 400 mg×1d Albendazole 400 mg×3d Mebendazole 200 mg×3d |
Albendazole: 400 mg PO once Mebendazole: 100 mg PO bid×3d or 500 mg once Pyrantel pamoate: 11 mg/kg (max 1 g) PO×3d |
| T. trichiura | Albendazole 400 mg×1d Albendazole 400 mg×3d Mebendazole 200 mg×3d |
Preferred drug: Mebendazole: 100 mg bid PO×3d or 500 mg once Alternatives: Albendazole: 400 mg PO×3d Ivermectin: 200 mcg/kg PO×3d |
| S. stercoralis | Albendazole 800 mg×3d Albendazole 400 mg×1d+200 mg×2d |
Preferred drug: Ivermectin: 200 mcg/kg/d PO×2d Alternative: Albendazole: 400 mg PO bid×7d |
| M. perstans | Albendazole 400 mg×1d | Albendazole: 400 mg PO bid×10d Mebendazole: 100 mg PO bid×30d |
| W. bancrofti | Diethylcarbamazine 6 mg/kg×1d | Diethylcarbamazine: 6 mg/kg/d PO in 3 doses×12d |
Key to abbreviations: d: Day; PO: By mouth; BID: Twice a day.
Source: Anon. (2007). Drugs for parasitic infections. Medical Letter on Drugs and Therapeutics, 5 (Suppl), e1–e15.
Table 3.
Weighted mean differences (WMDs) of CD4 count outcomes by species
| Worm | Author (Year) | Study design | Treatment | Control | CD4 WMD (95% CI) |
|---|---|---|---|---|---|
| A. lumbricoides | Walson (2008) | RCT | 26 | 28 | −120·0 (−190·9, −49·1) |
| Elliott (2003) | Observational | 2 | 34 | −47·0 (−368·2, 274·2) | |
| Modjarrad (20050 | Observational | 27 | 57 | 3·0 (−82·2, 88·2) | |
| Pooled | 55 | 119 | −60·4 (−159·9, 39·1) | ||
| S. mansoni | Kallestrup (2005) | RCT | 64 | 66 | −33·5 (−118·2, 51·2) |
| Brown (2004) | Observational | 118 | 171 | −8·0 (−70·9, 54·9) | |
| Elliott (2003) | Observational | 24 | 34 | −47·0 (−206·6, 112·6) | |
| Modjarrad (2005) | Observational | 5 | 57 | 10·0 (−176·3, 196·3) | |
| Pooled | 211 | 328 | −17·9 (−64·5, 28·7) | ||
| Hookworm | Walson (2008) | RCT | 76 | 72 | −22·0 (−73·5, 29·5) |
| Brown (20040 | Observational | 79 | 171 | 2·0 (−75·2, 79·2) | |
| Elliott (2003) | Observational | 14 | 34 | −80·0 (−278·2, 118·2) | |
| Modjarrad (2005) | Observational | 24 | 57 | 103·0 (−19·4, 225·4) | |
| Pooled | 193 | 334 | 0·14 (−51·1, 51·4) | ||
| T. trichiura | Walson (2008) | RCT | 12 | 12 | −41·0 (−190·1, 108·1) |
| Brown (2004)* | Observational | 15 | 171 | −6·0 (−136·1, 124·1) | |
| Elliott (2003) | Observational | 6 | 34 | −109·0 (−394·4, 176·4) | |
| Pooled | 18 | 46 | −55·6 (−187·7, 76·6) | ||
| S. stercoralis | Brown (2004) | Observational | 41 | 171 | −8·0 (−89·7, 73·6) |
| Modjarrad (2005) | Observational | 4 | 57 | −5·0 (−87·3, 77·3) | |
| M. perstans | Brown (2004) | Observational | 23 | 171 | −2·0 (−96·3, 92·3) |
| W. bancrofti | Nielsen (2007) | RCT | 6 | 6 | −5·2 (−17·6, 7·3) |
Not included in pooled estimate because of treatment regimen.
Table 4.
Weighted mean differences (WMDs) of viral load outcomes by species
| Worm | Author (Year) | Study design | Treatment | Control | VL WMD (95% CI) |
|---|---|---|---|---|---|
| A. lumbricoides | Walson (2008) | RCT | 26 | 28 | −0·85 (−1·46, −0·24) |
| Elliott (2003) | Observational | 2 | 34 | 0·19 (−0·73, 1·11) | |
| Modjarrad (2005) | Observational | 27 | 57 | −0·12 (−0·42, 0·18) | |
| Pooled | 55 | 119 | −0·29 (−0·83, 0·25) | ||
| S. mansoni | Kallestrup (2005) | RCT | 64 | 66 | −0·21 (−0·40, −0·02) |
| Brown (2004) | Observational | 79 | 126 | 0·04 (−0·19, 0·27) | |
| Elliott (2003) | Observational | 24 | 34 | −0·06 (−0·54, 0·42) | |
| Modjarrad (2005) | Observational | 5 | 57 | −0·09 (−0·68, 0·50) | |
| Pooled | 172 | 283 | −0·10 (−0·24 to 0·03) | ||
| Hookworm | Walson (2008) | RCT | 76 | 72 | 0·02 (−0·30, 0·34) |
| Brown (2004) | Observational | 55 | 126 | −0·11 (−0·37, 0·15) | |
| Elliott (2003) | Observational | 14 | 34 | 0·11 (−0·55, 0·77) | |
| Modjarrad (2005) | Observational | 24 | 57 | 0·03 (−0·35, 0·41) | |
| Pooled | 169 | 289 | −0·03 (−0·20, 0·15) | ||
| T. trichiura | Walson (2008) | RCT | 12 | 12 | 0·34 (−0·42, 1·10) |
| Brown (2004)* | Observational | 10 | 126 | −0·17 (−0·69, 0·35) | |
| Elliott (2003) | Observational | 6 | 34 | −0·05 (−0·69, 0·59) | |
| Pooled | 18 | 46 | 0·11 (−0·38, 0·60) | ||
| S. stercoralis | Brown (2004) | Observational | 26 | 126 | 0·00 (−0·31, 0·31) |
| Modjarrad (2005) | Observational | 4 | 57 | −0·07 (−0·60, 0·46) | |
| M. perstans | Brown (2004) | Observational | 18 | 126 | −0·04 (−0·47, 0·39) |
| W. bancrofti | Nielsen (2007) | RCT | 5 | 6 | −0·08 (−0·93, 0·77) |
Not included in pooled estimate because of treatment regimen.
Species-specific effects of treatment
A. lumbricoides
Three studies evaluated the effect of treatment for A. lumbricoides on CD4 count (n=174) and HIV viral load (n=174) (Elliott et al. 2003; Modjarrad et al. 2005; Walson et al. 2008). Details of the study design and methodology are summarized in Table 1. Using a random effects model, treatment for A. lumbricoides was not associated with CD4 count (WMD=−60·4; 95% CI: −159·9, 39·1) (Fig. 2A), or HIV viral load (WMD=−0·29; 95% CI: −0·83, 0·25) (Fig. 2B). The test for heterogeneity was statistically significant in both the analysis of HIV viral load (P=0·07, tau squared=0·14) and CD4 count (P=0·09, tau squared =0·00).
Fig. 2.
Treatment effect of A. lumbricoides on HIV disease progression. A. A. lumbricoides: Change in CD4 count after treatment or no treatment. B. A. lumbricoides: Change in Log10 HIV-1 RNA after treatment or no treatment.
S. mansoni
Four studies evaluated the effect of treatment for S. mansoni on CD4 (n=539) and HIV viral load (n=455) (Elliott et al. 2003; Brown et al. 2004; Kallestrup et al. 2005; Modjarrad et al. 2005) (Table 1). Using a random effects model, treatment for S. mansoni was not associated with CD4 count (WMD=−17·9; 95% CI: −64·5, 28·7) (Fig. 3A), however there was a trend towards an association between treatment for S. mansoni and a decrease in HIV viral load (WMD=−0·10; 95% CI: −0·24, 0·03) (Fig. 3B).
Fig. 3.
Treatment effect of S. mansoni on HIV disease progression. A. S. mansoni: Change in CD4 count after treatment or no treatment. B. S. mansoni: Change in Log10 HIV-1 RNA after treatment or no treatment.
Hookworm
Four studies evaluated the effect of treatment for hookworm on CD4 (n=527) and HIV viral load (n=458) (Elliott et al. 2003; Brown et al. 2004; Modjarrad et al. 2005; Walson et al. 2008) (Table 1). Using a random effects model, treatment for hookworm was not associated with CD4 count (WMD=0·14; 95% CI: −51·1, 51·4) (Fig. 4A) or HIV viral load (WMD=−0·03; 95% CI: −0·20, 0·15) (Fig. 4B).
Fig. 4.
Treatment effect of Hookworm on HIV disease progression. A. Hookworm: Change in CD4 count after treatment or no treatment. B. Hookworm: Change in Log10 HIV-1 RNA after treatment or no treatment.
T. trichiura
While 3 studies included in this review evaluated the effect of treatment for T. trichiura on CD4 and HIV viral load, the pooled data are limited to the 2 studies which gave the recommended treatment course of either 200 mg of mebendazole for 3 days (Elliott et al. 2003) or 400 mg of albendazole for 3 days (Walson et al. 2008). A total of 64 subjects are included in the viral load analysis and 64 are included in the analysis of CD4 count (Elliott et al. 2003; Brown et al. 2004; Walson et al. 2008) (Table 1). Using a random effects model, treatment for T. trichiura was not associated with CD4 count (WMD=−55·6; 95% CI: −187·7, 76·6) (Fig. 5A) or HIV viral load (WMD=0·11; 95% CI: −0·38, 0·60) (Fig. 5B).
Fig. 5.
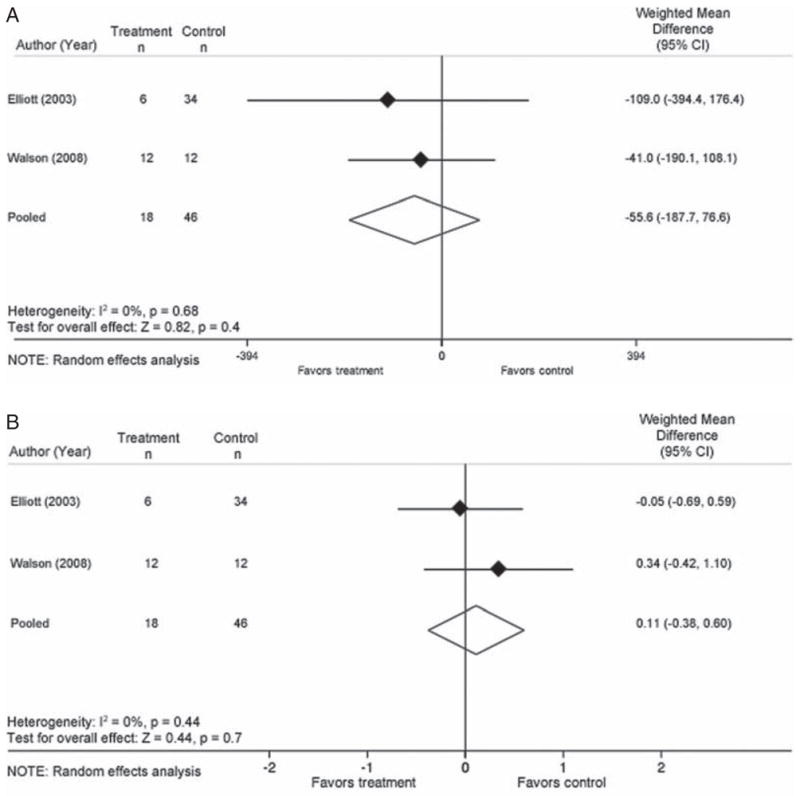
Treatment effect of T. trichiura on HIV disease progression. A. T. trichiura: Change in CD4 count after treatment or no treatment. B. T. trichiura: Change in Log10 HIV-1 RNA after treatment or no treatment.
M. perstans
One prospective cohort study evaluated the effect of treatment of M. perstans on CD4 count and HIV viral load (n=144) (Brown et al. 2004), therefore, pooled data are unavailable. Treatment in this study consisted of a single dose of 400 mg of albendazole. Treatment of M. perstans was not associated with CD4 count (WMD=−2·0; 95% CI: −96·3, 92·3) or HIV viral load in this study (WMD=−0·04; 95% CI: −0·47, 0·39).
W. bancrofti
One randomized clinical trial evaluated the effect of treatment of W. bancrofti on CD4 count and HIV viral load (n=17) (Nielsen et al. 2007), therefore pooled data are unavailable. Infections with treated with 6 mg/kg of diethylcarbamazine. Treatment of W. bancrofti was not associated with CD4 count (WMD=−5·2; 95% CI: −17·6, 7·3) or HIV viral load (WMD=−0·08; 95% CI: −0·93, 0·77) in this study.
S. stercoralis
Two cohort studies evaluated the effect of treatment for S. stercoralis on CD4 (n=273) and viral load (213) (Brown et al. 2004; Modjarrad et al. 2005). HIV-1-infected individuals in these studies were treated with either 800 mg of albendazole for 3 days (Brown et al. 2004), or 400 mg of albendazole on the first day and 200 mg for 2 subsequent days (Modjarrad et al. 2005). Because neither of these studies utilized the current recommended dose of 400 mg of albendazole per day for 7 days (Anonymous, 2007), these data were not pooled. Lastly, neither of these studies found a treatment effect on CD4 count or HIV viral load.
DISCUSSION
Data from several randomized trials have suggested species-specific differences exist in the observed benefit associated with de-worming, specifically among individuals infected with A. lumbricoides and S. mansoni. In this analysis, we stratified data from studies evaluating the effect of de-worming on markers of HIV-1 disease progression by helminth species and conducted a pooled analysis including data from both randomized and observational studies. Data from sub-group analyses from studies examining the treatment of A. lumbricoides, S. mansoni, hookworm, T. trichiura, M. perstans and W. bancrofti in HIV-co-infected individuals were included. In this pooled analysis, no significant benefits on markers of HIV-1 disease progression were observed with the treatment of any individual helminth species.
It is possible that the observation of benefit observed in the randomized trials following treatment of A. lumbricoides and S. mansoni was a result of type I error. However, as the randomized trial design provides the strongest level of evidence, the highly significant improvement in CD4 count and trend towards a reduction in viral load following de-worming in A. lumbricoides and HIV-1-co-infected individuals seen in one randomized trial (Walson et al. 2008), and the significant attenuation in viral load observed following treatment for schistosomiasis in another randomized trial (Kallestrup et al. 2005), suggest that type I error is unlikely. It appears more likely that the inclusion of observational studies in the pooled analysis may have resulted in type II errors, as many of these studies had notable limitations in their design and methodology.
There are several possible explanations for why benefit was observed in randomized trials following treatment of A. lumbricoides and S. mansoni, but not with other helminth species. A. lumbricoides-infected individuals, when compared to helminth uninfected controls, have been observed to have lower levels of TH1 cytokines and higher levels of TH2, pro-inflammatory, and immunosuppressive cytokines (Malla et al. 2006). These differences in TH2 polarization appear to be more prominent with A. lumbricoides infection than with hookworm or Trichuris (Cooper et al. 2000; Pit et al. 2001; Bradley and Jackson, 2004; Jackson et al. 2004; Geiger et al. 2007). HIV-1- infected individuals co-infected with A. lumbricoides who received albendazole displayed significantly larger reductions in interleukin-10 (IL-10) when compared to those receiving placebo, suggesting treatment of A. lumbricoides may reduce IL-10-mediated immunosuppression (Blish et al. 2010). Similarly, a predominant TH2 response is also thought to occur among individuals with early schistosomiasis, followed by IL-10 mediated hyporesponsiveness during chronic infection (Burke et al. 2009; Taylor et al. 2009). The strong TH2 polarization and IL-10-mediated hyporesponsiveness seen with these infections may significantly affect the hosts ability to control HIV-1 replication and may explain the observation of benefit following de-worming of A. lumbricoides and S. mansoni-infected individuals.
The failure to detect an association between de-worming and markers of HIV-1 progression may also have been influenced by the variation in efficacy of the treatment regimens used for each species. The efficacy of a single 400 mg dose of albendazole is significantly different for each of these individual helminth species (Fig. 6). While this treatment regimen is highly effective against A. lumbricoides (cure >94%) (Bennett and Guyatt, 2000; Horton, 2000; Keiser and Utzinger, 2008), cure rates are significantly lower for hookworm (cure 78%) (Horton, 2000) and T. trichiura (cure 28–48%) (Bennett and Guyatt, 2000; Horton, 2000; Keiser and Utzinger, 2008). The lower efficacy of treatment of hookworm and T. trichiura infection may have reduced the ability of this analysis to detect an effect, as many of these individuals who were randomized to albendazole may not actually have been cured of their infection. These individuals would effectively dilute the effect of treatment observed in the analysis.
Fig. 6.
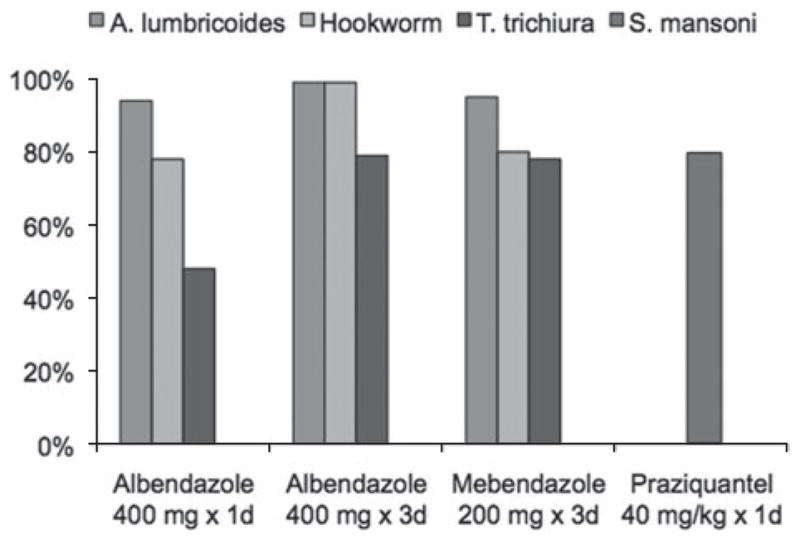
Previously published data on the proportion of patients cured by treatment regimen.(Footnote to Fig. 6): Data Sources: (Marti et al., 1996; Bennett and Guyatt, 2000; Horton, 2000; Ferrari et al., 2003; Keiser and Utzinger, 2008).
Finally, it is important to note that many studies conducted to date have not been designed to evaluate species-specific effects among individual sub-groups of patients. In retrospectively evaluating the power of published studies, we determined that less than 35% of sub-group analyses had adequate power (>80%) to detect meaningful differences when stratified by individual helminth species. It is plausible that species-specific differences have not consistently been observed due to inadequate power in these studies.
Due to the wide heterogeneity of methodologies used in HIV/helminth co-infection treatment studies, we applied stringent inclusion criteria, resulting in only six studies being included in this review. Many studies conducted to date have included inappropriate comparison groups, limiting the ability of these studies to make valid comparisons. Some studies have compared individuals who test negative for helminths at follow-up to those who test positive for helminths at follow-up. There may be important differences in the immunological responses to helminth infection between individuals who successfully clear helminth infection following therapy and those who do not (Mutapi, 2001; Anthony et al. 2007). These differences may also be related to individual immune control of HIV-1. Differences in factors associated with risk of re-exposure to helminth infection are also likely to exist between participants who are rapidly re-infected with helminths compared to those who are not. Given that both re-infection and/or decreased immune control of HIV-1 are likely to dilute the treatment effect, this methodology may result in a significant bias towards the null.
Insufficient data from individual trials exist to determine the effect of treating individual helminths on markers of HIV-1 progression. As a result, we sought to increase the effective sample size of each comparison by pooling data from multiple prospective studies. While this approach may add power to the analysis of each comparison, differences in study design and methodology between included studies are an important limitation of this analysis. In addition, while randomized trials of anthelminthic therapy provide the strongest level of evidence to determine the possible benefit of such treatment, the analysis of sub-groups within these cohorts reduces the benefit of randomization. We also included several observational studies, all of which may be limited by bias. The lack of standardized treatment regimens included in the analysis likely resulted in differences in treatment efficacy by study and by species. Finally, study sample sizes were relatively small and species-specific analyses from individual studies lacked power to detect meaningful effects.
While the implications of these data may be important for all HIV-1-infected individuals, HIV-1-infected children may benefit disproportionately. In addition to harbouring a large burden of helminth infections, HIV infection is often undiagnosed and untreated in young children in many resource-limited settings. Without effective antiretroviral therapy, mortality approaches 50% in the first two years of life for these children. It is critical that practical and effective approaches to delay immunosuppression be examined and implemented in pediatric populations in order to maximize benefit.
Conclusions
Understanding differences in the treatment effects among helminth species may lead to better clinical and therapeutic management of these infections. It is important to determine the relative benefit of treating individual helminth species on markers of HIV-1 progression. Further trials are needed to confirm the possible role of anthelmintic treatment in HIV-1 co-infected individuals, particularly in populations most likely to benefit, such as young children. Such studies should be rigorously designed and adequately powered to detect species-specific effects.
Acknowledgments
We would like to acknowledge the support of the Royal Society of Tropical Medicine and Hygiene and the British Society for Parasitology for providing funds for travel. In addition, we would like to acknowledge the support of the University of Washington Kenya Research Program and the University of Washington International AIDS Research and Training Program.
FINANCIAL SUPPORT
This work was supported by the University of Washington, Center for AIDS Research (CFAR), USA.
References
- Anonymous. Drugs for parasitic infections. Medical Letter on Drugs and Therapeutics. 2007;5 (Suppl):e1–e15. [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature Reviews. Immunology. 2007;7:975–987. doi: 10.1038/nri2199. nri2199 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggaley RF, Griffin JT, Chapman R, Hollingsworth TD, Nagot N, Delany S, Mayaud P, de Wolf F, Fraser C, Ghani AC, Weiss HA. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS. 2009;23:1005–1013. doi: 10.1097/QAD.0b013e32832aadf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitology Today. 2000;16:71–74. doi: 10.1016/s0169-4758(99)01544-6. S0169-4758(99)01544-6 [pii] [DOI] [PubMed] [Google Scholar]
- Blish CA, Sangaré L, Herrin BR, Richardson BA, John-Stewart G, Walson JL. Changes in plasma cytokines after treatment of Ascaris lumbricoides infection in individuals with HIV-1 infection. Journal of Infectious Diseases. 2010;201:1816–1821. doi: 10.1086/652784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CD, Maizels RM, Mutapi F. Acquired immune heterogeneity and its sources in human helminth infection. Parasitology. 2010:1–21. doi: 10.1017/S0031182010001216. S0031182010001216 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JE, Jackson JA. Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunology. 2004;26:429–441. doi: 10.1111/j.0141-9838.2004.00730.x. PIM730 [pii] [DOI] [PubMed] [Google Scholar]
- Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. American Journal of Tropical Medicine and Hygiene. 2007;77:88–98. 77/6_Suppl/88 [pii] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Smith JL, Mupfasoni D, Mwanje MT, Ndayishimiye O, Lwambo NJ, Mbotha D, Karanja P, Mwandawiro C, Muchiri E, Clements AC, Bundy DA, Snow RW. An updated atlas of human helminth infections: the example of East Africa. International Journal of Health Geographics. 2009;8:42. doi: 10.1186/1476-072X-8-42. 1476-072X-8-42 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Rowlands M, Haller L, Savioli L, Bundy DA. Towards an atlas of human helminth infection in sub-Saharan Africa: the use of geographical information systems (GIS) Parasitology Today. 2000;16:303–307. doi: 10.1016/s0169-4758(00)01687-2. S0169-4758(00)01687-2 [pii] [DOI] [PubMed] [Google Scholar]
- Brown M, Kizza M, Watera C, Quigley MA, Rowland S, Hughes P, Whitworth JA, Elliott AM. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. Journal of Infectious Diseases. 2004;190:1869–1879. doi: 10.1086/425042. JID32687 [pii] [DOI] [PubMed] [Google Scholar]
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunology. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. PIM1098 [pii] [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Chico ME, Sandoval C, Espinel I, Guevara A, Kennedy MW, Urban JF, Jr, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. Journal of Infectious Diseases. 2000;182:1207–1213. doi: 10.1086/315830. JID000478 [pii] [DOI] [PubMed] [Google Scholar]
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends in Parasitology. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. S1471492203002757 [pii] [DOI] [PubMed] [Google Scholar]
- Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. S0264-410X(08)00540-9 [pii] [DOI] [PubMed] [Google Scholar]
- Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Review of Anti-Infective Therapy. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, Watera C, Dunne DW, Whitworth JA. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:103–108. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- Ferrari ML, Coelho PM, Antunes CM, Tavares CA, da Cunha AS. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bulletin of the World Health Organization. 2003;81:190–196. S0042-96862003000300009 [pii] [PMC free article] [PubMed] [Google Scholar]
- Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Tropica. 2003;86:315–333. doi: 10.1016/s0001-706x(03)00063-9. S0001706X03000639 [pii] [DOI] [PubMed] [Google Scholar]
- Geiger SM, Caldas IR, McGlone BE, Campi-Azevedo AC, De Oliveira LM, Brooker S, Diemert D, Correa-Oliveira R, Bethony JM. Stage-specific immune responses in human Necator americanus infection. Parasite Immunology. 2007;29:347–358. doi: 10.1111/j.1365-3024.2007.00950.x. PIM950 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Molecular and Biochemical Parasitology. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. S0166-6851(09)00122-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121(Suppl):S113–S132. doi: 10.1017/s0031182000007290. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud CM, Bethony J, Correa–Oliveira R, Shu–Hua X, Fenwick A, Savioli L. Helminth Infections: Soil–Transmitted Helminth Infections and Schistosomiasis. In: Jamison Dean T, Breman Joel G, Measham Anthony R, Alleyne George, Claeson Mariam, Evans David B, Jha Prabhat, Mills Anne, Musgrove Philip., editors. Disease Control Priorities in Developing Countries. 2. New York: Oxford University Press; 2006. pp. 467–482. [DOI] [Google Scholar]
- Jackson JA, Turner JD, Rentoul L, Faulkner H, Behnke JM, Hoyle M, Grencis RK, Else KJ, Kamgno J, Bradley JE, Boussinesq M. Cytokine response profiles predict species-specific infection patterns in human GI nematodes. International Journal for Parasitology. 2004;34:1237–1244. doi: 10.1016/j. ijpara.2004.07.009. S0020-7519(04)00166-3 [pii] [DOI] [PubMed] [Google Scholar]
- Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, Gerstoft J, Erikstrup C, Ullum H. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. Journal of Infectious Diseases. 2005;192:1956–1961. doi: 10.1086/497696. JID34637 [pii] [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. Journal of the American Medical Association. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. 299/16/1937 [pii] [DOI] [PubMed] [Google Scholar]
- Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Neglected Tropical Diseases. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla N, Fomda BA, Thokar MA. Serum cytokine levels in human ascariasis and toxocariasis. Parasitology Research. 2006;98:345–348. doi: 10.1007/s00436-005-0081-z. [DOI] [PubMed] [Google Scholar]
- Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. American Journal of Tropical Medicine and Hygiene. 1996;55:477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. 00002030-200810180-00015 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K, Zulu I, Redden DT, Njobvu L, Lane HC, Bentwich Z, Vermund SH. Treatment of intestinal helminths does not reduce plasma concentrations of HIV-1 RNA in coinfected Zambian adults. Journal of Infectious Diseases. 2005;192:1277–1283. doi: 10.1086/444543. JID33853 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau E, Chauvin A. Immunity against helminths: interactions with the host and the intercurrent infections. Journal of Biomedicine & Biotechnology. 2010;2010:428593. doi: 10.1155/2010/428593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutapi F. Heterogeneities in anti-schistosome humoral responses following chemotherapy. Trends in Parasitology. 2001;17:518–524. doi: 10.1016/s1471-4922(01)02118-3. S1471-4922(01)02118-3 [pii] [DOI] [PubMed] [Google Scholar]
- Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Annals of Tropical Medicine and Parasitology. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NO, Simonsen PE, Dalgaard P, Krarup H, Magnussen P, Magesa S, Friis H. Effect of diethylcarbamazine on HIV load, CD4%, and CD4/CD8 ratio in HIV-infected adult Tanzanians with or without lymphatic filariasis: randomized double-blind and placebo-controlled cross-over trial. American Journal of Tropical Medicine and Hygiene. 2007;77:507–513. 77/3/507 [pii] [PubMed] [Google Scholar]
- Pit DS, Polderman AM, Baeta S, Schulz-Key H, Soboslay PT. Parasite-specific antibody and cellular immune responses in human infected with Necator americanus and Oesophagostomum bifurcum. Parasitology Research. 2001;87:722–729. doi: 10.1007/s004360100419. [DOI] [PubMed] [Google Scholar]
- Roussilhon C, Brasseur P, Agnamey P, Perignon JL, Druilhe P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS One. 2010;5:e9309. doi: 10.1371/journal.pone.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. Journal of Clinical Investigation. 2009;119:1019–1028. doi: 10.1172/JCI36534. 36534 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M, Berzins K. Immune interactions in malaria co-infections with other endemic infectious diseases: implications for the development of improved disease interventions. Microbes and Infection. 2008;10:948–952. doi: 10.1016/j. micinf.2008.07.014. S1286-4579(08)00191-3 [pii] [DOI] [PubMed] [Google Scholar]
- UNAIDS. 2007 AIDS Epidemic Update. UNAIDS; 2007. [Google Scholar]
- Walson JL, Otieno PA, Mbuchi M, Richardson BA, Lohman-Payne B, Macharia SW, Overbaugh J, Berkley J, Sanders EJ, Chung MH, John-Stewart GC. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. AIDS. 2008;22:1601–1609. doi: 10.1097/QAD.0b013e32830a502e. 00002030-200808200-00009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walson JL, John-Stewart G. Treatment of helminth co-infection in HIV-1 infected individuals in resource-limited settings. Cochrane Database of Systematic Reviews. 2008;(1):CD006419. doi: 10.1002/14651858.CD006419.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walson JL, Herrin BR, John-Stewart G. Deworming helminth co-infected individuals for delaying HIV disease progression. Cochrane Database of Systematic Reviews. 2009;(3):CD006419. doi: 10.1002/14651858.CD006419.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. First WHO Report on Neglected Tropical Diseases 2010: Working to Overcome the Global Impact of Neglected Tropical Diseases. 2010. WHO/HTM/NTD/2010.1. [Google Scholar]
- Wolday D, Mayaan S, Mariam ZG, Berhe N, Seboxa T, Britton S, Galai N, Landay A, Bentwich Z. Treatment of intestinal worms is associated with decreased HIV plasma viral load. Journal of Acquired Immune Deficiency Syndromes. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]