Abstract
Allografts of articular cartilage are both used clinically for tissue-transplantation procedures and experimentally as model systems to study the physiological behavior of chondrocytes in their native extracellular matrix. Long-term maintenance of allograft tissue is challenging. Chemical mediators in poorly defined culture media can stimulate cells to quickly degrade their surrounding extracellular matrix. This is particularly true of juvenile cartilage which is generally more responsive to chemical stimuli than mature tissue. By carefully modulating the culture media however it may be possible to preserve allograft tissue over the long-term while maintaining its original mechanical and biochemical properties. In this study juvenile bovine cartilage explants (both chondral and osteochondral) were cultured in both chemically defined medium and serum-supplemented medium for up to 6 weeks. The mechanical properties and biochemical content of explants cultured in chemically-defined medium were enhanced after 2 weeks in culture and thereafter remained stable with no loss of cell viability. In contrast, the mechanical properties of explants in serum-supplemented medium were degraded by (~70%) along with a concurrent loss of biochemical content (30~40% GAG). These results suggest that long-term maintenance of allografts can be extended significantly by the use of a chemically-defined medium.
1 Introduction
Fresh osteochondral allografts have demonstrated more than 75% clinical success in treatment of femoral condyle lesions, avascular necrosis and iatrogenic cartilage injury (Bugbee, 2002). However, concerns over the decrease in chondrocyte viability with storage time generally reduce their clinical use to within 28 days post-harvest. An increase in shelf life will have a very significant impact on the treatment of cartilage lesions by expanding the availability of osteochondral allografts. While Brighton and coworkers showed promising findings using tissue culture techniques for cartilage maintenance nearly two decades ago, cold storage (~4 C) is the current standard for osteochondral graft preservation and storage (Brighton et al., 1979). In the current study, we revisit the potential of using in vitro techniques for maintaining cartilage explants in long-term culture.
Explants of articular cartilage are used experimentally to study chondrocytes in their native extracellular environment (Asanbaeva et al., 2007; Sah et al., 1989). They have a potential advantage over alternative system such as in vivo models in that they provide a defined and controlled environment to study cartilage function and mechanoregulation. However, since many in vitro experiments on cartilage tissue can last for weeks; maintenance of the physiological functions of cartilage explants in the long term is vital to the validity of the experimental results. Long-term maintenance of allograft tissue is challenging as suboptimal culture conditions can result in the degradation of the surrounding matrix, particularly in media supplemented with fetal bovine serum (FBS). Previous studies have shown that FBS can induce excessive cell proliferation (Strehl et al., 2002), chondrocyte phenotypic instability (Garcia and Gray, 1995), induction of cell outgrowth (Luyten et al., 1988), and excessive tissue swelling (Sah, Trippel and Grodzinsky, 1996). This is particularly true of juvenile cartilage which is generally more responsive to chemical stimuli than mature tissue. Furthermore, the composition of serum is variable and its constituents are largely unknown. For greater consistency many researchers opt to use chemically defined serum-free medium for culture of cartilage explants (Dumont et al., 1999; Malpeli et al., 2004). By carefully modulating the culture medium it may be possible to preserve allograft tissue over the long-term while maintaining its original mechanical and biochemical properties. The objective of this study was to investigate the efficacy of adopting serum-free medium in maintaining the native properties of both chondral (Study 1) and osteochondral (Study 2) juvenile bovine cartilage explants in long-term culture. This serum-free medium (also referred to as chondrogenic medium, CM) was adapted from a well-established formulation known to foster chondrogenesis in bone marrow stem cells and de novo matrix formation in tissue engineered cartilage (Mauck, Yuan and Tuan, 2006).
2 Materials and methods
1. Sample preparation & culturing
In Study 1, bovine cartilage plugs were harvested from the femoral condyles of 2–6 month-old calves. Middle zone explant disks of (Ø4 × 2.2 mm) were obtained by removing both the superficial (0.25–0.5 mm) and deep zone layer. Explants were then cultured in either DMEM supplemented with 20% FBS or chemically-defined serum-free medium (DMEM, 1% ITS+ Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate) (Byers, Mauck and Tuan, 2006) and supplemented with ascorbate 2-phosphate (50μg/ml) (37 ºC, 5% CO2). The serum-free medium is also referred to as chondrogenic medium (CM) for its original use in inducing chondrogenesis of stem cells. The two experimental groups were: explants cultured in CM and explants cultured in FBS for 42 days. In Study 2, full-thickness osteochondral plugs (Ø3mm, Figure 2E) were harvested from bovine wrist joints, cleaned of bone marrow with a high velocity water pick, and incubated in CM at 37 ºC for 14 days. Media were changed three times a week.
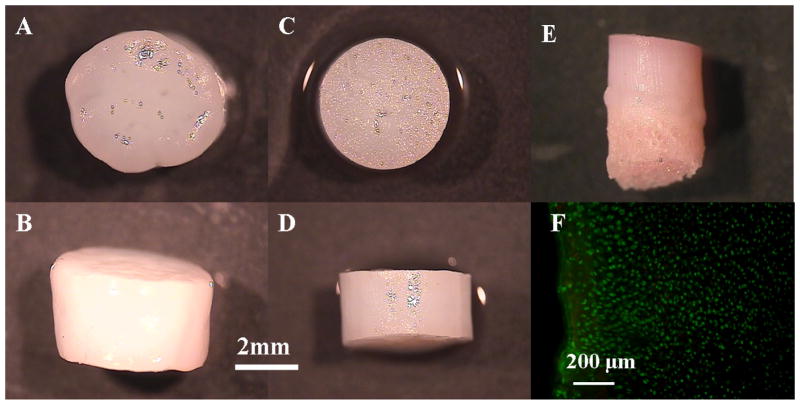
Figure 2.
top and side view of the chondral explants grown in serum-supplemented medium (A, B) and serum-free medium (C, D) on day 42. Side view of the osteochondral explants grown in serum-free medium on day 14 (E). Viability staining of the cartilage part of the osteochondral explants on day 14 (F).
2. Mechanical testing
The average mechanical properties of explant disks was evaluated at day 0, 14, 28, 42 of culture using a custom table top testing device for Study 1 and at day 0, 14 for Study 2 (Mauck et al., 2000). The equilibrium Young’s modulus (EY) was determined under unconfined compression at 10% strain, followed by tests for dynamic moduli at 0.1, 0.5, and 1 Hz and 1% strain. Following average property measurements, osteochondral explant disks in Study 2 were halved and tested for local axial mechanical properties under unconfined compression on a custom microscope testing device (Wang et al., 2003; Wang et al., 2002). Paired images of uncompressed and compressed tissue samples were obtained on the cut surface and correlated using an automated optimized digital image correlation technique to determine the local displacement field. To determine the axially varying tissue properties, the local compressive modulus was calculated from the strain derived from the 1st order derivative of the displacement profile and the measured force.
3. Biochemical analysis
One-half of each explant disk was weighed wet, lyophilized, reweighed dry, and digested in 0.5mg/ml Proteinase-K (Fisher Scientific; in 50mM Tris buffered saline containing 1mM EDTA, 1mM iodoacetamide and 10 mg/ml pepstatin A) at 56ºC for 16 hrs. The PicoGreen assay (Invitrogen, Molecular Probes) was used to quantify the DNA content of the explant disks with Lambda phage DNA (0–1 mg/ml) as a standard (McGowan et al., 2002). The GAG content was measured using dimethylmethylene blue (DMMB,Sigma Chemicals) dye-binding assay with shark chondroitin sulfate (0–50 mg/ml) as a standard (Farndale, Buttle and Barrett, 1986). The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio(Hollander et al., 1994). The collagen and GAG contents were normalized to the disk wet weight and DNA content.
4. Media collection and analysis
Multiple cartilage explant disks were cultured in petri dishes, with 4 mL of media per explant during the culture period. The media GAG, COMP or MMP in each dish were determined from media pooled over the culture day denoted plus two preceding days (i.e., 3 days), and then normalized by the number of explants in the dish to attain a constituent per disk value. The average of this quantity for multiple dishes is graphed in Figure 3. The MMP analysis was performed on media from explants in a separate, similarly performed study. GAG release into the media was analyzed using the DMMB assay described above. Cartilage oligomeric matrix protein (COMP) concentration was analyzed using the animal COMP elisa kit (MdBiosciences), with basal levels of COMP present in fresh media subtracted. Matrix Metalloproteinase-3 (MMP-3, stromelysin 1) was analyzed using the Fluorokine MAP Multiplex Human MMP assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol.
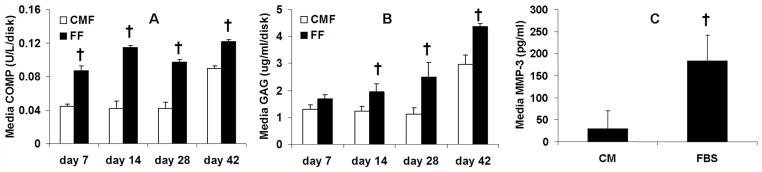
Figure 3.
(A) The COMP and (B) GAG levels pooled from medium of the culture day denoted plus two preceding culture days. (C) MMP-3 concentration in the spent medium collected on day 9 of culture in a separate study. † p<0.05 vs. CM
4. Histological analysis
The other half of each explant disk was fixed (5% acetic acid, 3.7% formaldehyde, 70% ethanol) for 24 hrs and stored in 70% ethanol solution. After serial dehydration in ethanol, the disks were embedded in paraffin (Fisher Scientific), sectioned to 8 μm, and mounted onto microscope slides. The samples were then dewaxed, rehydrated, and stained with Safranin-O (Sigma Chemical) and Picrosirius Red (Sigma Chemical) dyes to determine the distribution of GAGs and collagen, respectively.
5. Statistical analysis
Statistica (Statsoft, Tulsa, OK) was used to perform statistical analyses using two-way ANOVA and the Tukey HSD Post Hoc test (n= 4~6 per group) with culture duration, culture media type as independent variables.
3 RESULTS
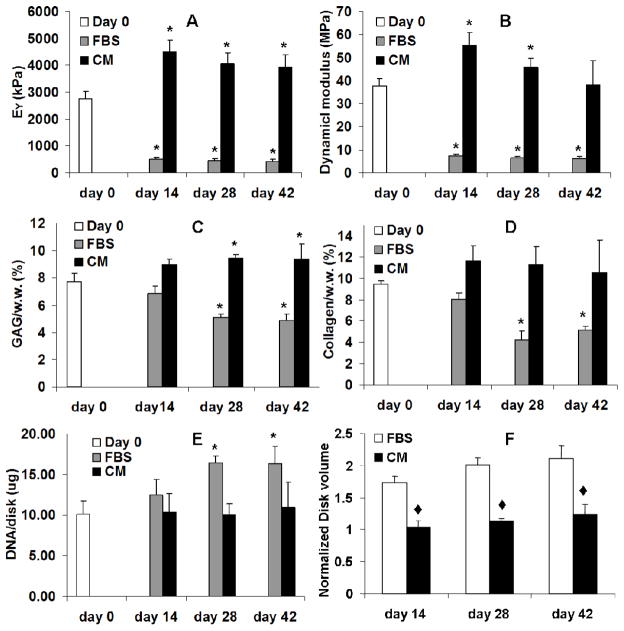
In Study 1 the equilibrium Young’s modulus and dynamic modulus of the middle-zone chondral explant disks cultured in serum-free medium (CM) increased from initial values (2.6 MPa and 37.3MPa, respectively) to 4.4 MPa and 55.2 MPa on day 14. By day 42 of culture the equilibrium modulus remained at day 14 levels and the dynamic modulus returned to day 0 values (Figure 1A, B). In contrast, by day 14 the moduli of the explants grown in FBS medium dropped to 20% of the day 0 values and remained low through the remainder of the 42 day culture period (Figure 1A, B). There was a significant increase in DNA per explant for FBS groups whereas CM explants remained at initial values (Figure 1E). The absolute content of GAG increased in both serum and CM explant cultures relative to initial values whereas collagen remained relatively constant (data not shown). However, unlike CM culture, explants in FBS culture swelled to nearly double their volume. Therefore the concentration of GAG and collagen (per wet weight) significantly decreased with culture time for FBS explants, but not CM explants (Figure 1F, C, D and Figure 2A-D). The volume of CM explants did not change. The concentration of GAG significantly increased whereas the concentration of collagen remained unchanged with culture time in the CM explants. (Figure 1C, D).
Figure 1.
Equilibrium Young’s modulus (EY) (A) and dynamic modulus (B) of the chondral explants. GAG content (C), collagen content (D) and DNA content (E) of the chondral explants by wet weight. The volume of the chondral explants (E, normalized to Day 0 value) * p<0.05 vs. Day 0, ◆ p<0.005 vs. FBS
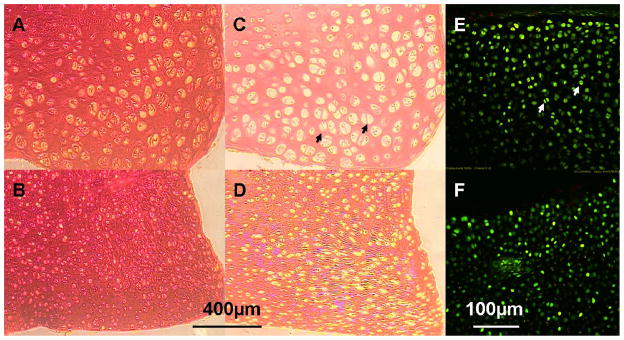
The COMP and GAG concentration in the FBS culture medium was significantly elevated compared to the CM medium (Figure 3). The MMP-3 level in the FBS medium was also significantly greater than the CM medium. Histology revealed that the CM group stained more intensely with Safranin-O and Picrosirius red than the FBS group (Figure 4), consistent with the quantitative assays for their content described above. Viability staining showed that cell death, when present, was mostly observed at the superficial layer of the cartilage explants (Figure 4). Although cells from the serum-supplemented cultures appeared larger (and also as doublets from cell division) in histology images (Figure 4A, C), this larger cell size did not appear as obvious for unfixed and un-embedded vital staining images (Figure 4E). As such, the apparent cell enlargement in the histology images may reflect processing of tissues having different compositions including water content. Importantly, both Von Kossa staining and EDAX analysis showed there was no mineralization in all CM samples that would have contributed to the increase in mechanical properties with culture time (data not shown).
Figure 4.
Safranin-O staining (for GAG) of the chondral explants grown in serum-supplemented medium (A) and serum-free medium (B) on day 42. Picrosirius-red staining (for collagen) of the chondral explants grown in serum-supplemented medium (C) and serum-free medium (D) on day 42. Viability staining of the chondral explants grown in serum-supplemented medium (E) and serum-free medium (F) on day 28. Arrows: Representative doublets of dividing cells in serum-supplemented cultures.
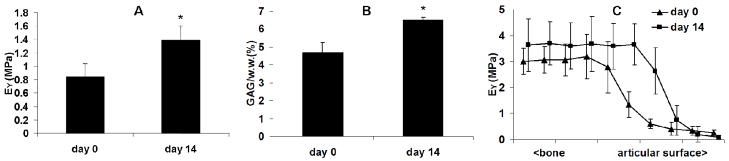
In Study 2 the equilibrium modulus and GAG content of the full-thickness osteochondral explants increased similarly as the chondral CM explants in Study 1 (Figure 5). The depth-dependent Young’s modulus plot showed an increase in compressive stiffness in the transition region from the superficial zone to the middle zone layer of cartilage after 14 days of culture (Figure 5C). There was no significant cell death observed in osteochondral explants as indicated by the viability staining (Figure 2F) and cell concentration (DNA) remained constant over the two week culture time (data now shown). Minimal swelling of the osteochondral explants was observed after 14 days of culture.
Figure 5.
Equilibrium Young’s modulus (A) and GAG content (B) of the osteochondral explants. (C) Depth-dependent (axial) Young’s modulus of the osteochondral explants. * p<0.05 vs. Day 0
4 DISCUSSION
The results of this study demonstrate that chondral and osteochondral explants can be maintained in serum-free chondrogenic medium at 37 C without decrease in Young’s modulus and dynamic modulus, GAG content and cell concentration from initial “fresh” levels for up to 6 weeks. In contrast, medium supplemented with serum resulted in excessive tissue swelling and significant degradation of explant properties that were anticipated from previous reports in the literature (Asanbaevaet al., 2007; Gray et al., 1988; Malpeliet al., 2004; Sahet al., 1989; Torzilli et al., 1997). The successful use of the serum-free medium for cartilage tissue maintenance using standard tissue culture techniques is significant for clinical application, reducing potential disease transmission as well as culture variability. We demonstrate that the stiffness and GAG content of cartilage explants can actually increase with tissue culture duration without inducing mineralization in the tissue. Our encouraging findings support the use of tissue culture as a preservation technique of chondral (Study 1) and osteochondral (Study 2) explants. The impact of these enhanced tissue properties on clinical outcome remains to be determined with in vivo studies.
No specific growth factors (such as transforming growth factor-beta 1 or insulin-like growth factor-1 typically applied) were added to the chondrogenic medium. The chondrogenic medium formulation is similar to that typically adopted for maintenance of bone marrow stem cells (BMSCs) and to support growth of chondrocyte-seeded agarose hydrogel constructs (Lima et al., 2007). The enhancement in mechanical properties of explants grown in serum-free medium over time can be explained in part by the suppressed MMP-3 activity, elevated GAG and collagen content, and probably interaction with the retained COMP. The MMP-3 enzyme is important in resorption and remodeling of the extracellular matrix and digests proteoglycan, fibronectin, and collagen and activates procollagenase (Milner, Elliott and Cawston, 2001; Sandya and Sudhakaran, 2007). COMP is an extracellular matrix protein belonging to the thrombospondin gene family and is predominantly found in cartilage, tendons, ligaments and bone growth plates (Di Cesare et al., 1999; Di Cesare et al., 2000; Saxne and Heinegard, 1992). It has been shown to bind to type II collagen fibers (Holden et al., 2001; Rosenberg et al., 1998) as well as aggrecan (Chen et al., 2007) and may therefore play a role in stabilizing the matrix. Altered levels of COMP and its degradation products in the serum and the synovial fluid are being studied as potential clinical markers of cartilage degradation in osteoarthritis and rheumatoid arthritis (Fernandes et al., 2007; Young-Min et al., 2007). At present, it is unclear whether COMP release is due to increased anabolic or catabolic activities by the chondrocytes. As this molecule binds to aggrecan (Chenet al., 2007), it is possible that COMP is lost concomitantly with GAG into the media. The much lower COMP, GAG and MMP-3 concentrations in the serum-free medium as compared with FBS medium also suggest that the serum-free medium provides a more conducive environment for maintaining the cartilage explants in terms of retaining matrix components and suppressing catabolic activities.
Accompanying the increased MMP activity and matrix loss was a significant swelling (two-fold increase from initial volume) of cartilage explants cultured with serum. This swelling resulted in an effective dilution of biochemical constituents in the tissue. This most likely contributed to the observed drop in properties. There was significantly more cell division noted from serum cultured DNA/explant data as well as histology that shows cell doublets in the outer regions of the explant. The surfaces of the cartilage explants appeared covered with layers of flattened cells. Thus, with serum, chondrocytes appeared more focused on cell division rather than matrix biosynthesis. In contrast, the volume of CM explants and cell concentration remained relatively constant with culture time, further suggesting the beneficial effects of serum-free chondrogenic medium in cartilage explant maintenance.
It is interesting to note that the serum-free medium formulation resulted in improvements in the mechanical and biochemical aspects of the cultured cartilage explants without added growth factors (such as transforming growth factor-beta 1 or insulin-like growth factor-1 typically applied). However, the formulation of the chondrogenic medium includes dexamethasone. Dexamethasone is a synthetic adrenal corticosteroid with potent anti-inflammatory properties and is used in the treatment of a wide variety of inflammatory conditions such as rheumatoid arthritis (Cuzzocrea et al., 2005). It has also been shown that dexamethasone reduces the expression of catabolic factors leading to the degradation of cartilage (Jafari et al., 1993; Morin et al., 1999), while at the same time increasing the expression of anabolic growth and differentiation factors, such as bone morphogenetic proteins (Martinovic et al., 2006; Oreffo et al., 1999). For these reasons, we speculate that dexamethasone plays a role in maintaining or enhancing the mechanical properties of the explants in serum-free medium.
We note that the promising results observed for the middle zone chondral explants (Study 1), appeared translatable to full-thickness osteochondral constructs (Study 2), and were not adversely affected by the presence of adjacent superficial and deep zone cartilage or bone in the culture. Our data indicates that culture conditions influence the maintenance of tissue properties in a zonal-dependent manner, with a stiffening of the transition region from the superficial layer to the middle zone. This resulted in an increase of average tissue properties. Future studies will elaborate further on this finding and the ability of tissue culture with chondrogenic, serum-free medium to preserve these more clinically-relevant osteochondral grafts. Additionally, we will explore the application of physiologic loading to further extend the tissue culture storage duration of cartilage explants. While the current studies were performed on juvenile bovine cartilage, a well-characterized in vitro model system, in lieu of adult human cartilage we are encouraged by the potential that serum-free culture techniques may hold for lengthening cartilage tissue storage periods, leading to improved clinical outcomes and availability of fresh osteochondral allografts. The development of long-term strategies for maintaining stable cartilage explants in culture will also provide a valuable tool for cartilage basic science studies.
Acknowledgments
This work was supported by National Institutes of Health grants AR46568 (CTH), AR53530 (JLC) and Musculoskeletal Transplant Foundation grant CU07-194 (CTH).
The authors thank Dr. Steve B. Doty (Hospital for Special Surgery, New York) for his assistance with the mineral content assay (EDAX & Von Kossa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Arthritis Rheum. 2007;56:188. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Shadle CA, Jimenez SA, Irwin JT, Lane JM, Lipton M. Arthritis Rheum. 1979;22:1093. doi: 10.1002/art.1780221008. [DOI] [PubMed] [Google Scholar]
- Bugbee WD. J Knee Surg. 2002;15:191. [PubMed] [Google Scholar]
- Byers BA, Mauck RA, Tuan RS. Trans ORS. 2006:31. [Google Scholar]
- Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. J Biol Chem. 2007;282:24591. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Paola RD, Genovese T, Muia C, Caputi AP, Salvemini D. Arthritis Rheum. 2005;52:1929. doi: 10.1002/art.21044. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Fang C, Leslie MP, Della Valle CJ, Gold JM, Tulli H, Perris R, Carlson CS. J Orthop Res. 1999;17:437. doi: 10.1002/jor.1100170321. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. J Orthop Res. 2000;18:713. doi: 10.1002/jor.1100180506. [DOI] [PubMed] [Google Scholar]
- Dumont J, Ionescu M, Reiner A, Poole AR, Tran-Khanh N, Hoemann CD, McKee MD, Buschmann MD. Connect Tissue Res. 1999;40:259. doi: 10.3109/03008209909000704. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fernandes FA, Pucinelli ML, da Silva NP, Feldman D. Scand J Rheumatol. 2007;36:211. doi: 10.1080/03009740601154186. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Gray ML. J Orthop Res. 1995;13:208. doi: 10.1002/jor.1100130209. [DOI] [PubMed] [Google Scholar]
- Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. J Orthop Res. 1988;6:777. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. J Biol Chem. 2001;276:6046. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari HS, Saez-Llorens X, Paris M, Rinderknecht S, Friedland I, Ehrett S, Severien C, Olsen KD, Burns DK, Harper CF, et al. J Infect Dis. 1993;168:1186. doi: 10.1093/infdis/168.5.1186. [DOI] [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Arch Biochem Biophys. 1988;267:416. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Malpeli M, Randazzo N, Cancedda R, Dozin B. Tissue Eng. 2004;10:145. doi: 10.1089/107632704322791790. [DOI] [PubMed] [Google Scholar]
- Martinovic S, Borovecki F, Miljavac V, Kisic V, Maticic D, Francetic I, Vukicevic S. Arch Histol Cytol. 2006;69:23. doi: 10.1679/aohc.69.23. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Yuan X, Tuan RS. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Osteoarthritis Cartilage. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- Milner JM, Elliott SF, Cawston TE. Arthritis Rheum. 2001;44:2084. doi: 10.1002/1529-0131(200109)44:9<2084::AID-ART359>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Morin I, Li WQ, Su S, Ahmad M, Zafarullah M. J Pharmacol Exp Ther. 1999;289:1634. [PubMed] [Google Scholar]
- Oreffo RO, Kusec V, Romberg S, Triffitt JT. J Cell Biochem. 1999;75:382. doi: 10.1002/(sici)1097-4644(19991201)75:3<382::aid-jcb4>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Morgelin M, Heinegard D. J Biol Chem. 1998;273:20397. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. J Orthop Res. 1989;7:619. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Sah RL, Trippel SB, Grodzinsky AJ. J Orthop Res. 1996;14:44. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- Sandya S, Sudhakaran PR. Exp Biol Med (Maywood) 2007;232:629. [PubMed] [Google Scholar]
- Saxne T, Heinegard D. Br J Rheumatol. 1992;31:583. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- Strehl R, Schumacher K, de Vries U, Minuth WW. Tissue Eng. 2002;8:37. doi: 10.1089/107632702753503036. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, Lust G. J Biomech. 1997;30:1. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chahine NO, Hung CT, Ateshian GA. J Biomech. 2003;36:339. doi: 10.1016/s0021-9290(02)00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Guo XE, Sun D, Mow VC, Ateshian GA, Hung CT. Biorheology. 2002;39:11. [PubMed] [Google Scholar]
- Young-Min S, Cawston T, Marshall N, Coady D, Christgau S, Saxne T, Robins S, Griffiths I. Arthritis Rheum. 2007;56:3236. doi: 10.1002/art.22923. [DOI] [PubMed] [Google Scholar]