Abstract
Objective
Chondrocyte-seeded agarose constructs of 4 mm diameter (2.34 mm thickness) develop spatially inhomogeneous material properties with stiffer outer edges and a softer central core suggesting nutrient diffusion limitations to the central construct region1. The effects of reducing construct thickness and creating channels running through the depth of the thick constructs were examined.
Methods
In Study 1, the properties of engineered cartilage of 0.78mm (thin) or 2.34mm (thick) thickness were compared. In Study 2, a single nutrient channel (1 mm diameter) was created in the middle of each thick construct. In Study 3, the effects of channels on larger 10 mm diameter, thick constructs was examined.
Results
Thin constructs developed superior mechanical and biochemical properties than thick constructs. The channeled constructs developed significantly higher mechanical properties versus control channel-free constructs while exhibiting similar GAG and collagen content. Collagen staining suggested that channels resulted in a more uniform fibrillar network. Improvements in constructs of 10mm diameter were similarly observed.
Conclusions
This study demonstrated that more homogeneous tissue engineered cartilage constructs with improved mechanical properties can be achieved by reducing their thickness or incorporating macroscopic nutrient channels. Our data further suggests that these macroscopic channels remain open long enough to promote this enhanced tissue development while exhibiting the potential to refill with cell elaborated matrix with additional culture time. Together with reports that <3 mm defects in cartilage heal in vivo and that irregular holes are associated with clinically used osteochondral graft procedures, we anticipate that a strategy of incorporating macroscopic channels may aid the development of clinically-relevant engineered cartilage with functional properties.
1 Introduction
Articular cartilage functions as a highly wear-resistant and low-friction weight bearing cushion in the diarthrodial joint 1, 2. Its poor healing capacity has motivated extensive research efforts aimed at developing functional tissue-engineered cartilage. It is well recognized that it is harder to supply nutrients to a larger sized tissue construct than a smaller one. From Fick’s law, the diffusion time of a solute is proportional to the square of the distance it needs to travel (Distance2 ∝ Diffusivity × Time)3, and this time is effectively increased by cell nutrient consumption along the diffusion path. Cartilage resides in a hypoxic environment, relying on nutrients via convective diffusion from joint loading-induced tissue deformation, thereby permitting diffusion distances in this avascular connective tissue to span several millimeters5, 6. While our laboratory has extensive experience with the application of physiologic deformational loading bioreactors for cultivating engineered cartilage, the current study was designed to explore strategies that optimize passive diffusion transport in developing engineered cartilage in vitro.
Agarose has been used extensively in cartilage biology because of its ability to promote and maintain the chondrocyte phenotype 7–10. It is being currently evaluated in human clinical trials as a scaffold component of a next generation autologous chondrocyte implantation strategy11, 12, showing promise for agarose-based engineered tissues for in vivo repair of cartilage. We have found that 2% agarose (Type VII, Sigma) permits robust cartilaginous tissue growth in culture and engineered cartilage with Young’s moduli (EY) and glycosaminoglycan (GAG) levels similar to native tissue in ≤8 weeks 16. While higher agarose concentrations yield initially stiffer tissue constructs, presumably due to their more efficient retention of matrix products, the long-term tissue properties become significantly inferior to those with 2% agarose 17, 18. This finding results likely from nutrient insufficiency caused by this early rapid matrix elaboration.
Despite our encouraging results with 2% agarose, closer examination reveals that the construct properties are spatially inhomogeneous, with typically softer central regions and stiffer peripheral surface regions 19. Thus while the hydraulic permeability and porosity of 2% agarose appears optimal for tissue growth at early times, tissue elaboration eventually hinders nutrient transport by producing a dense barrier to transport 20. Diffusion channels have been used in tissue engineering of bone 21 and cardiac tissue 22 and their positive effects have been well documented 23. Studies have also demonstrated potential applications of microchannels in hydrogel-based cartilage tissue engineering24–26. In this study, we hypothesize that stiffer engineered cartilage constructs can be achieved by fostering development of tissues that possess central regions with properties more similar to the outer regions. To test our hypothesis, we examined the effects of (1) decreasing the initial thickness of the engineered constructs or (2) creating nutrient channels in the constructs, thereby shortening the effective diffusion distance for tissue development. The results are presented in a series of three studies examining matrix content and mechanical properties. In Study 1, constructs of two different thicknesses (Thick vs. Thin) were compared; in Study 2 the efficacy of nutrient channels in thick constructs was investigated; in Study 3 the number of the channels was increased to study the effects of channels in larger diameter, thick constructs.
2 Material and methods
2.1 Sample preparation & tissue culture
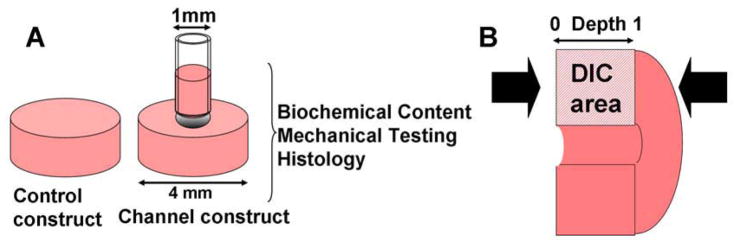
Chondrocyte-seeded agarose hydrogel disks were prepared as previously described using primary immature bovine chondrocytes (wrist joint) isolated via enzymatic digestion 14. Cells were encapsulated in 2% (w/v) low melting temperature agarose (Type VII, Sigma) in PBS at 30 × 106 cells/ml for Study 1 and 60 × 106 cells/ml for Study 2 and Study 3. In Study 1, the gel-cell mixture was cast into slabs of two different thicknesses: 0.78 (Thin) and 2.34 mm (Thick). Disks (∅4.00 mm) were cored from the slabs and cultured in defined serum-free chondrogenic medium (DMEM, 1% ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, antibiotics) 27, supplemented with ascorbate (50μg/ml). rhTGF-β3 (10ng/ml) (R&D Systems) was administered the first 2 weeks of culture. Media were changed 3 times a week. In Study 2, a ∅1 mm channel was created in the middle of the agarose disk (2.34 mm thick, ∅4.00mm) using a biopsy punch (Figure 1A) immediately after construct fabrication (day 0). Disks without a channel served as controls. For Study 3, ∅10 mm disks were punched and three ∅1 mm channels were sub-cored in a centered equilateral triangular pattern, with a mutual separation of 4.3 mm.
Figure 1.
Study 2: (A) Experimental groups. (B) DIC performed on compressed constructs. Arrow: compression direction
2.2 Mechanical testing
The spatially-averaged mechanical properties of construct disks were evaluated at selected time points using a custom table top testing device 14. The EY was determined under unconfined compression at 10% strain, followed by tests for dynamic moduli (G*) at 0.1, 0.5, and 1 Hz and 1% strain amplitude. The actual area of the channels was deducted from the total cross-sectional area of the constructs for the stress calculations. The relative error introduced by any overestimation of the actual channel size, proportional to the ratio of the area of a 1 mm hole over a 4 mm disk, was expected to be no greater than ~6%. Following average property measurements, constructs were halved and tested for local axial mechanical properties under unconfined compression on a custom microscope testing device and using optimized digital image correlation as previously described 28, 29 (Figure 1B).
2.3 Matrix molecule content analysis
One-half of each construct was weighed wet, lyophilized, reweighed dry, and digested in 0.5mg/ml Proteinase-K (Fisher Scientific) at 56°C for 16 hrs. The PicoGreen assay (Invitrogen) was used to quantify the DNA content of the constructs with Lambda phage DNA (0–1 mg/ml) as a standard 30. The GAG content was measured using dimethylmethylene blue (DMMB, Sigma) dye-binding assay with shark chondroitin sulfate (0–50 mg/ml) as a standard 31. The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio32. The collagen and GAG contents were normalized to the disk wet weight.
2.4 Histological analysis
The other halves of the constructs were fixed in a fixative solution (5% acetic acid, 3.7% formaldehyde, 70% ethanol) for 24 hrs and stored in 70% ethanol solution. After serial dehydration in ethanol, the constructs were embedded in paraffin, sectioned to 8 μm, and mounted onto microscope slides. The samples were then de-waxed, rehydrated, and stained with Safranin O (Sigma) and Picrosirius Red (Sigma) dyes to determine the distribution of GAG and collagen, respectively.
2.5 Finite Element Modeling (FEM)
Osmotic swelling of the tissue-engineered constructs was modeled using a custom finite element program 33. The objective was to identify conditions that could replicate experimental findings of central cracking in one of the tested groups. The cylindrical engineered construct was divided into two concentric regions; an inner core and outer peripheral region, with respective sizes determined from polarized light images of construct histological slices. This assumption was based on the fact that increasing birefringence was observed in the constructs with increasing culture time and the pattern of the histological staining roughly correlates with the pattern of the polarized light microscopy. A hexahedral mesh was created for one-eighth of the tissue engineered construct with boundary conditions prescribed based on symmetry. Several combinations of material properties were explored, consistent with experimental measurements, which might explain the crack formation. In the final analysis the tensile moduli of the respective regions were assigned values consistent with the intensity and distribution of Picrosirius red staining of collagen across the constructs, suitably scaled using an upper limit from experimental values obtained in our previous study 34, 35. A tensile stiffness of 2.5 MPa and 120 kPa were thus assigned to the periphery and core of the mesh, respectively. GAG content was estimated to be 8% of the wet weight in the core and 10% in the periphery of the constructs based on the results of the GAG quantification assay. Assuming two negative charges per chondroitin sulfate isomer and a molecular weight of 513 g/mol, a fixed charge density (cF) was calculated from the GAG content to be 458 mEq/L (10% GAG) and 367 mEq/L (8% GAG) 33. The water content was estimated to be 85% of wet weight based on the difference between the dry weight and wet weight of the constructs. The material properties used in FEM are summarized in Table 1.
Table 1.
Material properties used in FE modeling
| Model Parameters | Core | Periphery |
|---|---|---|
| GAG/ww (%) | 8 | 10 |
| Charge density (mEq/L) | 367 | 458 |
| Tensile modulus (MPa) | 0.12 | 2.5 |
| Water content (%) | 85 | 85 |
2.6 Statistical analysis
Statistica (Statsoft) was used to perform statistical analyses using two-way ANOVA and the Tukey HSD Post Hoc test of the means (n= 4~6 samples per group) with culture duration and experimental groups as independent factors.
3 Results
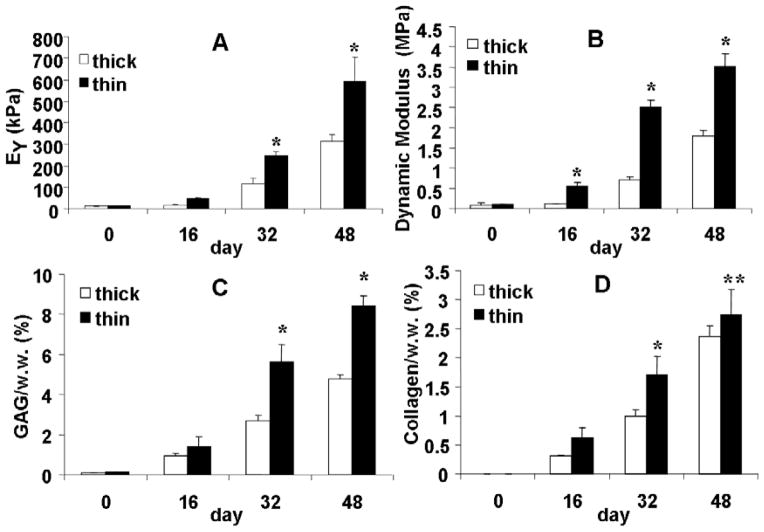
In Study 1, EY of the thin constructs were two-fold greater than that of the thick constructs, reaching values of 246±21 and 592±111 kPa on day 32 and 48, respectively (p<0.005, Figure 2A, B). G* (frequency = 0.5 Hz) of the thin constructs were 3.5±0.3 MPa on day 48, twice as much as that of the thick constructs (1.8±0.1 MPa) at the same time points. The thin constructs also developed significantly higher GAG and collagen content than the thick constructs after day 14 (p<0.005 Figure 2C, D). On day 48, the GAG content of the thin constructs reached 8.4 ± 0.49 % wet weight (%ww) compared to thick constructs 4.8 ± 0.23 %ww. The collagen content of the thin constructs was significantly higher than that of the thick constructs on both day 32 (1.71 ± 0.31 %ww versus 1.00 ± 0.10 % ww, p<0.005) and day 48 (2.73 ± 0.43 %ww versus 2.36 ± 0.19 %ww, p<0.05). Note that the difference in collagen content between the two groups on day 48 was less than that on day 32. The thin constructs had a greater DNA content and GAG/DNA ratio as compared to the thick constructs after day 16. Increased GAG content in the thin constructs could be attributed to both increased cell proliferation and elevated GAG production of individual cells (data not shown).
Figure 2.
Study 1: (A) Equilibrium modulus and (B) Dynamic modulus (0.1 Hz) of Thick and Thin construct groups. (C) GAG content of the constructs (normalized to wet weight). (D) Collagen content of the constructs (normalized to wet weight). *p<0.005 vs. Thick group.
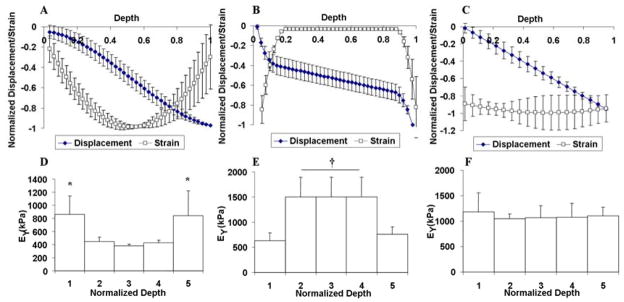
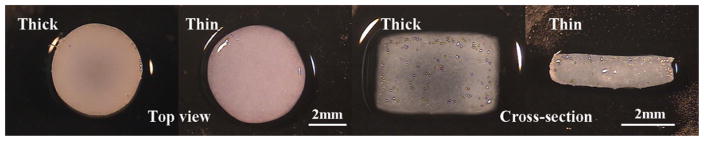
Spatial EY profiles across the longitudinal depth of the cylindrical disks varied among the groups. The thin constructs had a significantly softer layer on the surface taking up a large part of the applied compressive strain and a uniformly stiff center with minimal strain (Figure 3B, E). In contrast, thick constructs developed significantly stiffer edges and a softer central core as indicated by the U-shaped strain profile across the construct depth (Figure 3A, D), a trend reported previously 19. Top and cross-sectional views of the constructs showed that the thick constructs were more opaque at the peripheral regions than in the center whereas the thin constructs appeared homogeneous in translucency (Figure 4). Immunohistochemistry indicated that the cells produced predominantly Type II collagen with minimal Type I collagen (data not shown).
Figure 3.
Study 1: Displacement and strain (normalized by minima) profiles of the Thick (A) and Thin (B) construct groups in Study 1 and for the Channel group (C) in Study 2 across the depth (normalized by the full disk thickness) of the disks on day 48. Averaged local equilibrium modulus of the Thick (D) and Thin (E) construct groups in Study 1 and Channel group (F) in Study 2 for five layers of equal thickness across the depth of the disks. *p<0.05 vs. the central region (2–4); †p<0.001 vs. the peripheral axial surfaces (1, 5).
Figure 4.
Study 1: Top view and cross-sectional view of the Thick and Thin constructs on day 48.
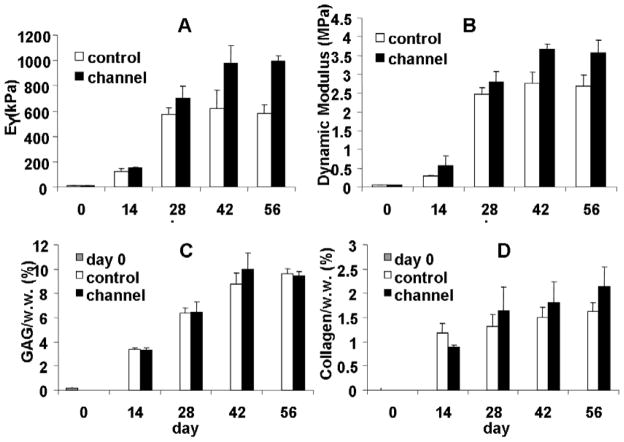
The process of punching channels did not affect cell viability at the cutting surface or other areas of the constructs (data not shown). For Study 2, the moduli of the constructs with a channel were significantly higher than controls from day 28 onward (Figure 5). Furthermore, the moduli of the control disks (without a channel) started to plateau after day 28 and stagnated afterwards until day 56, reaching values of EY=600 kPa and G*=2.7 MPa, respectively, whereas those of the disks with a channel continued to increase until day 42 and plateaued at a higher level of EY=1000 kPa and G*=3.6 MPa (Figure 5A, B). The channel constructs also possessed more uniform local stiffness along the axial direction, whereas constructs without channels developed significantly stiffer edges and a softer central core as indicated by the U-shaped strain profile across the depth of the constructs (Figure 3C, F). However, overall GAG and collagen content of the two groups were not statistically different (Figure 5C, D).
Figure 5.
Study 2: (A) Equilibrium modulus of Control and Channel construct groups. (B) Dynamic modulus of Thick and Thin construct groups (1 Hz). (C) GAG content of the constructs. (D) Collagen content of the constructs. *p<0.0005 vs. Control group, †p<0.05 vs. Control group.
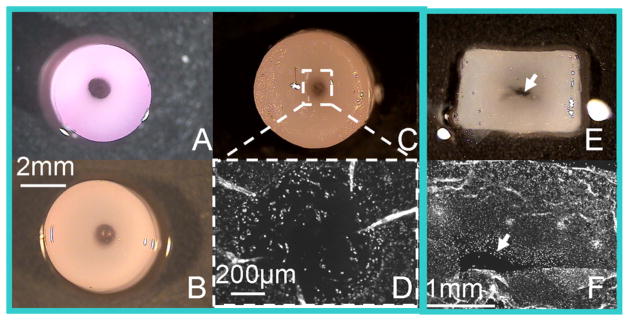
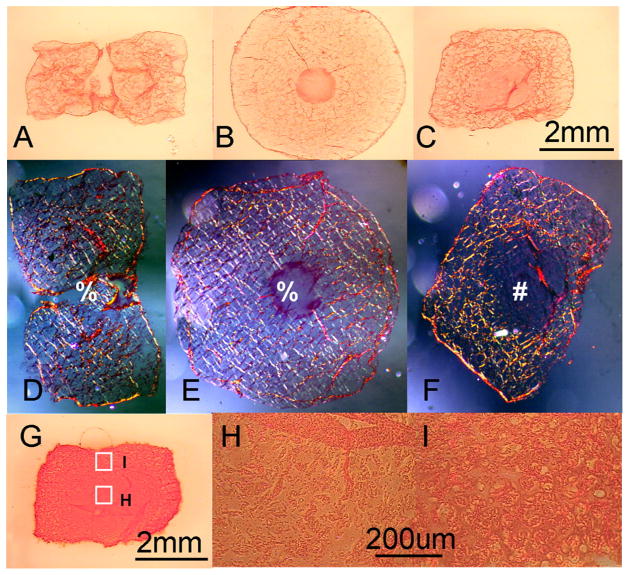
The channels were gradually filled in with translucent material and infiltrated by cells (Figure 6A–C, F). Picrosirius red staining revealed that the control constructs exhibited a mesh-like extracellular matrix structure in the peripheral regions, not apparent in the center. In contrast, the channeled disks exhibited more uniform staining throughout their cross-section (Figure 7A–C). This disparity in structural organization between the construct types is even more pronounced in polarized microscopy images of these same tissue sections (Figure 7D–E). Histology also showed more intense Safranin-O staining in the periphery of the control constructs than in the center (Figure 7G–I).
Figure 6.
Study 2: Pictures of the channel disk taken on day 0, 28, and 56 (A–C). Axial cross-sectional view of the control disk on day 56 (E). Hoechst staining of transverse slice of the channeled disk (D) and axial slice of a control disk (F) on day 56. Arrow: crack observed in control disks on day 56.
Figure 7.
Study 2: Picrosirius red staining of the channeled disk (A, B) and control disk (C) on day 42. (D) to (F) are the Polarized light pictures of the same samples shown in (A) to (C) in order. Picture b, e are transverse slices, the rest are all axial slices. Safranin-O staining of the control constructs (G) on day 42 and local staining intensity at the center (H) and periphery (I) of the constructs. % central channel; # central interior region of the control disk.
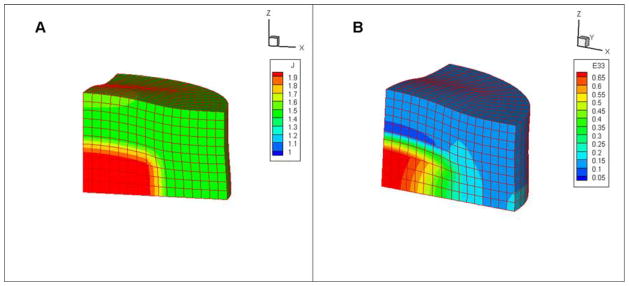
A large crack parallel to the axial disk faces was observed in the center of the control constructs on day 56 (Figure 6D, E), likely resulting from the osmotic swelling due to GAG as well as low tensile stiffness in the center of the control constructs due to the absence of an organized collagen network (as seen in the periphery), as deduced from the finite element analysis. On day 56 the constructs exhibited swelling to ~70% of initial volume. The finite element modeling results predicted a similar amount of volume expansion for the model variables chosen. The volume of the elements expanded by about 90% in the center of the constructs and by about 50% in the periphery region due to osmotic swelling (Figure 8A). The Lagrangian strain in the axial direction of the constructs was also significantly higher in the core region of the constructs, reaching a value of 0.55 whereas it remained below 0.15 in the periphery (Figure 8B). Though the finite element analysis did not explicitly model crack formation, the larger strains observed at the center is consistent with the experimental observation of cracking.
Figure 8.
(A) Volume expansion and (B) the Lagrangian strain in the axial direction (z axis), for model representative of tissue constructs in Study 2. From symmetry considerations, only one-eighth of the construct was modeled.
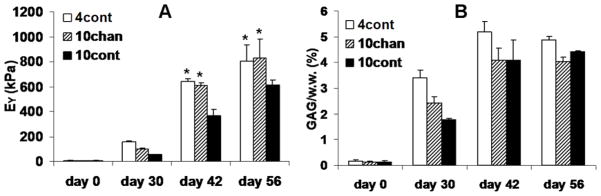
For Study 3, large constructs of 10 mm diameter with channels developed significantly higher mechanical stiffness as compared to the control constructs without channels (Figure 9A). However, in analogy to observations in ∅4 mm constructs, the GAG content is similar between the two groups (Figure 9B). The ∅10 mm control constructs exhibited lower mechanical stiffness and GAG content as compared to the ∅4 mm control constructs (Figure 9A, B).
Figure 9.
Study 3: (A) Equilibrium modulus of Control and Channel group. (B) GAG content of the constructs. *p<0.005 vs. 10 mm Control group.
4 Discussion
Various strategies to produce engineered cartilage constructs with more axially-homogeneous tissue properties were explored, including altering the construct dimensions (Study 1) and improving transport of nutrients and waste products via channels (Study 2 and Study 3). Insights gained from these fundamental studies provide rational approaches for successfully scaling up tissue engineering techniques from smaller to larger tissue constructs (Study 3). As anticipated, the results of Study 1 show that reducing the thickness of agarose constructs by one-third promotes more uniform material properties through the construct depth, as a result of the reduced transport path length (Figure 3). The mechanism underlying the softer outer layers observed in the thin constructs is unclear, and may be related to diffusive loss of GAG from the periphery. Interestingly, Klein and co-workers have observed similar strain profiles for 1 mm thick scaffold-free cartilage constructs for both zonally layered or mixed chondrocyte populations 38. Together with our findings, it appears that there may be a critical length >0.78 mm where diffusion limitations will lead to inhomogeneous cartilaginous tissue development under free-swelling culture conditions.
While Study 1 showed that thin constructs developed superior material properties, there remains a clinical need for thicker tissue constructs (e.g., ~ 5 mm for the human patella or tibial plateau 39–41). In Study 2 a single nutrient channel and in Study 3 a parallel array of ∅1 mm channels were studied for their influence on tissue elaboration in ∅4 mm and ∅10 mm constructs, respectively. Single ∅1 mm channels were chosen for Study 2 based on preliminary work (not shown) that examined the efficacy of nutrient channels with diameters ranging from 0.8 to 1.5 mm on construct tissue elaboration. It was found that channels with a diameter <1 mm became occluded within one week of culture; ∅1.5 mm channels remained completely open after 28 days; and ∅1 mm channels gradually shrank in size and were partially sealed up by day 28. Channels introduced at a late stage of culture, when the cells had already produced significant extracellular matrix, rather than immediately after construct fabrication on day 0, did not influence construct properties 14 days later.
For the application of channels to be efficacious, the channels must remain open long enough to play a role in providing greater nutrient access to chondrocytes when significant tissue matrix elaboration has occurred (and where a plateauing of tissue properties are often noted). While microchannels (0.1 mm diameter in alginate, Choi and co-workers) can modulate diffusion of solutes in constructs at early culture times24, our findings would suggest that channel sealing would occur, limiting the benefits of these microchannels channels to short-term culture prior to significant matrix elaboration.
In Study 2, constructs (2.34 mm thick) were seeded with 60 million cells/ml, which is twice that of Study 1. This culture condition is expected to increase the effect of nutrient limitations to tissue development, with increases in construct dimension and nutrient consumption 42. Our previous study found that at this high seeding density the mechanical stiffness of the agarose constructs quickly increased and plateaued43. The introduction of nutrient channels in the current study not only elevated construct stiffness but also delayed the plateauing of the mechanical properties of the constructs (Figure 5), probably due to the improved nutrient delivery to the center of the constructs. The average content of GAG and collagen, the two major matrix constituents of articular cartilage, were similar for constructs with and without a central channel. However, there was a striking difference in the structural organization of the fibrillar network between the construct types (Figure 7). As a result of the introduced channels, the provision of nutrients through the construct periphery and center increased the surface area for diffusion by only 10.5 % but decreased the path for radial diffusion by 50%; this led to a more uniform fibrillar network of extracellular matrix, which was in contrast to the presence of an organized network of fibers only in the outer peripheral regions as well as occasional cracking of the control constructs.
The central crack observed in the control constructs (Figure 6) likely resulted from osmotic swelling due to GAG as well as low tensile stiffness in the center of the control constructs due to absence of organized collagen network as compared to the periphery (Figure 7). This crack is not typically found in low seeding density (30 million cells/ml) constructs. Its occurrence in the high-seeding density constructs is reflective of the higher GAG content achieved with the higher seeding density (compare Thick samples in Figure 2C to control samples in Figure 5C). The finite element model provides insights to the structure-function relationships developed in the engineered cartilage tissue. In the engineered cartilage, GAG levels (Figure 5C) were similar to, or even higher than native levels (~6% ww), producing significant osmotic pressures (estimated at ~0.12 MPa when using ideal Donnan law) and swelling. Whereas swelling is resisted by the collagen matrix in native tissue, the constructs’ collagen levels (Figure 5D) were only a fraction of the native values (~20% ww), and mostly deficient at the center of the construct (Figure 7F). The model of a construct deficient in collagen at its center predicted a swelling strain there that was four times greater than in the peripheral region. As the yield strain for 2% w/v type VII agarose is ~0.2, these results suggest that exceedingly high swelling-strains in the central region of the constructs may give rise to construct cracking. Therefore, the elevated GAG content and non-uniform distribution of collagen created conditions that supported internal cracking. As strategies evolve for increasing collagen content in constructs and promoting development of more uniform tissue properties, as shown in the channeled constructs, cracking can be avoided. Indeed, this study shows that channels created on day 0 clearly demonstrate many advantages, such as improved material properties and more homogeneous composition, and no apparent adverse effects.
The characteristic solute diffusion time τ in a disk whose lateral and top axial surfaces are exposed to the culture medium depends on the radius a and thickness h of the disk. According to Fickian diffusion, this characteristic time constant varies according to τ ∝ D−1[(π/2h)2 + (γ0/a2)]−1, where D is the solute diffusion coefficient in the tissue and γ0 is the first root of the Bessel function of the first kind, of order zero (γ0 ≈ 2.4 )47. Based on this relation it can be deduced that the characteristic solute diffusion time τ4 in the ∅4 mm disks ( a = 2 mm, h = 2.34 mm) is ~3 times shorter than τ∅10 in the ∅10 mm disks ( a = 5 mm, h = 2.34 mm). Taking into account nutrient consumption and/or binding by cells (reaction-diffusion effects), it is therefore not surprising that in Study 3 there was a negative effect associated with the larger radial dimension on the development of tissue properties (despite scaling up the cell-to-medium volume proportionately). With the diameter of the constructs 2.5-fold greater than those of Study 2, the compressive moduli were ~25% lower and the GAG content of the larger constructs was also significantly lower (Figure 5, Figure 9). This lower GAG content, which resulted in lower swelling pressure, may explain why the crack seen in Study 2 was not observed in 10 mm control constructs in Study 3. With the incorporation of an array of channels, however, the tissue properties were restored, consistent with the resulting reduction in the characteristic solute diffusion time constant.
We believe that nutrient limitations are a significant impediment to cultivation of engineered cartilage with native functional properties. While applied mechanical loading has been shown by our group and by other researchers to promote solute transport in to cartilage and engineered constructs44, 45, we have found that with an optimized medium recipe that loading has showed less beneficial effects (only about 15–20% stiffer than the free swelling grouping our most recent media optimization16). Furthermore, the enhancement of nutrient transport into engineered constructs with dynamic loading will be less significant in constructs of larger dimension (such as targeted for repair of an entire articular surface) or with more elaborated matrix, which can hinder the transport of the nutrients. While mechanical loading continues to be attractive in promoting the growth of engineered cartilage (via a biophysical stimulus and enhanced transport), supplementing loading with nutrient channels may generate synergistic effects.
While the introduction of channels or “holes” in the engineered cartilage may raise some concerns, we anticipate these channels to completely seal themselves with additional culturing as the channels are beginning to be filled in with tissue by culture day 56. If constructs with unsealed channels were to be implanted, we may also expect they would seal in vivo, as observations in the literature indicate that cartilage defects of <3 mm diameter heal spontaneously46. In addition, clinically accepted cartilage repair strategies that use single or multiple osteochondral grafts also introduce irregular holes to the articular surface. For these reasons, we are encouraged by the incorporation of large diameter channels as a strategy to develop clinically-relevant engineered cartilage tissue. Ultimately, it remains to be seen with in vivo studies how such channels are tolerated.
Previously we have found that mature bovine chondrocytes produce much less matrix components as compared to juvenile bovine chondrocytes cultured under similar conditions. Through optimized expansion protocols however, mature chondrocytes can be primed to produce more matrix molecules48 and engineered cartilage constructs with compressive stiffness of about 200 kPa are possible with adult chondrocytes 49.
5 Conclusion
This study demonstrated that more homogeneous tissue engineered cartilage constructs with improved mechanical properties can be achieved by reducing their thickness or incorporating macroscopic nutrient channels. An array of channels as proposed above may permit cultivation of single constructs having appropriate thickness, with the channels eventually filling naturally. The application of physiologic deformational loading with concomitant convective transport would be expected to further enhance the passive strategies to increase nutrient diffusion described in this investigation.
Acknowledgments
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR46568, CTH) of the National Institutes of Health, USA. The help of Mr. Vikram Rajan and Ms. Clare E. Canal with the finite element program is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Philadelphia: 1997. pp. 179–207. [Google Scholar]
- 2.Mow VC, Bachrach NM, Setton LA, Guilak F. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mow VC, Guilak F, Hayes WC, Tran-Son-Tay R, Hochmuth RM, editors. Cell Mechanics and Cellular Engineering. New York: 1994. pp. 345–379. [Google Scholar]
- 3.Atkins PW. Physical Chemistry. 5. New York: W. H. Freeman; 1994. [Google Scholar]
- 4.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A:1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Potter K, Spencer RG, McFarland EW. Magnetic resonance microscopy studies of cation diffusion in cartilage. Biochim Biophys Acta. 1997;1334:129–139. doi: 10.1016/s0304-4165(96)00083-9. [DOI] [PubMed] [Google Scholar]
- 6.Schiller J, Naji L, Trampel R, Ngwa W, Knauss R, Arnold K. Pulsed-field gradient-nuclear magnetic resonance (PFG NMR) to measure the diffusion of ions and polymers in cartilage: applications in joint diseases. Methods Mol Med. 2004;101:287–302. doi: 10.1385/1-59259-821-8:287. [DOI] [PubMed] [Google Scholar]
- 7.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 8.Aydelotte MB, Schleyerbach R, Zeck BJ, Kuettner KE. Articular chondrocytes cultured in agarose gel for study of chondrocytic chondrolysis. In: Kuettner K, editor. Articular Cartilage Biochemistry. New York: Raven Press; 1986. pp. 235–256. [Google Scholar]
- 9.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 10.Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828–835. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 11.Selmi TA, Neyret, Verdonk PCM, Barnouin L. Autologous Chondrocyte Transplantation in Combination With an Alginate-Agarose Based Hydrogel (Cartipatch) Techniques in Knee Surgery. 2007;6:253–258. [Google Scholar]
- 12.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: OUTCOME AT TWO YEARS. J Bone Joint Surg Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 13.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 14.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 15.Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kugler LE, Ng KW, O’Conor CJ, Ateshian GA, Hung CT. Scaffold properties play a critical role in the retention of Synthesized glycosaminoglycans in tissue engineered cartilage. ASME 2007 Summer Bioengineering Conference; Colorado, USA. 2007. [Google Scholar]
- 18.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- 21.Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74:782–788. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- 22.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 24.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 25.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 26.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 27.Byers BA, Mauck RA, Tuan RS. Temporal exposure of TGF-β3 under serum-free conditions enhances biomechanical and biochemical maturation of tissue-engineered cartilage. Trans ORS. 2006:31. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CC, Chahine NO, Hung CT, Ateshian GA. Optical determination of anisotropic material properties of bovine articular cartilage in compression. J Biomech. 2003;36:339–353. doi: 10.1016/s0021-9290(02)00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CC, Deng JM, Ateshian GA, Hung CT. An automated approach for direct measurement of two-dimensional strain distributions within articular cartilage under unconfined compression. J Biomech Eng. 2002;124:557–567. doi: 10.1115/1.1503795. [DOI] [PubMed] [Google Scholar]
- 30.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 31.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 32.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01027.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kelly TN, Lima EG, Ateshian GA, Clark TH. Tension-compression nonlinearity in engineered cartilage. 2008 submitted to Journal of biomechanical engineering. [Google Scholar]
- 35.Kelly TN, Ateshian GA, Clark TH. Effect of dynamic loading on tension-compression nonlinearity in engineered cartilage. Orthopaedic Research Society; San Diego, CA: 2007. [Google Scholar]
- 36.Ng KW, Defrancis JG, Kugler LE, Kelly TA, Ho MM, O’Conor CJ, et al. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2007 doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng KW, Saliman JD, Lin EY, Statman LY, Kugler LE, Lo SB, et al. Culture duration modulates collagen hydrolysate-induced tissue remodeling in chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2007;35:1914–1923. doi: 10.1007/s10439-007-9373-z. [DOI] [PubMed] [Google Scholar]
- 38.Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Draper CE, Besier TF, Gold GE, Fredericson M, Fiene A, Beaupre GS, et al. Is cartilage thickness different in young subjects with and without patellofemoral pain? Osteoarthritis Cartilage. 2006;14:931–937. doi: 10.1016/j.joca.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Eckstein F, Muller-Gerbl M, Putz R. Distribution of subchondral bone density and cartilage thickness in the human patella. J Anat. 1992;180 ( Pt 3):425–433. [PMC free article] [PubMed] [Google Scholar]
- 41.Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech. 1991;24:761–776. doi: 10.1016/0021-9290(91)90340-s. [DOI] [PubMed] [Google Scholar]
- 42.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 44.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 45.Quinn TM, Studer C, Grodzinsky AJ, Meister JJ. Preservation and analysis of nonequilibrium solute concentration distributions within mechanically compressed cartilage explants. J Biochem Biophys Methods. 2002;52:83–95. doi: 10.1016/s0165-022x(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Crank J. The mathematics of diffusion. 2. Oxford, Eng: Clarendon Press; 1979. [Google Scholar]
- 48.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 49.Ng KW, O’Conor CJ, Lima EG, Lo SB, Ateshian GA, Cook JL, et al. Trans ORS. Vol. 33. San Francisco CA: 2008. Primed mature canine chondrocytes can develop an engineered cartilage tissue with physiologic properties. [Google Scholar]
- 50.Gleghorn JP, Lee CS, Cabodi M, Stroock AD, Bonassar LJ. Adhesive properties of laminated alginate gels for tissue engineering of layered structures. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31565. [DOI] [PubMed] [Google Scholar]