Abstract
The intake of the n-3 fatty acids alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) has been related to testosterone levels in epidemiological analyses. The aim of this study was to assess whether the n-3 fatty acids affects testosterone levels in post-myocardial infarction (MI) patients, who are at risk of testosterone deficiency. In a double-blind, placebo-controlled trial of low-dose supplementation of n-3 fatty acids, we included 1850 male post-MI patients aged 60–80 y who participated in the Alpha Omega Trial. Patients were randomly allocated to margarines that provided 400 mg/d of EPA–DHA (n=453), 2 g/d of ALA (n=467), EPA–DHA plus ALA (n=458), or placebo (n=472). Serum testosterone levels were assessed at baseline and after 41 months using whole day blood samples obtained at the subjects' home or at the hospital. Subjects were on average age of 68.4 (SD 5.3) years old and had baseline mean serum total testosterone of 14.8 (SD 5.6) nmol/L. The four randomized groups did not differ for baseline characteristics. ALA, EPA–DHA, and EPA–DHA plus ALA supplementation did not affect serum total testosterone compared to placebo. Moreover, n-3 fatty acid supplementation did not affect the risk of incident testosterone deficiency (n=76 with total testosterone <8.0 nmol/L). We conclude that n-3 fatty acids supplementation did not affect serum total testosterone in men who had had a MI.
Key terms: n-3 polyunsaturated fatty acids, alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, testosterone
Introduction
Long-chain n-3 polyunsaturated fatty acids include eicosapentaenoic acid (EPA, C20:5n3) and docosahexaenoic acid (DHA, C22:6n-3) derived from fish, and alpha-linolenic acid (ALA, C18:6n3) from vegetable origin. Supplementation with these n-3 fatty acids has become popular in Western countries, because of reports suggesting a variety of health benefits including a reduced risk of cardiovascular diseases (Léon et al., 2008). Whereas testosterone administration decreases (Giltay et al., 2004b) and estrogen administration increases (Giltay et al., 2004b; Giltay et al., 2004a) DHA levels, there are also indications from in vitro studies and studies in animals and humans n-3 fatty acid intake affect androgen secretion and metabolism (Nagata et al., 2000; Gromadzka-Ostrowska, 2006). If so, several aspect of male physiology would be affected. Testosterone is not only involved in male reproduction and sexual arousal, but also affects bone mineral density, prostate gland physiology, and erythropoiesis (Kaufman and Vermeulen, 2005; Dandona and Dhindsa, 2011). Although n-3 fatty acids may affect male fertility (Wathes et al., 2007) and the risk of (androgen-sensitive) prostate cancer (Akaza et al., 2009; Rose, 1997; Norrish et al., 1999; Terry et al., 2001; Leitzmann et al., 2004; Augustsson et al., 2003; De Stefani et al., 2000; Gann et al., 1994; Harvei et al., 1997; Newcomer et al., 2001; Brouwer et al., 2004), it is unclear whether these effects are partially mediated through altered testosterone levels.
An observational study in 69 Japanese men aged 43–88 years showed that EPA and DHA intake was associated with lower total testosterone levels. Only one small experimental study did assess the effects of n-3 fatty acids on serum testosterone in men. Using a cross-over design in 13 men with hypertension and 13 nonhypertensive men, a very high dose of 5 g of EPA-DHA did not affect testosterone levels after 30 days (Hughes et al., 1990). Two other trials were conducted in women with the polycystic ovary syndrome and showed inconsistent results on serum total testosterone. (Phelan et al., 2011; Vargas et al., 2011)
In the present randomized, double-blind, placebo-controlled study we assessed the effects of EPA–DHA (400 mg/d), ALA (2 g/d), or both on serum testosterone levels during 41 months of follow-up in 1850 male patients who survived a myocardial infarction (MI). We aimed to assess whether in these older men with coronary heart disease, who are at risk of testosterone deficiency (Malkin et al., 2010), n-3 fatty acids affect serum testosterone compared to placebo.
Subjects and methods
Patients
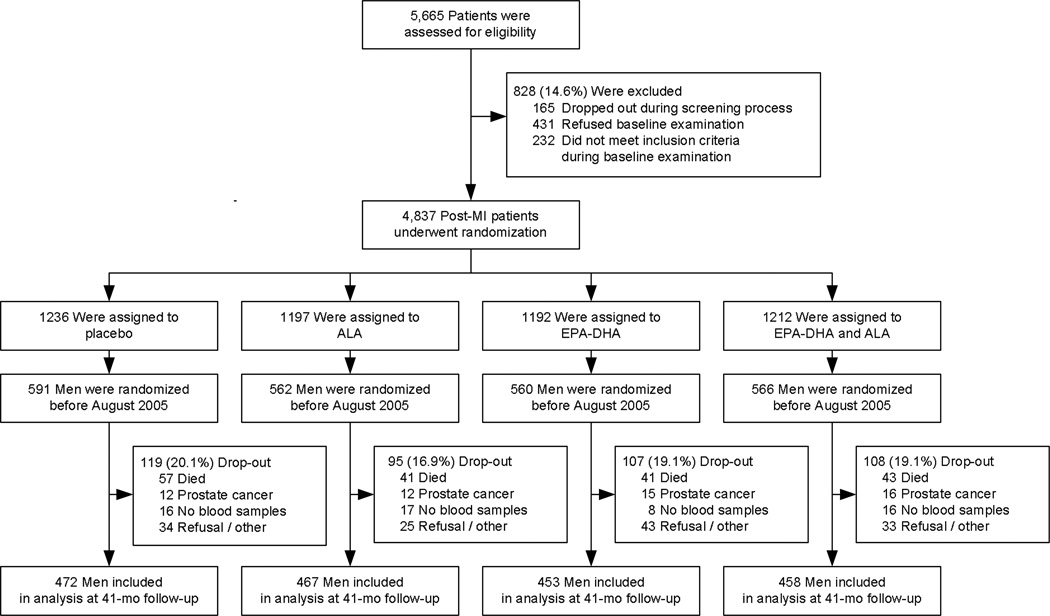
The present study made use of the design and infrastructure of the Alpha Omega Trial, a double-blind placebo-controlled trial aimed to examine whether cardiovascular diseases can be prevented by low-dose supplementation of omega-3 fatty acids in margarines during 41 months, as described in detail elsewhere (Geleijnse et al., 2010; Kromhout et al., 2010). In brief, 4,837 free-living Dutch post-MI patients aged 60–80 years were randomized to receive one of four margarines. Blood was drawn twice in 2,531 patients (52.3% of the cohort). Of these participants, 2279 were men who were randomized before August 2005 and scheduled to complete the intervention before 1 January 2009 (Figure 1). After 1 January 2009, blood was no longer collected because of budgetary constraints (Geleijnse et al., 2010), and changes in testosterone levels could not be assessed. Of the 2279 men, 429 did not participate in the present analysis because 182 men died, 55 had prevalent or incident prostate cancer, or received androgen(-deprivation) therapy, 57 were scheduled to but did not provide blood samples at both time points, and 135 refused a final examination for various reasons (Figure 1). 1850 patients remained for the present study. Baseline examination took place between May 15th 2002 until August 1 2005, while the final examination took place between October 12th 2005 until January 27th 2009. The mean time interval between the two blood samples was 41.4 (SD 1.2) months. The Alpha Omega Trial was conducted in accordance with the Helsinki Declaration and approved by a central medical ethics committee in the Netherlands. Written informed consent was obtained from all patients. The data and safety monitoring board monitored the safety of the patients.
Figure 1.
Flow chart of the Alpha Omega Trial. Prostate cancer was defined as non-fatal prevalent or incident prostate cancer (or the use of antiandrogenic or other androgen-modulating medication).
Intervention with n-3 fatty acids
Patients were randomly allocated to daily intake of ~20 grams of trial margarines that provided 400 mg of EPA–DHA (ratio 3:2), 2 grams of ALA, 400 mg of EPA–DHA plus 2 grams of ALA (EPA–DHA + ALA), or placebo, for 41 months. Oleic acid was exchanged for n-3 fatty acids. Actual treatment was preceded by 4–6 weeks on placebo margarine for logistic reasons. Trial margarines were completely identical, except for n-3 fatty acids and oleic acid. Compliance was monitored via margarine tub counts, telephone interviews, patient diaries, as well as in randomly selected patients after 20 and 41 months, in whom n-3 fatty acids in plasma cholesteryl esters were assessed as an objective measure of compliance.
Data collection and follow-up procedures
Patients were interviewed and physically examined by trained research nurses at home or in the hospital. Information on demographic variables, lifestyle habits, current health status and medical history were collected by self-administered questionnaires (Geleijnse et al., 2010). Diabetes was considered to be present if a patient reported having received the diagnosis from a physician, was taking antidiabetic drugs, or had an elevated plasma glucose level (≥7.8 mmol per liter [140.5 mg per deciliter] in the case of patients who had fasted more than 4 hours or ≥11.1 mmol per liter [200.0 mg per deciliter] in the case of nonfasting patients). Anthropometric measurements included the body mass index (BMI; kg/m2). Subjects were categorized for the highest attained level of education, current smoking and alcohol use. Medication was coded according to the Anatomical Therapeutic Chemical Classification System (ATC), with C02, C03, C07, C08, and C09 for blood pressure lowering drugs and C10 for lipid modifying drugs. Dietary data were collected by a 203-item food frequency questionnaire, including the intake of different types of fish (Geleijnse et al., 2010).
After 41 months, patients were re-invited for an interview and physical examination. The vital status of all patients was monitored via municipal registries and by telephone interviews after 41 months of follow-up, and no loss to follow-up occurred. Incident prostate cancer was monitored and included fatal cases and verified hospital admissions for prostate cancer. Men with prevalent and incident prostate cancer were excluded from the present analysis, because of the common use of anti-androgenic treatment (Akaza et al., 2009).
Testosterone assessment
Standardized blood handling procedures for the Alpha Omega Trial are described in detail elsewhere (Giltay et al., 2003). Briefly, two single whole day blood samples were obtained at the subjects' home or at the hospital at baseline and after 41 months. Tubes were packaged in sealed envelopes and sent via standard postal service to a central laboratory. Of blood samples collected during the pilot study, 89% was delivered at the laboratory within 24 hours and 96% within 48 hours. Blood samples were immediately processed and stored at −80°C. Testosterone levels was measured using the 2nd generation testosterone immunoassay (Architect i2000 analyzer; Abbott Diagnostics, Abbott park, IL, USA). This testosterone assay showed a very strong correlation with our ID-LC-MS/MS testosterone assay (Pearson’s r=0.97; testosterone levels >4.0 nmol/L) (Bui et al., 2010). Intra- and inter-assay variation of the assay above the levels of 2 nmol/L was 4% and 6%, respectively, and the lower limit of quantification was 0.1 nmol/L.
Data analysis
Baseline characteristics in the four groups are presented as mean ± standard deviation, median with interquartile range (IQR) or percentage. Differences between groups were tested by one-way analysis of variance (ANOVA) or chi-squared test, depending on the variable. Pearson’s correlation coefficient was used to analyze the association between baseline and 41 month testosterone levels.
Changes in testosterone levels after 41 months in the three n-3 fatty acid groups versus the placebo group were assessed by ANOVA, and analysis of covariance (ANCOVA) for multivariable models that included age, body mass index, education level, marital status, smoking status, alcohol use, baseline testosterone, and times of blood sampling. Crude and adjusted treatment effects are presented with 95% confidence intervals (CI). The effect of n-3 fatty acids on risk of testosterone deficiency (total testosterone levels < 8.0 nmol/L) (Wang et al., 2009; Wu et al., 2010) was assessed by logistic regression after exclusion of 144 (7.8%) patients with testosterone deficiency at baseline. Crude and adjusted odds ratios (OR) are presented with 95% CI. The main analyses were repeated after exclusion of non-compliant patients, who consumed <80% of the time trial margarine. A two-sided P-value <0.05 was considered statistically significant. No adjustments were made for multiple comparisons. SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA) was used for data-analysis.
Results
Baseline characteristics of the 1850 included male post-MI patients were well-balanced over the groups due to randomization (Table 1). The average intake of trial margarine was 20.2 (SD 3.5) g/d and 96.8% of the patients consumed ≥80% of the time the supplied margarines. The median intervention period was 41.3 (IQR, 40.7–41.9) months, including 4–6 weeks on placebo margarine. At baseline, 144 (7.8%) men had a serum total testosterone level below 8 nmol/L and 460 (24.9%) between 8 and 12 nmol/L. The average time of blood sampling was 11:20 h at baseline (1st and 99th percentiles 8:00 and 15:54 h), and 11:43 h at 41 months follow-up (1st and 99th percentiles 8:30 and 16:15 h).
Table 1.
Baseline characteristics of 1850 male CHD patients to the assigned n-3 fatty acids.
| Variables | Placebo (N=472) |
ALA (N=467) |
EPA-DHA (N=453) |
EPA-DHA and ALA (N=458) |
P valuea |
|---|---|---|---|---|---|
| Age, mean (SD), y | 68.2 ± 5.2 | 68.4 ± 5.5 | 68.6 ± 5.4 | 68.7 ± 5.4 | 0.56 |
| Time since MI, mean (SD), y | 4.6 ± 3.3 | 4.6 ± 3.3 | 4.7 ± 3.3 | 4.5 ± 2.9 | 0.73 |
| Diabetesb, No. (%) | 92 (19.5%) | 78 (16.7%) | 74 (16.3%) | 78 (17.0%) | 0.57 |
| Body mass indexc, mean (SD), kg/m2 | 27.7 ± 3.6 | 27.3 ± 3.1 | 27.5 ± 3.2 | 27.4 ± 3.2 | 0.46 |
| Higher education, No. (%) | 222 (47.3%) | 212 (45.6%) | 190 (42.2%) | 213 (46.6%) | 0.42 |
| Married, No. (%) | 407 (86.2%) | 406 (86.9%) | 388 (85.7%) | 393 (85.8%) | 0.94 |
| Current smoker, No. (%) | 77 (16.3%) | 80 (17.1%) | 76 (16.8%) | 64 (14.0%) | 0.56 |
| Alcohol used, No. (%) | 381 (80.7%) | 374 (80.3%) | 372 (82.1%) | 376 (82.3%) | 0.82 |
| Medication use, No. (%)e | |||||
| Blood pressure lowering drugs | 402 (85.2%) | 401 (85.9%) | 398 (87.9%) | 386 (84.3%) | 0.46 |
| Lipid modifying drugs | 404 (85.6%) | 408 (87.4%) | 385 (85.0%) | 398 (86.9%) | 0.70 |
| High fish intake (≥10 g/d), No. (%) | 292 (66.1%) | 299 (68.6%) | 261 (62.6%) | 295 (68.8%) | 0.19 |
| Total testosterone level (nmol/L) | 14.8 ± 5.5 | 14.8 ± 5.4 | 14.9 ± 6.1 | 14.6 ± 5.6 | 0.94 |
| Time of blood sampling (h:min) | 11:24 ± 2:04 | 11:21 ± 2:16 | 11:17 ± 2:04 | 11:17 ± 2:00 | 0.77 |
| Testosterone deficiency (<8 nmol/L), No. (%)f | 38 (8.1%) | 31 (6.6%) | 37 (8.2%) | 38 (8.3%) | 0.76 |
Abbreviations: ALA, alpha-linolenic acid (ALA); DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MI myocardial infarction.
Numbers may not total to the column total because of missing values for some variables.
Data are reported as no. (%) or mean ± standard deviation (SD).
Chi-square tests and analysis of variance was used to determine statistical significance.
Diabetes was considered to be present if a patient reported having received the diagnosis from a physician, was taking antidiabetic drugs, or had an elevated plasma glucose level (≥7.8 mmol per liter [140.5 mg per deciliter] in the case of patients who had fasted more than 4 hours or ≥11.1 mmol per liter [200.0 mg per deciliter] in the case of nonfasting patients).
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Alcohol use was defined as ≥1 glass/week. Higher education was defined as higher vocational education, college or university.
Lipid modifying drugs ATC codes C10; Blood pressure lowering drugs ATC codes C02, C03, C07, C08 and C09.
Testosterone deficiency was defined as a serum total testosterone level <8.0 nmol/L.(Wang et al., 2009; Wu et al., 2010)
There were large changes in n-3 fatty acid levels in plasma cholesteryl esters in the three n-3 FA groups versus placebo, that were maintained throughout the intervention period. Serum ALA was 67% and 71% higher than baseline values after 41 months of ALA and EPA–DHA plus ALA supplementation, respectively. Serum EPA was 55% and 119% higher, and serum DHA was 29% and 36% higher after EPA–DHA and EPA–DHA plus ALA supplementation, respectively. Blinding was successful, because 74.6% patients could not tell which margarine they had used and the remaining patients were unable to do better than chance. The four groups did not differ significantly in gastrointestinal complaints, skin problems, or other side effects (P’s>0.2) (Kromhout et al., 2010). None of the visits to an emergency department, hospitalizations, or other serious adverse events could be related to use of trial margarines, as judged by the data and safety monitoring board.
The post-MI patients had a mean baseline testosterone level of 14.8 (SD 5.6) nmol/L, which did not differ among the four groups (p=0.94; Table 1). Baseline and 41 month testosterone levels were strongly correlated in the placebo group (r=0.73; P<0.001). Table 2 shows the effect of n-3 fatty acid treated groups vs. placebo on changes in testosterone levels. We found no effects for ALA, EPA-DHA, nor for the combination on changes in total testosterone levels, although there was a tendency of borderline statistical significance for a decline in testosterone levels in the group treated with ALA compared to placebo (of −0.50 nmol/L; SD 0.26; P=0.052). In a sensitivity analysis in 691 men with both baseline and follow-up blood sampling between 8–11 am findings remained similar, with no statistically significant differences in any of n-3 fatty acid treated groups compared to placebo (all P’s in adjusted analyses >0.2).
Table 2.
Effects of n-3 fatty acids on serum total testosterone levels in 1850 male CHD patients.
| Variables | Placebo (N=472) |
ALA (N=467) |
EPA-DHA (N=453) |
EPA-DHA and ALA (N=458) |
|---|---|---|---|---|
| Baseline total testosterone, nmol/L | ||||
| Mean (SE) | 14.79 ± 0.25 | 14.79 ± 0.25 | 14.87 ± 0.28 | 14.64 ± 0.26 |
| Median (interquartile range) | 14.0 (10.8–17.9) | 14.4 (11.1–17.7) | 13.9 (11.0–17.6) | 14.1 (11.1–17.3) |
| 41 months total testosterone, nmol/L | ||||
| Mean (SE) | 15.35 ± 0.27 | 14.97 ± 0.26 | 15.13 ± 0.28 | 15.28 ± 0.29 |
| Median (interquartile range) | 14.7 (11.1–18.5) | 14.5 (11.1–18.0) | 14.5 (11.3–18.2) | 14.3 (11.0–19.0) |
| Mean (SE) changes in total testosterone, nmol/L | 0.56 ± 0.18 | 0.18 ± 0.18 | 0.26 ± 0.21 | 0.64 ± 0.20 |
| Mean (SE) changes in total testosterone vs. placebo, nmol/L | 0 (ref) | −0.38 ± 0.27 | −0.31 ± 0.27 | 0.08 ± 0.27 |
| P-value | 0.16 | 0.26 | 0.77 | |
| Adjusted mean changes in total testosterone vs. placebo, nmol/La | −0.50 ± 0.26 | −0.26 ± 0.26 | 0.05 ± 0.26 | |
| P-value | 0.052 | 0.26 | 0.84 | |
| No. of subjects without testosterone deficiency at baseline | 434 | 436 | 416 | 420 |
| Testosterone deficiency at 41 months | 21 (4.8%) | 19 (4.4%) | 19 (4.6%) | 17 (4.0%) |
| Odds ratio vs. placebo for testosterone deficiency at 41 monthsb | 1.0 (ref) | 0.90 (0.48–1.69) | 0.94 (0.50–1.78) | 0.83 (0.43–1.60) |
| P-value | 0.74 | 0.85 | 0.58 | |
| Adjusted odds ratio vs. placebo for testosterone deficiency at 41 monthsa,b | 1.0 (ref) | 0.86 (0.42–1.74) | 1.04 (0.52–2.08) | 0.84 (0.41–1.74) |
| P-value | 0.67 | 0.92 | 0.64 | |
Abbreviations: ALA, alpha-linolenic acid (ALA); DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MI myocardial infarction.
Numbers may not total to the column total because of missing values for some variables.
Data are reported as no. (%), mean ± standard error (SE), or odds ratio (95% confidence interval).
Adjusted for age, body mass index, education level, marital status, smoking status, alcohol use, baseline testosterone levels, and both times of blood collection.
Testosterone deficiency was defined as a serum total testosterone level <8 nmol/L,(Wang et al., 2009; Wu et al., 2010) and 136 men with testosterone deficiency at baseline were excluded.
There were 76 incident cases of testosterone deficiency (4.5%), who had a mean testosterone level of 6.6 [SD 1.3] nmol/L. These 76 men had a mean decline of 3.8 (SE 0.3) nmol/L over 41 months of follow-up. N-3 fatty acid supplementation had no effect on incident testosterone deficiency (Table 2). Similar findings were obtained in multivariable analysis and after exclusion of 60 non-compliers.
Discussion
We found no effects of EPA–DHA or ALA supplementation on serum total testosterone levels in post-MI patients. This can be considered reassuring, because significant modulation of serum testosterone would have raised clinical concerns with respect to male reproduction function (e.g., erectile function and sperm quality) or other androgen-related physiology such as body composition (e.g., bone mineral density, muscle development, fat distribution), prostate gland physiology, and erythropoiesis (Kaufman and Vermeulen, 2005; Dandona and Dhindsa, 2011). Moreover, low-dose n-3 fatty acids in margarines were well tolerated. These findings further supports the safety of the consumption of n-3 fatty acids.
Our findings of effects on serum total testosterone is consistent with a cross-over trial with high-dose n-3 fatty acids in 26 men (Hughes et al., 1990). Two other trials were done in women with the polycystic ovary syndrome characterized by hyperandrogenism. In the first randomized trial in 22 women, 1.9g EPA and DHA per day for 6 weeks did reduce bioavailable testosterone levels compared to placebo oil, while total testosterone levels decreased nonsignificantly (Phelan et al., 2011). The other trial with either fish oil, flaxseed oil or soybean oil for 6 weeks in 51 women did not show differential effects among the 3 randomized groups on serum total testosterone levels (Vargas et al., 2011).
Testosterone deficiency has been linked to poor cardiovascular health, especially in old age (Araujo et al., 2011). Serum testosterone levels in men decline after age 30 years. In healthy young men (aged 20 to 30 years) morning levels of serum total testosterone vary between 12 and 35 nmol/L (i.e., 350 and 1000 ng/dL) (Harman et al., 2001), and these values are generally also applied as reference values for elderly men. At age 75, morning total testosterone levels have decreased by one third, while the sensitivity of target tissues to testosterone is also lowered with increasing age (Wu et al., 2008). Low testosterone has been associated with increases in abdominal fat mass, dyslipidaemia, hypertension, insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome (Stellato et al., 2000; Oh et al., 2002; Dandona and Dhindsa, 2011; Brand et al., 2011), and predicted future cardiovascular events and overall mortality in several prospective cohort studies in men from the general population (Haring et al., 2010; Jones, 2010; Araujo et al., 2011). In a prospective cohort study among 930 British men with coronary heart disease, testosterone deficiency (defined as a total testosterone levels <8.1 nmol/L) was found in 17% and predicted overall mortality (Malkin et al., 2010). A low testosterone level is strongly related to obesity, and especially when extra fat is present in the abdominal regions. Waist circumference, a reliable indicator of abdominal obesity, was inversely associated with total testosterone levels (Svartberg et al., 2004). It is however unclear whether testosterone deficiency is causally related to obesity, as central fat deposits have a high degree of aromatase activity that metabolizes testosterone into estradiol. Nevertheless, testosterone replacement reduces waist circumference and BMI in hypogonadal obese men (Kalinchenko et al., 2010; Jones, 2010). In our study, we found that 8% of post-MI patients had a testosterone level below 8 nmol/L and 33% below 12 nmol/L, but that n-3 fatty acids supplementation did not affect these levels nor the risk of testosterone deficiency.
Our findings of no effect of EPA–DHA and ALA on total testosterone are of importance to androgen-sensitive carcinogenesis of prostate cancer, one of the most common cancers in men. Androgens are believed to play a central role in prostate cancer development and progression, illustrated by the therapeutic effects of androgen-receptor blocking (Akaza et al., 2009). However, meta-analyses have shown inconsistent associations between serum total testosterone and a higher risk of prostate cancer.(Roddam et al., 2008) An in vitro study suggested that the n-3 fatty acids (especially EPA-DHA) inhibit prostate cancer growth (Rose, 1997). Most (Norrish et al., 1999; Terry et al., 2001; Leitzmann et al., 2004; Augustsson et al., 2003) but not all (Newcomer et al., 2001) prospective cohort and case-control studies showed inverse associations between EPA and DHA levels or fish intake and the risk of prostate cancer. Yet, a positive association between ALA intake and risk of prostate cancer was found in some (De Stefani et al., 2000), though not all epidemiological studies (Leitzmann et al., 2004). Plasma ALA levels were also positively associated with prostate cancer among participants of the Physician’s Health Study (Gann et al., 1994) and in several case control studies (Harvei et al., 1997; Newcomer et al., 2001). In a meta-analysis, a high versus low intake or blood levels of ALA was associated with a 70% increased risk of prostate cancer (Brouwer et al., 2004). Thus, there might be a protective effect of EPA–DHA intake on prostate cancer incidence and mortality, and a possible detrimental effect of ALA. Our findings do not support the hypothesis that ALA increases and EPA–DHA decreases testosterone levels, and thereby affect the risk of prostate cancer.
Limitations and strengths
The Alpha Omega Trial was designed for CVD endpoints (Kromhout et al., 2010; Geleijnse et al., 2010), and we had only data on total testosterone levels. Therefore, the effects of n-3 fatty acids on bioavailable and free testosterone, the biologically active fractions, could not be examined. Furthermore, we tested for the effects of low-dose supplementation of n-3 fatty acids, that corresponded to the recommended dietary allowances of those fatty acids (Elmadfa and Kornsteiner, 2009). It is therefore unclear whether higher doses of n-3 fatty acids would have yielded similar results. Non-fasting serum samples were used and whole day blood sampling was performed, and therefore extra biological variability in total testosterone levels throughout the day was present. Finally, a clinical diagnosis of hypogonadism could not be made, as we had not assessed symptoms of testosterone deficiency and testosterone was not assessed in more than one separate blood sample. Our study also has several strengths. Blinding was maintained throughout our study and compliance was excellent, as reflected in substantial increases in serum EPA, DHA and ALA in active treatment arms. Moreover, we are only aware of one small cross-over trial of n-3 fatty acid supplementation on serum total testosterone levels in men (Hughes et al., 1990).
Conclusion
In conclusion, we found no effect of EPA–DHA and ALA supplementation on changes in serum total testosterone. The risk of testosterone deficiency in elderly men who had had a MI was not affected by low-dose n-3 fatty acids.
Acknowledgements
The authors express their gratitude to Eveline Waterham and Annemarie Teitsma-Jansen for their invaluable contribution to the Alpha Omega Trial. Lucy Okma and Liesbeth Smit are gratefully acknowledged for collection and processing of data and blood samples. We thank the research nurses for physical examination of trial participants. A list of members of the Alpha Omega Trial Group has been published elsewhere (Kromhout et al., 2010) and is available at www.alphaomegatrial.com. We thank Abbott Diagnostics for providing us with the 2nd generation testosterone reagents.
This work was supported by the Netherlands Heart Foundation, US National Institutes of Health (NIH), Unilever R&D, The Netherlands, and Abbott Diagnostics. The grant of the Netherlands Heart Foundation covered baseline examinations and mortality follow-up. The NIH grant covered mid-term and final examinations and the verification of nonfatal cardiovascular events. Unilever provided an unrestricted grant for distribution of trial margarines to the patients. Part of this study was funded by a grant from The Netherlands Brain Foundation (Hersenstichting, Nederland, grant number 15F07(2).24). The funding organizations had no role in the design of the study, data collection, data analysis, interpretation, writing of the report or the decision to submit.
Conflicting interests / financial disclosure: Netherlands Heart Foundation (grant# 2000T401), US National Institutes of Health (NIH/NHLI and ODS, grant# R01-HL076200) and Unilever, The Netherlands (production of trial margarines and unrestricted grant for margarine distribution to the patients). The measurements of testosterone levels was financially supported by a grant from The Netherlands Brain Foundation (Hersenstichting, Nederland (grant# 15F07(2).24) and by Abbott Diagnostics (Abbott park, IL, USA).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author contributions
D.K., J.M.G., and E.J.G. designed the Alpha Omega Trial. The statistical analysis were done by E.J.G. E.J.G., J.d.G., and L.M.O.G. were responsible for the data collection. A.C.H. and M.A.B. were responsible to the testosterone measurements. E.J.G. wrote the first draft of the manuscript. All authors critically reviewed, edited, and approved the manuscript.
References
- Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S, Hirao Y. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–3445. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–67. [PubMed] [Google Scholar]
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40:189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- Bui HN, Struys EA, Martens F, de Ronde W, Thienpont LM, Kenemans P, Verhoeven MO, Jakobs C, Dijstelbloem HM, Blankenstein MA. Serum testosterone levels measured by isotope dilution-liquid chromatography-tandem mass spectrometry in postmenopausal women versus those in women who underwent bilateral oophorectomy. Ann Clin Biochem. 2010;47:248–252. doi: 10.1258/acb.2010.009171. [DOI] [PubMed] [Google Scholar]
- Dandona P, Dhindsa S. Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani E, Deneo-Pellegrini H, Boffetta P, Ronco A, Mendilaharsu M. Alpha-linolenic acid and risk of prostate cancer: a case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 2000;9:335–338. [PubMed] [Google Scholar]
- Elmadfa I, Kornsteiner M. Fats and fatty acid requirements for adults. Ann.Nutr Metab. 2009;55:56–75. doi: 10.1159/000228996. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Oude Griep LM, Teitsma-Jansen AM, Katan MB, Kromhout D. Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J. 2010;159:539–546. doi: 10.1016/j.ahj.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Duschek EJ, Katan MB, Zock PL, Neele SJ, Netelenbos JC. Raloxifene and hormone replacement therapy increase arachidonic acid and docosahexaenoic acid levels in postmenopausal women. J Endocrinol. 2004a;182:399–408. doi: 10.1677/joe.0.1820399. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Schouten EG, Katan MB, Kromhout D. High stability of markers of cardiovascular risk in blood samples. Clin Chem. 2003;49:652–655. doi: 10.1373/49.4.652. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJG, Toorians AWFT, Katan MB, Zock PL. Levels of docosahexaenoic acid are higher in women than in men through estrogenic effects. Am J Clin Nutr. 2004b;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Gromadzka-Ostrowska J. Effects of dietary fat on androgen secretion and metabolism. Reprod Biol. 2006;6(Suppl 2)(6):13–20. [PubMed] [Google Scholar]
- Haring R, Volzke H, Steveling A, Krebs A, Felix SB, Schofl C, Dorr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010;31:1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–551. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hughes GS, Ringer TV, Watts KC, DeLoof MJ, Francom SF, Spillers CR. Fish oil produces an atherogenic lipid profile in hypertensive men. Atherosclerosis. 1990;84:229–237. doi: 10.1016/0021-9150(90)90095-z. [DOI] [PubMed] [Google Scholar]
- Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21:496–503. doi: 10.1016/j.tem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blind placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- Léon H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008;337:a2931. doi: 10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–1825. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Kawakami N, Shimizu H. Relationships between types of fat consumed and serum estrogen and androgen concentrations in Japanese men. Nutr Cancer. 2000;38:163–167. doi: 10.1207/S15327914NC382_4. [DOI] [PubMed] [Google Scholar]
- Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- Phelan N, O'Connor A, Kyaw TT, Correia N, Boran G, Roche HM, Gibney J. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am J Clin Nutr. 2011;93:652–662. doi: 10.3945/ajcn.110.005538. [DOI] [PubMed] [Google Scholar]
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr. 1997;66:1513S–1522S. doi: 10.1093/ajcn/66.6.1513S. [DOI] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Svartberg J, von Mühlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. The Tromso study. Eur J Epidemiol. 2004;19:657–663. doi: 10.1023/b:ejep.0000036809.30558.8f. [DOI] [PubMed] [Google Scholar]
- Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- Vargas ML, Almario RU, Buchan W, Kim K, Karakas SE. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism. 2011;60:1711–1718. doi: 10.1016/j.metabol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12:5–12. doi: 10.1080/13685530802389628. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]