Introduction
Heterotrimeric G protein subunit Gβ5 is expressed primarily in the nervous system and retina and, among Gβ isoforms, is unique in its ability to heterodimerize with regulator of G protein signaling (RGS) proteins of the R7 subfamily (R7-RGS) [see [1, 2] for recent reviews]. The R7-RGS subfamily consists of RGS proteins 6, 7, 9, and 11 which can form tight heterodimers with the G protein β5 subunit through a Gγ-like (GGL) domain [1, 2]. R7-RGS subfamily members are unstable in the absence of Gβ5 [3]. Previous study from our laboratory showed that in addition to its expression in brain and neural cell lines, Gβ5 was also expressed in the αT3-1 gonadotrophic pituitary cell line [4]. We show now that the Gβ5/R7-RGS complex is expressed in corticotroph-derived pituitary AtT-20 cells, pituitary gland and purified pancreatic islets. The expression of Gβ5 in various endocrine cell lines and native tissues raises the possibility that hormone secretion, and not just neurotransmission and phototransduction, may be regulated by the Gβ5/R7-RGS complex.
Materials and methods
Antibodies employed in immunoblots and immunohistochemistry included normal rabbit IgG, rabbit anti-Gβ5 polyclonal N-terminal antibody ATDG [5], anti-β-actin mouse monoclonal (Sigma, A-5315), mouse anti-α-tubulin (Calibiochem, CP06), mouse anti-ACTH monoclonal antibody (Abcam, ab8615), mouse anti-p84/N5 [5E10] monoclonal antibody (GeneTex, GTX70220), rabbit anti-RGS7 polyclonal antibody 7RC-1 (cross-reacts with RGS6) [6], and rabbit anti-RGS9-2 polyclonal antibody RGS9CT (raised against the synthetic peptide 31-mer H-CRSPRK PFASPSRFIRRPSIAICPSPSRVAL-NH2). Secondary antibodies included HRP-conjugated goat anti-mouse (no. 1858413) and anti-rabbit (no. 1858415) antibodies from Pierce. AtT-20 cells were grown in 75-cm2 flasks at 37°C and 5% CO2 containing DMEM supplemented with 10% bovine serum, 5% horse serum, 4 mM L-glutamine, and penicillin/streptomycin (Biofluids, Rockville, MD). MIN6 cells were cultured in DMEM medium containing 4.5 g/L glucose, 10% fetal bovine serum and penicillin/streptomycin. Fractions of cultured cells were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, 78833) according to the manufacturer’s instructions. For isolation of pancreatic islets the procedure of Saeki et al. [7] was followed with minor modification. For immunohistochemistry and immunofluorescence, pituitary glands were excised from the ventral portion of mouse brain and fixed overnight in 4% paraformaldehyde, and then incubated in 70% ethanol. The preserved pituitary was processed for paraffin embedding and microscopic sectioning (Histoserv, Inc., Germantown, MD). Slides were prepared for immunohistochemistry according to the manufacturer’s recommendations (Bethyl Labs, Immunohistochemistry Accessory Kit, no. IHC-101). Confocal laser microscopy employed a Leica SP2-UV 405 confocal microscope (Leica Microsystems, Exton, PA, USA). Mice with a heterozygous deletion of exon 3 of Gnb5 in the germline were kindly provided by Dr. Ching-Kang Jason Chen (Virginia Commonwealth University Medical Center, Richmond, VA). Gnb5 KO pups were generated from heterozygotes as previously described [8].
Results
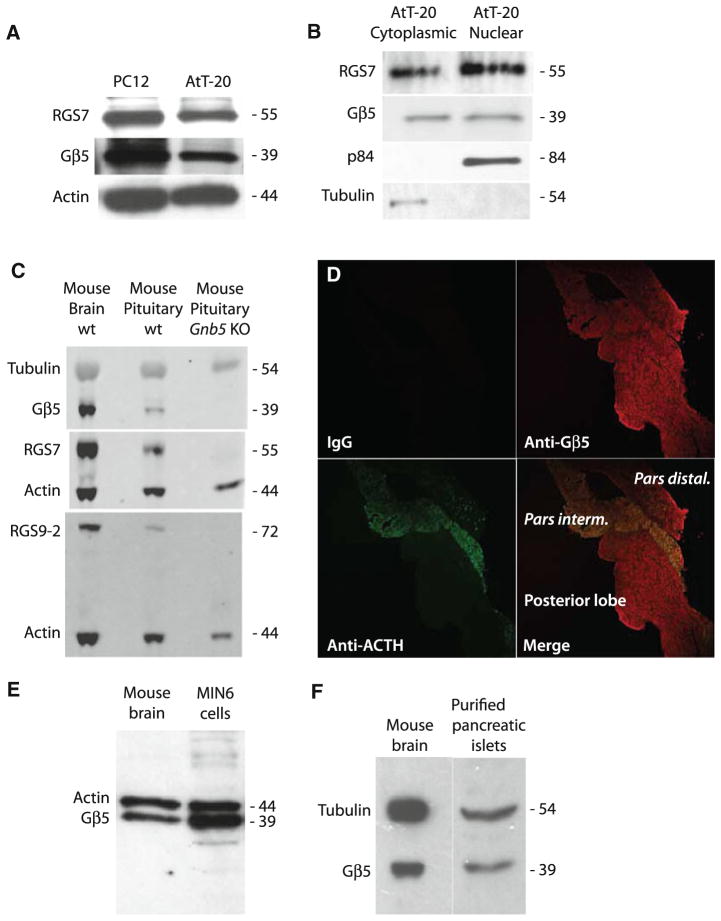
We looked for expression of Gβ5 and R7-RGS protein in AtT-20 cells, a well-characterized mouse pituitary tumor-derived cell line that expresses adrenocorticotropic hormone (ACTH). Gβ5 and RGS7 were expressed in whole cell lysates of AtT-20 cells comparable to their expression in neuron-like PC12 cells (Fig. 1a). Subcellular fractionation showed that in AtT-20 cells, as in mouse brain and PC12 cells [9], Gβ5 and RGS7 are targeted to both the cytoplasm and the nucleus (Fig. 1b).
Fig. 1.
Expression of Gβ5 in AtT-20 cells, mouse pituitary, MIN6 insulinoma cells, and purified mouse pancreatic islets. a Whole cell lysates from AtT-20 and PC12 cells were prepared and analyzed for Gβ5 and RGS7 by SDS-PAGE followed by immunoblotting. The corresponding β-actin levels are shown as loading controls. b Subcellular distribution of Gβ5 and RGS7 in AtT-20 cells and PC12 cells are shown by immunoblotting using p84/N5 as marker for the nuclear fraction and α-tubulin as a marker for the cytoplasmic fraction. c Whole lysates were prepared from mouse brain and wild-type or Gβ5-knockout mouse pituitary gland and analyzed for Gβ5, RGS7, or RGS9-2 by immunoblotting with the corresponding tubulin or β-actin levels shown as loading controls. d Slides of mouse pituitary were stained using control IgG, anti-Gβ5, or anti-ACTH antibodies as indicated. The primary antibodies were visualized with Cy3-conjugated secondary antibody (red) for Gβ5 and with FITC-conjugated secondary antibody (green) for ACTH. The slides were analyzed using confocal laser microscopy. Magnification is ×40. e Whole cell lysates from MIN6 mouse insulinoma cells or mouse brain were prepared and analyzed for Gβ5 by immunoblotting. The corresponding β-actin levels are shown as loading controls. f Whole lysates from mouse brain or a purified preparation of pancreatic islets were prepared and analyzed for Gβ5 by immunoblotting. The corresponding α-tubulin levels are shown as loading controls
Because AtT-20 cells were derived from a pituitary tumor, we studied Gβ5 expression in native mouse pituitary gland. The expression of Gβ5 as well as the Gβ5-dependent R7-RGS proteins RGS7 and RGS9-2 was evident in wild-type, but not Gnb5 KO [3], pituitary lysate (Fig. 1c). To investigate the regional expression of Gβ5 in mouse pituitary, we performed dual immunofluorescence staining using anti-Gβ5 and anti-ACTH antibodies (Fig. 1d). Gβ5 is widely expressed in both anterior and posterior pituitary lobes and it co-localizes with ACTH in the pars intermedia of the anterior murine pituitary gland (Fig. 1d).
Since Gβ5 was expressed widely throughout both the anterior and posterior mouse pituitary we wondered whether it might also be expressed in neuroendocrine islet cells of the pancreas. Immunoblots of insulinoma-derived MIN6 mouse pancreatic islet β-cells and a purified preparation of mouse pancreatic islets demonstrated robust expression of Gβ5 to a level comparable to that of neuronal controls (Fig. 1e, f).
Discussion
The expression of Gβ5 and R7-RGS subfamily RGS proteins in brain and retina is well documented (reviewed in [1, 2]), yet previous study from our laboratory suggested that Gβ5 was also expressed in cells of neuroendocrine origin, including the αT3-1 pituitary cell line [4]. In this study, besides documenting the expression of Gβ5 in corticotroph and islet β-cell derived cell lines, we demonstrate the unequivocal expression of Gβ5 in native endocrine tissue including cells of the pituitary and in pancreatic islet cells. The pancreatic islet cells derive from embryonic endodermal anlage, while the anterior and posterior pituitary develop from oral and neural ectoderm respectively. The expression of Gβ5 in tissues of such disparate embryologic origins implies an important role for the Gβ5/R7-RGS complex in neuroendocrine cells and in the physiology of endocrine systems. Indeed, targeted disruption of the Gβ5 partner RGS7 in Rgs7tm1Lex mice results in a phenotype of impaired glucose tolerance (Mutant Mouse Regional Resource Center, strain 011655; see http://www.mmrrc.org/strains/11655/011655.html). Furthermore, while this manuscript was being completed, Slepak and co-workers published evidence suggesting a role for Gβ5 in the regulation of energy metabolism at the organismal level and confirming the presence of the Gβ5 complex in cultured MIN6 cells and isolated pancreatic islets [10].
Even in the nervous system, few details of the function of the Gβ5/R7-RGS complex at the cell biological level are understood. It is known that the Gβ5/R7-RGS complex exhibits GTPase activating protein (GAP) activity for heterotrimeric Gα subunits and thus can function as a negative regulator of signaling to effectors from GPCRs. Studies in vitro have found that the GAP activity of Gβ5/R7-RGS complexes is most efficacious against pertussis-sensitive Goα subunits [11]. The expression of Gβ5 in pituitary and islet cells makes it likely that modulation of such GPCR-coupled pertussis-sensitive pathways by the Gβ5/R7-RGS complex may control hormone release and endocrine function in a physiologically important, and perhaps pharmacologically manipulable, fashion.
Acknowledgments
We thank Drs. Dinesh Gautam, Jean-Marc Guettier, and Jürgen Wess (NIDDK) and Dr. Tao Xie (NIAMS) for their expert assistance with pancreatic islet isolation and MIN6 cell culture. We thank Dr. Ching-Kang Jason Chen (Virginia Commonwealth University Medical Center) for the Gnb5 mutant mice. We also thank Drs. Hina Mehta (NIDDK) and Lily Koo (NIAID) for their help. The Intramural Research Program of the NIDDK supported this research.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Jayaraman M, Zhou H, Jia L, Cain MD, Blumer KJ. R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling. Trends Pharmacol Sci. 2009;30(1):17–24. doi: 10.1016/j.tips.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54(1–3):33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CK, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI. Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc Natl Acad Sci USA. 2003;100(11):6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JH, Lai ZN, Simonds WF. Differential expression of the G protein β5 gene: analysis of mouse brain, peripheral tissues, and cultured cell lines. J Neurochem. 2000;75:393–403. doi: 10.1046/j.1471-4159.2000.0750393.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JH, Simonds WF. Copurification of brain G-protein β5 with RGS6 and RGS7. J Neurosci. 2000;20:RC59, 1–5. doi: 10.1523/JNEUROSCI.20-03-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojkova AM, Woodard GE, Huang TC, Combs CA, Zhang JH, Simonds WF. Gγ subunit-selective G protein β5 mutant defines regulators of G protein signaling protein binding requirement for nuclear localization. J Biol Chem. 2003;278(14):12507–12512. doi: 10.1074/jbc.M207302200. [DOI] [PubMed] [Google Scholar]
- 7.Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51(6):1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JH, Pandey M, Seigneur EM, Panicker LM, Koo L, Schwartz OM, Chen W, Chen CK, Simonds WF. Knockout of G protein beta5 impairs brain development and causes multiple neurologic abnormalities in mice. J Neurochem. 2011;119(3):544–554. doi: 10.1111/j.1471-4159.2011.07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JH, Barr VA, Mo Y, Rojkova AM, Liu S, Simonds WF. Nuclear localization of Gβ5 and regulator of G protein signalling 7 in neurons and brain. J Biol Chem. 2001;276:10284–10289. doi: 10.1074/jbc.M009247200. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Levay K, Chanturiya T, Dvoriantchikova G, Anderson KL, Bianco SD, Ueta CB, Molano RD, Pileggi A, Gurevich EV, Gavrilova O, Slepak VZ. Targeted deletion of one or two copies of the G protein {beta} subunit G{beta}5 gene has distinct effects on body weight and behavior in mice. Faseb J. 2011;25:3949–3957. doi: 10.1096/fj.11-190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posner BA, Gilman AG, Harris BA. Regulators of G protein signaling 6 and 7-purification of complexes with Gβ5 and assessment of their effects on G protein-mediated signaling pathways. J Biol Chem. 1999;274:31087–31093. doi: 10.1074/jbc.274.43.31087. [DOI] [PubMed] [Google Scholar]