Abstract
Background
Evidence-based guidelines recommend routine surveillance, including office visits and testing, to detect new and recurrent disease among breast and colorectal cancer survivors. The extent to which surveillance practice is consistent with guideline recommendations or may vary by age is not known.
Methods
Cohorts of adult patients diagnosed with breast (n=6,205) and colorectal (n=2,297) cancer between 2000 and 2008 and treated with curative intent in four, geographically diverse, managed care environments were identified via tumor registries. Kaplan-Meier estimates were used to describe time to initial and subsequent surveillance service receipt. Cox proportional hazards models evaluated the relationship between patient characteristics and receipt of metastatic screening.
Results
Within 18-months of treatment, 87.2% of breast cancer survivors received recommended mammograms, with significantly higher rates for patients aged 50–65. Among colorectal cancer survivors, only 55.0% received recommended colon examinations, with significantly lower rates for those ≥ aged 75. Most breast (64.7%) and colorectal (73.3%) cancer survivors received non-recommended metastatic disease testing. In breast cancer, factors associated with metastatic disease testing include white race (HR=1.13), comorbidities (HR=1.17), and younger age. In colorectal cancer, these factors included younger age and comorbidities (HR=1.10).
Conclusions
Among an insured population, we found wide variation in the use of surveillance care by age and relative to guideline recommendations. Breast cancer survivors have high rates of both guideline recommended recurrence testing and non-guideline recommended metastatic testing. Only about half of colorectal cancer survivors receive recommended tests but over two thirds received metastatic testing.
Keywords: cancer survivors, breast cancer, colorectal cancer, guideline concordance
INTRODUCTION
In 2006, the Institute of Medicine (IOM) recommended that cancer survivors receive ongoing surveillance care based on a follow-up plan that is clearly and effectively explained1. Evidence- and consensus-based guidelines from the National Comprehensive Cancer Network (NCCN)2,3, the American Society of Clinical Oncology (ASCO)4,5, and other specialty-oriented medical societies6–9 outline recommended schedules for ongoing surveillance care after cancer treatment with curative intent. Although recommendations vary to some extent in terms of type and timing of care, NCCN and ASCO guidelines for breast and colorectal cancer survivors recommend a course that consists of periodic physical examinations and screening for recurrent and metastatic disease via imaging and other procedures.
Relatively little is known about the quality of care delivered to cancer survivors. Prior studies among breast and colorectal cancer survivors have found deviations in surveillance care patterns relative to evidence-based guidelines as well as variation in surveillance care use by patient socio-demographic characteristics, including race and income. However, such studies have been limited to survivors receiving care from one delivery organization10–16 or those enrolled in Medicare17–21. As such, the care received by survivors who are aged 64 years or younger is not well documented. Furthermore, no studies have been conducted since the IOM report was issued.
Using cohorts of breast and colorectal cancer survivors receiving care from four geographically diverse health maintenance organizations between 2000 and 2008, we evaluated the extent to which surveillance care use was consistent with guideline recommendations. Of specific interest was evaluation of the variability in surveillance care use by age at diagnosis.
METHODS
Study Population and Setting
This study was conducted within four large non-profit integrated health systems: Group Health Cooperative, Health Alliance Plan/Henry Ford Health System, Kaiser Permanente Colorado, and Kaiser Permanente Northwest. These plans are all members of the Cancer Research Network (CRN) (NCI Cooperative Agreement No. U19 CA79689, Increasing Effectiveness of Cancer Control Interventions), an initiative of the National Cancer Institute designed to conduct research on cancer prevention, early detection, treatment, long-term care, surveillance, and cancer communication and dissemination and implementation research.
Data available from each organization’s tumor registry were used to identify patients aged ≥ 18 years who were diagnosed between January 1, 2000 and December 31, 2008 with non-metastatic breast (ICD O C50.0–C50.9) or colorectal (ICD O C18.0–C18.9, C19.9, C20.9, and C26.0) cancer. Patients eligible for study inclusion were those continuously enrolled in the health plan for the 1-year period preceding their date of cancer diagnosis. Patients for whom no stage of disease information was available at the time of diagnosis were excluded. Patients were also excluded if they had a previous diagnosis of invasive cancer, had distant metastases at time of diagnosis, or did not receive treatment with curative intent (i.e., surgery). The breast cancer cohort was limited to females and excluded women who had a bilateral mastectomy or two separate occurrences of a unilateral mastectomy on different dates.
Sample patients were followed from an “index date,” defined as three months following the last curative surgical procedure, to the first of the following end points (“end date”): death; tumor recurrence; diagnosis of a second primary cancer; health plan disenrollment, five years after initial cancer diagnosis; or the end of the follow-up period (December 31, 2008). Previous findings indicate that altering assignment of the index date to exclude from consideration care received within the first six and nine months after surgical treatment (vs. three months), does not alter results or conclusions13. Institutional Review Boards at each of the four participating organizations approved all aspects of the study protocol.
Data Sources and Measures
Automated electronic medical record (EMR) and claims data linked to tumor registry data were accessed to obtain patient demographic characteristics, date of cancer diagnosis, stage at diagnosis, and comorbidities in the 12-month period preceding diagnosis for each patient. Patient demographics included age at diagnosis, gender, and race (black, white, and other). Using patients’ residential street address combined with 2000 US Census tract data we estimated the median household income for each patient’s residential neighborhood. Clinical measures included stage of disease at diagnosis, date of any cancer recurrence(s) (defined as either local recurrence or the occurrence of a second primary tumor) within five years of the index date, and date of death. We measured recurrence(s) using the algorithm for measuring disease-free survival described in Lamont et al. (2006).22 We summarized disease stages into three groups: in situ (AJCC general stage 0), localized (AJCC general stage 1), and regional (AJCC general stages 2–5; i.e., regional by direct extension, lymph nodes, both, or not otherwise specified).
In addition to data available via the tumor registries, EMR and claims data were reviewed between the patient’s “index date” and “end date.” For each organization, these data sources contain comprehensive visit and procedure information for any outpatient encounter to a physician, laboratory, and imaging department. These data were used to compute receipt of testing services, and treatment with surgery, chemotherapy, hormone therapy, radiation, and combination therapies. The Deyo adaptation of the Charlson comorbidity index and each of its component diagnostic subgroups were constructed using inpatient and outpatient diagnostic information available in the 12-month period preceding diagnosis.23
Surveillance Care Receipt
The primary outcome of interest is the time to receipt of recommended surveillance care. For both breast and colorectal cancer, we observed three distinct types of surveillance: physical examinations; testing for local recurrence; and testing for metastatic disease. Physical examinations performed by a primary care provider, medical oncologist, radiation oncologist, general surgeon or gastroenterologist (for colorectal cancer only) were included. For breast cancer, local recurrence tests included mammography, magnetic resonance imaging (MRI), and ultrasound, whereas for colorectal cancer, local recurrence testing included colonoscopy, sigmoidoscopy, and barium enema. Procedures to detect metastatic disease recurrence included chest radiograph, computerized tomography of the chest, abdomen, pelvis or head, MRI of the chest, abdomen, pelvis or head, bone scan, gallium scan, liver/spleen scan, and abdominal or pelvic ultrasound. Because surveillance care guidelines recommend not only type of care, but also its timing, we evaluated the frequency of care receipt and the time interval between initial and subsequent receipt of the same type of examination/test.
Finally, we evaluated whether the patient received NCCN and ASCO guideline-recommended surveillance care. This was done by estimating the time (in days) from index date to the date by which a patient had received the minimum amount and type of surveillance testing recommended by the guideline. For patients with breast cancer, this included two physical examinations and one mammogram, while for colorectal cancer this included two physical examinations and one complete examination of the colon. In addition to time, we also report the proportion of patients who received recommended care within 18 months following treatment with curative intent. This was done because care receipt in practice rarely falls neatly within 12-month periods.
Analytical Approach
Because of the differing lengths of follow up among sample members, we used Kaplan-Meier estimates to evaluate the median time to initial and subsequent care receipt by type of examination/test. We report the proportion of patients receiving care within the corresponding 18 months using actuarial results. Kaplan-Meier estimates were also used to determine cumulative incidences of the receipt of the minimum recommended surveillance care. Estimates were evaluated for the entire population of survivors as well as separately for four age groups: <50, 50–64, 65–74, and ≥75. We plotted values as the probability of the complement so that graphs reflected time to receipt of care. We used Cox proportional hazards models which also account for differing length of follow up among sample members to quantify the effects of baseline clinical and sociodemographic patient characteristics, including patient age group, on the risk of receiving metastatic testing (a non-recommended care type) within the initial 18 months. In addition to age, models adjusted for the patient’s race, sex (only for the colorectal cancer sample), stage, neighborhood household income, Charlson-Deyo comorbidity index, and health plan. All analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC). Statistical results achieving p < 0.05 were considered statistically significant.
RESULTS
Cohort Characteristics
The characteristics of the 6,205 breast cancer patients and 2,297 colorectal cancer patients by health plan are shown in Table 1. The mean ages of the cohorts were 62.4 (±12.6) for breast cancer patients and 68.6 (±12.2) for colorectal cancer patients with 57% of the breast cancer patients under the age of 65 and 35% of the colorectal cancer patients under the age of 65. While the study included only female breast cancer patients, the gender distribution for colorectal cancer was 49% male and 51% female. African Americans represented 8% of the breast cancer sample and 8% of the colorectal cancer sample. For breast cancer, most tumors were classified as local stage (80%), while among the colorectal cancer group there was a more even distribution between local (49%) and regional disease (51%). Per study eligibility criteria, all patients received curative surgical treatment. The overall median length of observation was 49.5 months for breast cancer and 40.9 months for colorectal cancer patients. Evidence of recurrent cancer or a second primary tumor occurred in 13% of breast cancer patients and 22% of colorectal cancer patients. Almost 96% of breast cancer patients survived through the end of follow-up, compared with 90% for colorectal cancer patients. This survival rate was 99% for breast cancer patients aged <65 years versus 91% for those aged ≥65 years. In colorectal cancer, the survival rate for patients aged <65 years was 97% compared with 86% for patients aged ≥65 years. Survival differences by age group (under/over 65) in both the breast and colorectal cancer cohorts were statistically significant (data not shown).
Table 1.
Sample characteristics by cancer site
| Breast n = 6,205 |
Colorectal n = 2,297 |
|
|---|---|---|
| Mean age at diagnosis (SD) | 62.4 (12.6) | 68.6 (12.2) |
| Age at diagnosis (%) | ||
| < 50 years | 16 | 6 |
| 50–64 years | 41 | 29 |
| 65–74 years | 24 | 29 |
| ≥ 75 years | 19 | 36 |
| Sex (%) | ||
| Male | - | 49 |
| Female | 100 | 51 |
| Race (%) | ||
| Black | 8 | 8 |
| White | 81 | 81 |
| Other | 11 | 11 |
| Median household income (SD) | $53,906 ($20,354) |
$51,580 ($19,237) |
| Stage (%) | ||
| In situ | 24 | 4 |
| Localized | 56 | 45 |
| Regional | 20 | 51 |
| Site of care (%) | ||
| Health plan 1 | 22 | 18 |
| Health plan 2 | 31 | 32 |
| Health plan 3 | 19 | 18 |
| Health plan 4 | 28 | 32 |
| Treatment (%) | ||
| Surgery | 100 | 100 |
| Chemotherapy | 29 | 33 |
| Hormone therapy | 49 | - |
| Radiation therapy | 61 | 12 |
| Combination treatments | 70 | 33 |
| Median follow-up in months | 49.5 | 40.9 |
| Recurred within 5 years (%) | 13 | 22 |
| Survived 5 years (%) | 96 | 90 |
SD: Standard deviation.
Surveillance Care Receipt
Results from the Kaplan-Meier survival analysis for time to initial and subsequent receipt of specific examinations and procedures are shown in Table 2. These results are presented for all ages combined, and separately for each of the four aforementioned age groups. For both cancer sites, the overwhelming majority of patients (97.1% of breast cancer and 98.2% of colorectal cancer patients) received the minimum recommended number of physical examinations within the initial 18 months after treatment with curative intent. The median time to receipt of the recommended physical examinations ranged from 1.6 months among breast cancer patients to 1.8 months among colorectal cancer patients. If we consider the time to subsequent receipt of physical examinations, then a majority of cancer survivors received at least twice the number of recommended physical examinations within the initial year following treatment. Among both breast and colorectal cancer patients, the median time to receipt of a subsequent physical examination was just over two months, implying that many patients received two physical examinations within the initial four months following treatment.
Table 2.
Time to receipt of physical examinations and local recurrence and metastatic disease testing among cancer survivors by service type and age, among breast (n=6,205) and colorectal (n=2,297) cancer survivors.
| Age Group | ||||||
|---|---|---|---|---|---|---|
| Median time in months to initial receipt | All | <50 | 50–64 | 65–74 | ≥75 | |
| Breast Cancer | Two physical exams | 1.6 | 1.2 | 1.5 | 1.7 | 2.1 |
| One mammogram | 7.2 | 7.9 | 7.0 | 6.6 | 7.8 | |
| Any metastatic disease testing | 9.4 | 9.1 | 9.5 | 8.8 | 9.9 | |
| Colorectal Cancer | Two physical exams | 1.8 | 1.6 | 1.7 | 1.8 | 1.9 |
| One complete exam of colon | 13.3 | 10.5 | 10.7 | 11.5 | 28.8 | |
| Any metastatic disease testing | 6.1 | 4.3 | 6.1 | 5.9 | 6.9 | |
| Proportion of patients in receipt within 18 months of treatment | All | <50 | 50–64 | 65–74 | ≥75 | |
| Breast Cancer | Two physical exams | 97.1 | 96.3 | 96.6 | 98.1 | 97.6 |
| One mammogram | 87.2 | 81.6 | 89.3 | 91.1 | 82.5 | |
| Any metastatic disease testing | 64.7 | 61.6 | 63.4 | 67.4 | 67.1 | |
| Colorectal Cancer | Two physical exams | 98.2 | 98.6 | 97.0 | 98.6 | 99.2 |
| One complete exam of colon | 55.0 | 63.0 | 64.1 | 62.1 | 41.0 | |
| Any metastatic disease testing | 73.3 | 73.3 | 72.9 | 73.2 | 74.8 | |
| Median time in months between initial and subsequent receipt | All | <50 | 50–64 | 65–74 | ≥75 | |
| Breast Cancer | Two physical exams | 2.1 | 1.6 | 2.1 | 2.1 | 2.4 |
| One mammogram | 12.3 | 12.4 | 12.2 | 12.2 | 12.6 | |
| Any metastatic disease testing | 15.9 | 16.8 | 21.0 | 13.8 | 13.8 | |
| Colorectal Cancer | Two physical exams | 2.3 | 2.1 | 2.1 | 2.1 | 2.6 |
| One complete exam of colon | (n=412)* | (n=34)* | (n=103)* | (n=111)* | (n=86)* | |
| Any metastatic disease testing | 7.4 | 6.9 | 8.4 | 7.5 | 7.1 | |
| Proportion of patients in receipt within 18 months of initial receipt | All | <50 | 50–64 | 65–74 | ≥75 | |
| Breast Cancer | Two physical exams | 94.3 | 93.2 | 93.2 | 96.6 | 95.1 |
| One mammogram | 75.4 | 69.4 | 79.0 | 80.5 | 66.7 | |
| Any metastatic disease testing | 51.3 | 50.8 | 48.6 | 53.5 | 55.3 | |
| Colorectal Cancer | Two physical exams | 90.8 | 92.0 | 86.7 | 93.0 | 93.0 |
| One complete exam of colon | 16.7 | 26.4 | 17.8 | 19.8 | 12.2 | |
| Any metastatic disease testing | 62.5 | 62.1 | 60.9 | 62.4 | 64.7 | |
When sample size did not permit estimation of median time, number of cohort members receiving service of interest during follow-up period is reported.
Most breast cancer patients received recommended local recurrence testing within 18 months. Among this group, 87.2% received mammograms within 18 months of treatment, with a median time of 7.2 months. Only 55% of colorectal cancer survivors received a complete examination of the colon within the first 18 months. The median time to initial receipt of this test was 13.3 months. Table 2 also shows the relatively common use of metastatic disease testing among cancer survivors. Almost 65% of breast cancer survivors and 73% of colorectal cancer survivors received some type of metastatic disease testing within 18 months after treatment with curative intent. The median time to initial receipt of metastatic testing in breast cancer was 9.4 months, and 6.1 months in colorectal cancer.
When comparing the four age groups among breast cancer survivors, the proportion of patients receiving a mammogram within 18 months of curative intent treatment was similar among the middle two age groups, with 89% and 91% respectively, but significantly lower with the aged <50 group (82%) and the aged ≥75 group (82%). Among colorectal cancer survivors, the younger three age groups had similar rates of colon examinations within 18 months of curative intent treatment, with 63%, 64%, and 62% respectively, but a significantly lower rate among the aged ≥75 (41%). Meanwhile we observe a small, but significant increase in the receipt of metastatic testing within the first 18 months following curative intent associated with increasing age, for both breast and colorectal cancer survivors.
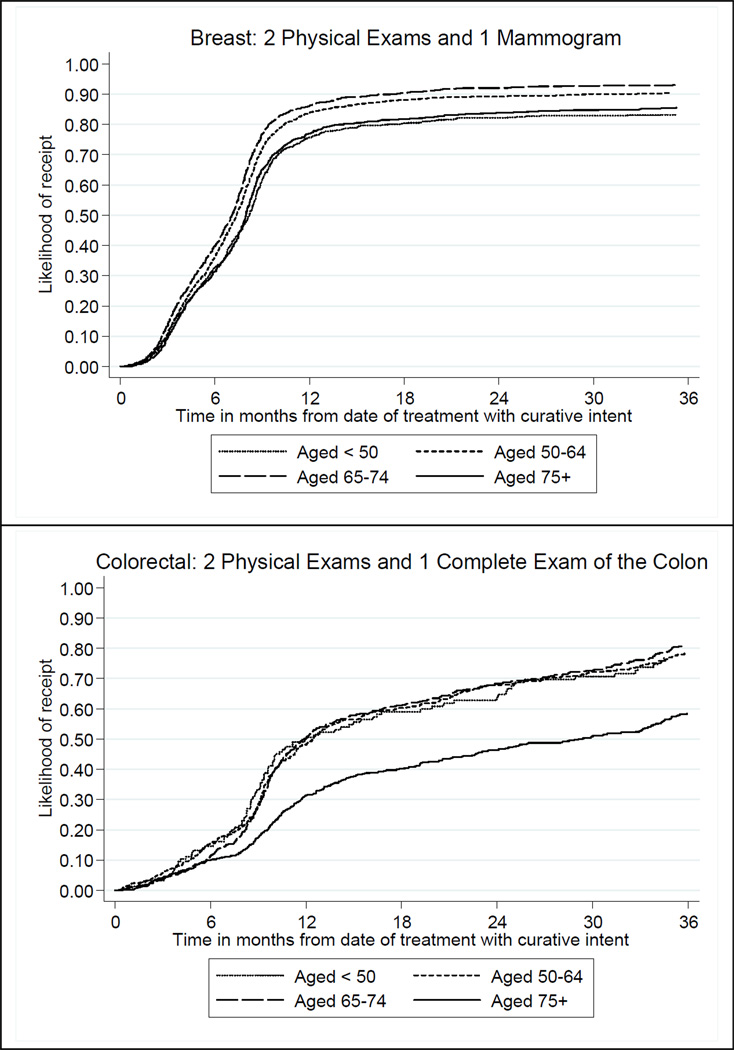
Figure 1 shows estimates of the time to minimum guideline recommended surveillance care during the initial year (i.e. two physical examinations and one mammogram for breast cancer survivors; and two physical examinations and one complete examination of the colon for colorectal cancer survivors), stratified by age group. Overall, almost 90% of breast cancer survivors receive the recommended surveillance care within 18 months of treatment with curative intent, although the rates are higher for the patients aged 50 through 74 years compared to their younger and older counterparts. Of note, is the fact that, regardless of age, the overwhelming majority of these patients received that care well before 12 months following their treatment with curative intent. On the other hand, just over half of colorectal cancer survivors received recommended surveillance care within 18 months of treatment with curative intent, with all age groups having similar rates except for the aged ≥75 years who recorded significantly lower rates of receipt.
Figure 1.
Likelihood of initial year minimum guideline recommended service receipt for breast and colorectal cancer survivors
Factors Associated with Metastatic Disease Testing
Results of the Cox proportional hazards models for receipt of metastatic disease testing within 18 months of curative intent treatment, among breast cancer patients, are presented in Table 3. As shown in the table, a diagnosis in any of the three younger age groups, compared to those aged ≥75, was associated with a higher likelihood of receiving metastatic testing for aged <50 (HR, 1.13), for aged 50–64 (HR, 1.15), and for aged 65–74 (HR, 1.13). The diagnosis of in situ (HR, 0.31) or regional (HR, 0.57) disease decreased the likelihood of receiving metastatic disease surveillance testing. White breast cancer survivors (HR, 1.13), those with a higher Charlson-Deyo comorbidity index (HR, 1.17), and those receiving care at Health Plan 1 (HR, 1.76) had a greater likelihood of receiving metastatic testing. We also found significant (p<0.01) variation in the use of metastatic testing by health plan. Table 4 shows the results of the Cox proportional hazards models for receipt of metastatic disease testing within 18 months of curative intent treatment, among colorectal cancer patients. Testing for metastatic disease was more likely to occur among patients aged <50 years (HR, 1.31), or aged 50–64 years (HR, 1.25), or aged 65–74 years (HR, 1.11). Patients with more comorbidities (HR, 1.10) were also more likely to receive metastatic testing, but less likely if they were diagnosed with in situ (HR, 0.30) or local (HR, 0.60) disease. Testing for metastatic disease also varied significantly by health plan (p<0.01).
Table 3.
Cox proportional hazards model: Metastatic disease testing within 18 months of treatment, among breast cancer survivors.
| Hazard Ratio (95% CI) | P-value | |
|---|---|---|
| Sociodemographic factors | ||
| Age at diagnosis | ||
| < 50 years | 1.13 (1.01–1.27) | 0.03 |
| 50–64 years | 1.15 (1.05–1.26) | <0.01 |
| 65–74 years | 1.13 (1.03–1.25) | 0.01 |
| ≥ 75 years | reference | - |
| White | 1.13 (1.03–1.23) | 0.01 |
| Median household income ($1000s) | 1.00 (1.00–1.00) | 0.97 |
| Disease stage | ||
| In situ | 0.31 (0.28–0.35) | <0.01 |
| Localized | 0.57 (0.52–0.61) | <0.01 |
| Regional | reference | - |
| Charlson comorbidity index | 1.17 (1.13–1.20) | <0.01 |
| Site of care | ||
| Health plan 1 | 1.76 (1.61–1.93) | <0.01 |
| Health plan 2 | 0.78 (0.71–0.85) | <0.01 |
| Health plan 3 | 0.98 (0.89–1.08) | 0.66 |
| Health plan 4 | reference | - |
χ2 = 791.2, 11 df (p < 0.001).
Table 4.
Cox proportional hazards model: Metastatic disease testing within 18 months of treatment, among colorectal cancer survivors.
| Hazard Ratio (95% CI) | P-value | |
|---|---|---|
| Sociodemographic factors | ||
| Age at diagnosis | ||
| < 50 years | 1.31 (1.06–1.63) | 0.01 |
| 50–64 years | 1.25 (1.10–1.42) | <0.01 |
| 65–74 years | 1.11 (0.98–1.26) | 0.10 |
| ≥ 75 years | reference | - |
| Female | 1.08 (0.98–1.19) | 0.13 |
| White | 1.08 (0.95–1.24) | 0.25 |
| Median household income ($1000s) | 1.00 (1.00–1.00) | 0.55 |
| Disease stage | ||
| In situ | 0.30 (0.22–0.40) | <0.01 |
| Localized | 0.60 (0.54–0.67) | <0.01 |
| Regional | reference | - |
| Charlson comorbidity index | 1.10 (1.07–1.14) | <0.01 |
| Site of care | ||
| Health plan 1 | 1.78 (1.53–2.07) | <0.01 |
| Health plan 2 | 1.07 (0.94–1.21) | 0.29 |
| Health plan 3 | 0.88 (0.75–1.03) | 0.11 |
| Health plan 4 | reference | - |
χ2 = 225.6, 12 df (p < 0.001).
DISCUSSION
Among geographically diverse cohorts of breast and colorectal cancer survivors, we found deviations in the surveillance care received relative to guideline recommendations for physical examinations, and local recurrence and metastatic disease screening. Although many cancer survivors appear to be receiving the minimum recommended surveillance care, a large number of cancers survivors, regardless of age, are still not receiving minimum care recommendations while others are receiving non-recommended metastatic disease testing, as well as recommended care at a greater frequency than what is recommended.
In the integrated delivery systems studied here, the overwhelming majority of breast and colorectal cancer survivors received the minimum recommended number of post-treatment physical exams. In fact, the majority of both breast and colorectal cancer survivors, regardless of age or location of care, appear to receive physical exams more frequently than is recommended by national guidelines. However, despite such exams and national guideline recommendations, almost half of the colorectal cancer cohort failed to receive a complete examination of the colon within 18 months of treatment. On the other hand the majority of breast cancer survivors received recommended recurrence testing. Regardless of cancer site, more than two thirds of survivors particularly those who are younger received some type of metastatic disease testing within 18 months—none of which is recommended by evidence-based guidelines. Furthermore, there were significant differences in the receipt of metastatic testing among health plans that may be a result of regional and practice variations. In addition to these organizational differences, our findings highlight the patient factors associated with metastatic disease testing use. In fact, very little is known about what clinical factors such as type and duration of treatment that clinicians might consider when making surveillance care recommendations to their patients. Clearly there is more to be understood about not only the multi-level factors associated with the receipt of under- and over-care relative to guideline recommendations, but also the health and economic implications of such care use.
Compared to prior studies,10,12,14,16,18 our results seem to illustrate an increase in the receipt of recommended mammograms within 18 months of curative intent treatment as well as a declinein the use of non-recommended metastatic testing among both breast and colorectal cancer survivors. However, these improvements in care quality seemed to occur concurrently with a decrease in receipt of recommended colon examinations among colorectal cancer patients. Furthermore, consistent with prior studies,10,15,21,24 we found that age, stage, race (in breast cancer only), and comorbidities at diagnosis impacted the receipt of metastatic disease testing. Additional clinical evidence is needed to justify whether such variations reflect an opportunity for quality improvement from a public health perspective.
The IOM recommends that all cancer survivors receive a survivorship care plan that includes clear and effective recommendations regarding preventive practices and specific information about the timing and content of recommended follow-up1. European studies have highlighted that patients exhibit a positive attitude towards follow-up care and want follow-up plans to be tailored to their specific needs26–29. There is growing evidence that survivors in the US have similar preferences, and that they wish to collaborate with providers in making medical decisions and want to receive care plan information directly30–32. It remains unknown whether the patterns of follow-up care observed here are consistent with those preferences. However, as we have no evidence that such plans were routinely used during the time of observation in any of the organizations studied here, it is likely safe to assume that the observed patterns of follow-up are as much a result of a lack of explicit follow-up planning and care coordination as a coordinated and well-planned effort. Given that each of the participating organizations is an integrated delivery system with a comprehensive electronic medical record system, and each routinely scores well on publicly available quality metrics33, it seems clear that while substantive quality gains may have been made at either end of the cancer care continuum, as the IOM report points out, care delivered during the transition from cancer patient to cancer survivor more often than not is inadequate.
These conclusions should be interpreted in the light of several important limitations. First, caution should be used when generalizing study results and conclusions to other populations and settings. Study cohort members were limited to insured individuals who received their cancer care from one of four integrated health care delivery systems. Although these samples are generally similar to the general populations in their respective communities in terms of demographics, they may differ in other unmeasured respects. In particular, care should be taken in generalizing our findings to those who are uninsured. Another limitation is that we are unable to ascertain from EMR or claims-based sources whether care received was for surveillance versus other purposes. Thus our findings of surveillance care overuse may be partially explained by our lack of information on symptoms and laboratory findings and our inability to differentiate between procedures received for screening versus diagnostic purposes. However, a prior study found that claims data capturing procedures and visit use for characterizing guideline adherence was comparable with documentation found in the medical record, and that administrative data could be used to describe patterns of follow up care.13 A further limitation stems from grouping patients, with heterogeneity in prognoses, into general disease stages. Patients within these broad groups and the clinicians treating them may resort to different surveillance practices based on perceived differences in prognosis. Yet, it is important to note that current guidelines do not differ in their recommendations for surveillance care based on these or other factors.
Compared to other phases of cancer control and prevention, surveillance care among cancer survivors is understudied. Findings here highlight the wide variations that exist in cancer surveillance care among seemingly clinically similar patients and across different age groups. This variation combined with the overall lack of concordance with established clinical practice guidelines, highlights the need for research exploring both whether observed variations are driven by patient preferences and reflect informed decision making, and how survivorship planning as outlined by the IOM can impact such variation. Furthermore, given the survival advantage for patients <65, it is important to consider the care trajectory and its implications among this subset of cancer patients.
Acknowledgments
Funding for this research was provided by NCI Grants R01 CA114204, R01 CA114204-03S1, R25 CA116339, and NCI Cooperative Agreement U19 CA79689. The following staff members provided data processing and analysis support for this study: KPNW: Erin Keast, Erin Masterson, Donald Bachman, and Arthur Dixon; GHC: Christi Hanson and Arvind Ramaprasan; KPCO: Stephanie Latimer and Gwyn Saylor; HFHS: Nonna Akkerman and Elizabeth Dobie.
Contributor Information
Ramzi G. Salloum, Health Policy and Management, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC and Center for Health Policy and Health Services Research, Henry Ford Health System, Detroit, MI..
Mark C. Hornbrook, Center for Health Research, Kaiser Permanente Northwest, Portland, OR. mark.c.hornbrook@kpchr.org.
Paul A. Fishman, Group Health Research Institute, Group Health Cooperative, Seattle, WA. fishman.p@ghc.org.
Debra P. Ritzwoller, Institute for Health Research, Kaiser Permanente Colorado, Denver, CO. debra.ritzwoller@kp.org.
Maureen C. O’Keeffe Rossetti, Center for Health Research, Kaiser Permanente Northwest, Portland, OR. maureen.rosetti@kpchr.org.
Jennifer Elston Lafata, Social and Behavioral Health and Massey Cancer Center, School of Medicine, Virginia Commonwealth University, Richmond, VA and Center for Health Policy and Health Services Research, Henry Ford Health System, Detroit, MI. jelstonlafat@vcu.edu.
References
- 1.IOM (Institute of Medicine) From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academy Press; 2006. [Google Scholar]
- 2.National Comprehensive Cancer Network. [Accessed March 8, 2011];Breast Cancer. Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 3.National Comprehensive Cancer Network. [Accessed March 8, 2011];Colon Cancer. Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 4.Desch CE, Benson AB, 3rd, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999;17(4):1312. doi: 10.1200/JCO.1999.17.4.1312. [DOI] [PubMed] [Google Scholar]
- 5.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17(3):1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130(6):1865–1871. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 7.The University of Texas, MD Cancer Center. [Accessed March 8, 2011];Clinical & Scientific Resources. Guidelines by Disease Site. http://www.mdanderson.org/education-and-research/resources-for-professionals/index.html. Updated 2011.
- 8.Grunfeld E, Dhesy-Thind S, Levine M. Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172(10):1319–1320. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 10.Lash TL, Silliman RA. Medical surveillance after breast cancer diagnosis. Med Care. 2001;39(9):945–955. doi: 10.1097/00005650-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Elston Lafata J, Cole Johnson C, Ben-Menachem T, Morlock RJ. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39(4):361–372. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Cooper GS, Johnson CC, Lamerato L, et al. Use of guideline recommended follow-up care in cancer survivors: routine or diagnostic indications? Med Care. 2006;44(6):590–594. doi: 10.1097/01.mlr.0000215902.50543.77. [DOI] [PubMed] [Google Scholar]
- 13.Cooper GS, Schultz L, Simpkins J, Lafata JE. The utility of administrative data for measuring adherence to cancer surveillance care guidelines. Med Care. 2007;45(1):66–72. doi: 10.1097/01.mlr.0000241107.15133.54. [DOI] [PubMed] [Google Scholar]
- 14.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43(6):592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 15.Rolnick S, Hensley Alford S, Kucera GP, et al. Racial and age differences in colon examination surveillance following a diagnosis of colorectal cancer. J Natl Cancer Inst Monogr. 2005:96–101. doi: 10.1093/jncimonographs/lgi045. (35)(35) [DOI] [PubMed] [Google Scholar]
- 16.Rulyak SJ, Mandelson MT, Brentnall TA, Rutter CM, Wagner EH. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59(2):239–247. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 17.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100(2):418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors : a population-based analysis. Cancer. 2008;113(8):2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 19.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 20.Ellison GL, Warren JL, Knopf KB, Brown ML. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38(6 Pt 2):1885–1903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38(3):281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Lamont EB, Herndon JE, 2nd, Weeks JC, et al. Measuring disease-free survival and cancer relapse using Medicare claims from CALGB breast cancer trial participants (companion to 9344) J Natl Cancer Inst. 2006;98(18):1335–1338. doi: 10.1093/jnci/djj363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Geller BM, Kerlikowske K, Carney PA, et al. Mammography surveillance following breast cancer. Breast Cancer Res Treat. 2003;81(2):107–115. doi: 10.1023/A:1025794629878. [DOI] [PubMed] [Google Scholar]
- 25.Ko CW, Kreuter W, Baldwin LM. Effect of Medicare coverage on use of invasive colorectal cancer screening tests. Arch Intern Med. 2002;162(22):2581–2586. doi: 10.1001/archinte.162.22.2581. [DOI] [PubMed] [Google Scholar]
- 26.Stiggelbout AM, de Haes JC, Vree R, et al. Follow-up of colorectal cancer patients: quality of life and attitudes towards follow-up. Br J Cancer. 1997;75(6):914–920. doi: 10.1038/bjc.1997.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kjeldsen BJ, Thorsen H, Whalley D, Kronborg O. Influence of follow-up on health-related quality of life after radical surgery for colorectal cancer. Scand J Gastroenterol. 1999;34(5):509–515. doi: 10.1080/003655299750026254. [DOI] [PubMed] [Google Scholar]
- 28.Kiebert GM, Welvaart K, Kievit J. Psychological effects of routine follow up on cancer patients after surgery. Eur J Surg. 1993;159(11–12):601–607. [PubMed] [Google Scholar]
- 29.Papagrigoriadis S, Heyman B. Patients' views on follow up of colorectal cancer: implications for risk communication and decision making. Postgrad Med J. 2003;79(933):403–407. doi: 10.1136/pmj.79.933.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270–2273. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 31.Partridge AH, Winer EP, Burstein HJ. Follow-up care of breast cancer survivors. Semin Oncol. 2003;30(6):817–825. doi: 10.1053/j.seminoncol.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Kantsiper M, McDonald EL, Geller G, Shockney L, Snyder C, Wolff AC. Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med. 2009;24(Suppl 2):S459–S466. doi: 10.1007/s11606-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Committee for Quality Assurance. [Accessed 10/01, 2011];NCQA Health Plan Report Card. http://reportcard.ncqa.org/plan/external/plansearch.aspx.