Abstract
APOL1 gene variants are associated with end-stage renal disease in African Americans. Here we investigate the impact of recipient APOL1 (apolipoprotein L-1) gene distributions on kidney allograft outcomes. We conducted a retrospective analysis of 119 African American kidney transplant recipients, and found that 58 (48.7%) carried two APOL1 kidney disease risk variants. Contrary to the association seen in native kidney disease, there is no difference in allograft survival at 5 years post-transplant for recipients with high-risk APOL1 genotypes. Thus, we were able to conclude that APOL1 genotypes do not increase risk of allograft loss after kidney transplantations, and carrying 2 APOL1 risk alleles should not be an impediment to transplantation.
Keywords: African-Americans, kidney graft survival, gene polymorphism
Introduction
Specific “risk” variants of the APOL1 gene located on chromosome 22 are strongly associated with end-stage renal disease (ESRD) in African Americans (AAs). The variants are G1 (rs73885319), a single nucleotide polymorphism leading to a non-synonymous amino acid substitution (S342G) and G2 (rs71785313), a 6 base pair deletion leading to the deletion of two amino acids (delN388/Y389), in the last exon of the APOL1 gene (1). The variants are common in people of African ancestry (allele frequency G1 0.18-0.21, G2 0.15-0.13) and especially so in AAs with ESRD (allele frequency G1 0.41-0.52, G2 0.21-0.23). Individuals that are carriers of two APOL1 risk alleles, possible genotypes include G1/G1, G1/G2, or G2/G2, have much higher odds of developing ESRD (FSGS odds ratio is 10.5, 95% CI 6.0-18.4; hypertensive ESRD odds ratio is 7.3, 95% CI 5.6-9.5) (1). APOL1 variants are generally not seen in European populations (2, 3).
African Americans have higher rates of kidney failure, lower rates of transplantation, and decreased allograft survival compared to Caucasian Americans (4, 5). The adjusted 10-year graft survival for deceased donor and living donor kidney allografts have been found to be worse for AA patients (32.2% and 45.3%, respectively) compared to white individuals (44.8% and 60.2%) (5). Similar to the development of ESRD, the reasons for decreased allograft survival in African Americans are not fully explained by co-morbid disease or socioeconomic factors (6). A recent retrospective study reported shorter allograft survival in transplant recipients when the genotype of the donor kidney carried 2 APOL1 risk variants (HR 3.84, p= 0.008) (7). However, the study had only 2 years of post-transplant follow-up, and did not study the impact of the recipient's APOL1 genotype on clinical outcomes.
Here, we evaluated the prevalence of the APOL1 variant genotypes in a heterogeneous kidney transplant population and studied 5-year allograft survival in kidney transplant recipients with 0, 1, and 2 APOL1 risk alleles.
Materials and Methods
Study Participants
Subjects were kidney transplant recipients at the Brigham and Women's Hospital and the University of Alabama at Birmingham, transplanted between 1988 and 2002. The participants were originally enrolled in a study of single nucleotide polymorphisms in the gene encoding β3 integrin and it's role in the pathogenesis of acute rejection (8). Of the original cohort (n=424) we were able to identify 124 remnant DNA samples from kidney transplant recipients of recent African ancestry. We were unable to locate the clinical follow-up data on five subjects, leaving 119 self-identified African American subjects from whom we were able to locate remnant DNA samples and post-transplant clinical information.
The study protocols were approved by the Institutional Review Board at Brigham and Women's Hospital (Protocol #: 2011-P-001553/1) and at the University of Alabama at Birmingham, and are in accordance with the Declaration of Helsinki. Informed consent was waived because of the infeasibility of contacting patients transplanted 20 year ago, as many had lost their allograft, were no longer followed by the transplantation centers, and/or had died. In addition, we used remnant DNA samples and removed all identifiers after the initial transcription of the clinical information.
Data Collection
Clinical data was collected retrospectively and included age at transplant, cause of native kidney disease, type of donor allograft (deceased vs. living donor), donor race, results of allograft biopsy, years of allograft and patient survival post-transplant, and serum creatinine at last follow-up where applicable.
Genotyping
Genotyping was performed using a 7300 Real Time PCR System from Applied Biosystems (Life Technologies Corporation, Carlsbad, CA). Primers were made by a custom SNP genotyping assay from Applied Biosystems for G1 (rs73885319; S342G) and G2 (rs71785313, 6 base-pair deletion of N388 and Y389) variants of the APOL1 gene. Each sample was tested in triplicates with a positive and a non-template control on each PCR plate.
Statistical Analysis
The primary outcome was allograft survival. Differences between subjects in the 2-risk allele group and the 0 or 1 risk allele group were tested using a two-tailed unpaired t test. Kaplan-Meier survival curves were generated using the software program Prism (GraphPad Software, San Diego, CA). Comparisons of survival curves were evaluated with the Log-rank (Mantel-Cox) test. Multivariate analysis was done to control for differences in age and the prevalence of diabetes mellitus using a Cox proportional hazards model on JMP Pro v9.0.0 (SAS, Cary, NC). Age was used as a categorical variable and was determined to best fit the statistical model by calculation of the Akaike Information Criterion (SAS version 9.3, Cary NC). Age groups were defined by the following, Group 1: age <30 (reference group); Group 2: age ≥ 30, but less than 40; Group 3: age ≥ 40, but less than 50; Group 4: age ≥ 50, but less than 60; Group 5: age ≥ 60.
Results
Half of all AA patients who are transplanted have 2 APOL1 risk alleles. DNA samples from 119 African-American kidney transplant recipients were genotyped for the G1 and G2 variants of APOL1. Of the 119 subjects 58 (48.7%) carried 2 APOL1 risk alleles (G1/G1, G1/G2, or G2/G2; Table 1). The proportions of APOL1 genotypes were similar to those for African-American subjects with end-stage kidney disease reported by Genovese, et al. (1). Approximately one quarter of the subjects had been documented as having end-stage diabetic kidney disease, another quarter had hypertension-related kidney failure, a third quarter had focal segmental glomerulosclerosis (FSGS) or glomerulonephritis (GN), and the final quarter had either unknown native kidney disease or another form of kidney disease not previously listed. The proportion of recipients with 2 APOL1 risk alleles was for 25%, 60%, 82%, and 52% for AA kidney recipients with diabetes mellitus (DM), FSGS, GN, and hypertension (HTN), respectively. There is a greater number of subjects with FSGS and GN that carry 2 APOL1 risk alleles, compared to subjects with any other form of native kidney disease.
Table 1. Number of APOL1 genotypes organized by native end-stage renal disease in kidney transplant recipients.
| DM | FSGS | GN | HTN | Other/UNK | Total (%) | |
|---|---|---|---|---|---|---|
| WT/WT | 8 | 2 | 2 | 5 | 8 | 25 (21) |
| WT/G1 | 8 | 2 | 0 | 6 | 8 | 24 (20.2) |
| WT/G2 | 2 | 2 | 1 | 4 | 3 | 12 (10.1) |
| G1/G1 | 2 | 7 | 8 | 6 | 6 | 29 (24.2) |
| G1/G2 | 3 | 2 | 6 | 10 | 6 | 27 (22.7) |
| G2/G2 | 1 | 0 | 0 | 0 | 1 | 2 (1.7) |
| Total (%) | 24 (20.2) | 15 (12.6) | 17 (14.3) | 31 (26.1) | 32 (26.9) | 119 |
High-risk variants appear in bold. DM=diabetes mellitus, FSGS=focal segmental glomerulosclerosis, GN=glomerulonephritis, HTN=hypertension, UNK=unknown, WT=wild type
Patients with 2 APOL1 risk alleles were transplanted at an earlier age. The mean age of the 2-risk allele group was 40 compared to 47 in the 0/1-risk allele group (Table 2). The difference in age was statistically significant. The number of deceased and living donor transplants was similar in both groups. There was a higher proportion of black donor kidneys in the 2-risk allele group compared to the 0/1 risk allele group, 43.1% and 32.8% respectively. The number of deaths, subjects with allograft loss, and subjects with allograft loss not including death with a functioning graft, were roughly equivalent between the two groups.
Table 2. Characteristics of kidney transplant recipients.
| 2 Risk alleles | 0 or 1 Risk alleles | |
|---|---|---|
| Number of subjects | 58 (48.7%) | 61 (51.3%) |
| Mean age at transplant (years) p value 0.0003 |
39.82 | 46.80 |
| Cause of ESRD | ||
| Attributed to HTN | 15 | 14 |
| Diabetes mellitus | 7 | 19 |
| FSGS | 9 | 6 |
| Membranous nephropathy | 2 | 1 |
| MPGN | 3 | 0 |
| Other GN | 9 | 3 |
| SLE | 2 | 5 |
| PKD | 0 | 3 |
| Other (Lithium, reflux, horseshoe kidney) | 0 | 3 |
| IgA nephropathy | 1 | 0 |
| Unknown | 10 | 7 |
| Type of donor kidney | ||
| Deceased | 44 | 48 |
| Living related | 14 | 13 |
| Donor ethnicity is Black | 25 (43.1%) | 20 (32.8%) |
| Allograft loss (%) | 13 (22.4%) | 13 (21.3%) |
| Allograft loss censored by death (%) | 13 (22.4%) | 11 (18.0%) |
| Patient death | 1 | 2 |
| Acute rejection within 1yr post-transplant | 2 | 2 |
HTN=hypertension, FSGS=focal segmental glomerulosclerosis, MPGN=membranoproliferative glomerulonephritis, GN=glomerulonephritis, SLE=systemic lupus erythematosus, PKD=polycystic kidney disease, IgA=immunoglobulin
Unadjusted allograft survival (time to allograft loss or death) and allograft survival censoring for patient death (time to allograft loss not including death with a functioning graft) are shown in Figures 1A and 1B respectively. The number of subjects at risk is listed below each figure. There was also no difference between the subjects with 0 risk alleles and 1 risk allele (Figure 2).
Figure 1.
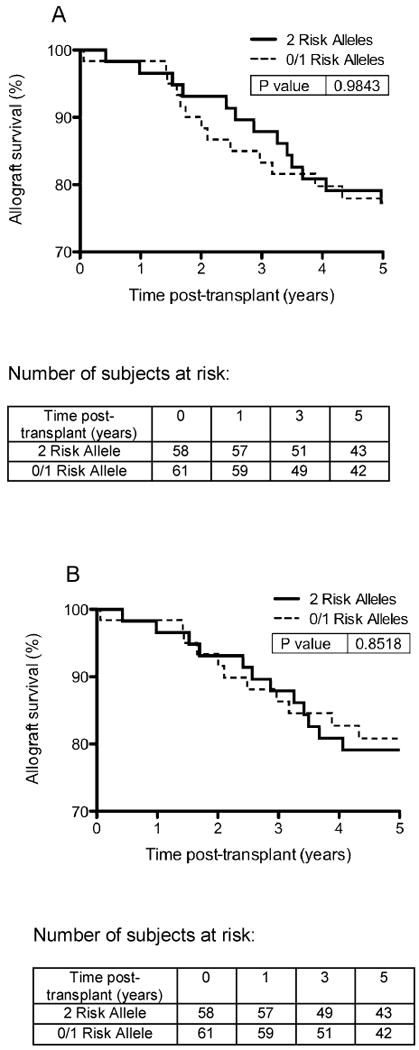
A. Unadjusted allograft survival (time to allograft loss or death) in African American kidney transplant recipients with 2 APOL1 risk alleles compared to 0/1 APOL1 risk alleles. B. Allograft survival censoring for patient death (time to allograft loss not including death with a functioning graft).
Figure 2.
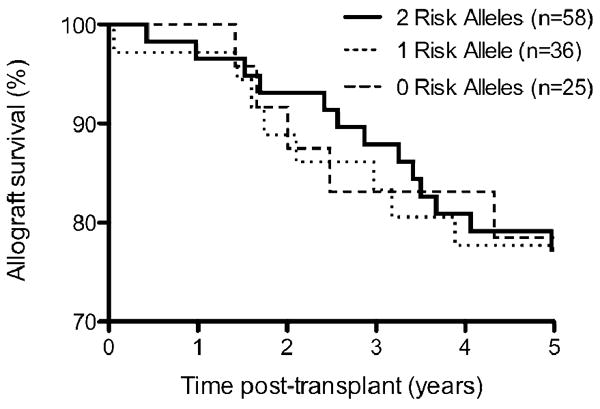
Allograft survival of subjects with 0, 1, or 2 risk alleles.
Age and DM would be expected to account for differences in allograft survival, and when controlling for age, categorized by decades, and DM there was no statistically significant difference in the allograft survival of the high risk 2 APOL1 allele group compared to the low risk 0 or 1 APOL1 allele group (HR 0.96, 95% CI 0.61-1.49, p=0.840). When censoring for death, not including subjects that died with a functioning kidney allograft, there was also no statistically significant difference in allograft survival between the two groups (HR 0.96, 95% CI 0.61-1.48, p=0.841).
Donor genotype would also be expected to be a risk factor for allograft loss. However, information on the donor genotype was not available for our analysis. However, we were able to ascertain the ethnicity of the donor. In an explorative analysis, we compared the recipients of non-AA donors, thus eliminating the possible effects of donor genotypes, and found no difference in allograft survival between carriers of 2 APOL1 risk alleles (n=33) vs. 0/1 APOL1 risk alleles (n=41) (p-value 0.522), but this may be because the comparison was underpowered to detect a potential difference.
Discussion
While the association of allelic variations of APOL1 and increased risk of FSGS and hypertensive ESRD in AA patients has been made, the causal mechanism has yet to be determined. ApoL1 is a soluble protein found in plasma bound to high-density lipoproteins (9). Although ApoL1 has been localized to podocytes and proximal tubular epithelial cells in the kidney (10), the trypanolytic role of ApoL1 in defending against African Sleeping Sickness is through the circulating serum protein. In addition, ApoL1 is also expressed on vascular endothelial cells (11). The present study examined the prevalence of APOL1 risk alleles in AA recipients of kidney transplants and their impact on transplant outcomes.
The distribution of APOL1 genotypes in a heterogeneous population of kidney transplant recipients is similar to the distribution seen in the hypertension associated end-stage kidney disease cohort studied by Genovese, et al. [219 G1/G1, 203 G1/G2, 44 G2/G2, and 536 (53%) with only 0 or 1 APOL1 risk allele] (1). The younger mean age at transplant of the 2-risk allele group in our cohort also mirrors a recent report by Kanji, et al., where subjects with 2 APOL1 risk alleles were on average 7 years younger at dialysis initiation than subjects with 0 or 1 risk allele (49 years vs. 56 years, respectively) (12). That our cohorts were younger than the ESRD cohort studied by Kanji, et al. is likely due to the fact that transplantation preselects younger, healthier patients. The two studies may also not be directly comparable because of the difference in the time period studied.
Overall, half of our cohort were found to be carriers of 2 APOL1 kidney disease variants. However, there were a disproportionate number of individuals with FSGS or glomerulonephritis native kidney disease who had 2 APOL1 risk alleles. It appears that certain non-diabetic glomerular disease may have a different and more profound susceptibility to APOL1 gene variants than other forms of kidney disease, as has been shown in previous studies of hypertension associated ESRD and HIV associated nephropathy ESRD (13-17). Fourteen of 17 subjects (82%) with GN and 9 of 15 subjects (60%) with FSGS had 2 APOL1 risk alleles. In contrast, only 25% of subjects with diabetic native kidney disease were carriers of 2 APOL1 risk variants.
Both the unadjusted and the adjusted for age and DM analyses show that there is no increased risk of allograft loss at 5 years post-transplant in African American kidney transplant recipients with 2 APOL1 variant alleles compared individuals with 0 or 1 risk alleles. Patients with 2 risk alleles are not at risk of earlier graft loss, and therefore, having 2 APOL1 risk alleles should not be an impediment to kidney transplantation.
Donor genotype may be a risk factor for allograft loss. While we were unable to ascertain the donor genotype in this study, we did have information on donor race. Given that only 10-12% of the general AA population carry 2 APOL1 risk alleles, only 2-3 donor kidneys of the 25 and 20 AA donors in both the 2 and the 0/1 risk allele, respectively, would be expected to carry 2 APOL1 risk alleles. While lacking the donor genotype is a limitation, the small numbers of AA donors that would have a high-risk genotype is small and would likely not have a large impact on the outcomes observed.
Future work needs to be done to study the genotypes of kidney transplant recipients and donors together. In addition, our small sample size limits our statistical analysis. A larger cohort study with extended clinical follow-up to examine long-term allograft function and survival in African Americans with APOL1 gene variants would be useful.
Acknowledgments
Sarah B. Goldberg, MD, Dana-Farber Cancer Institute, Boston, MA, and Bernard Rosner, PhD, Brigham and Women's Hospital, Harvard School of Public Health provided counsel and aided in the statistical analysis.
Research work supported by National Institutes of Health grant R24DK092158.
Abbreviations
- APOL1
apolipoprotein L1 (gene)
- ApoL1
apolipoprotein L1 (protein)
- ESRD
end-stage renal disease
- AA
African American
- FSGS
focal segmental glomuerulosclerosis
- GN
glomerulonephritis
- DM
diabetes mellitus
- HTN
hypertension
- HR
hazard ratio
- CI
confidence interval
- TTL
time to allograft loss
- TTLD
time to allograft loss or death
- SLE
systemic lupus erythematosus
- PKD
polycystic kidney disease
- IgA
immunoglobulin A
- UNK
unknown
- WT
wild type
Footnotes
Disclosure: The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation: MP, DF and GG are named on a provisional patent for APOL1 genotyping.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Seaghdha CM, Parekh RS, Hwang SJ, Li M, Kottgen A, Coresh J, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet. 2011;20(12):2450–2456. doi: 10.1093/hmg/ddr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int. 2010;78(7):698–704. doi: 10.1038/ki.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 5.2009 Annual Report U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. [Accessed: November 7, 2011]; http://www.ustransplant.org/annual_reports/current/all_data_tables.htm.
- 6.Fan PY, Ashby VB, Fuller DS, Boulware LE, Kao A, Norman SP, et al. Access and outcomes among minority transplant patients, 1999-2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10(4 Pt 2):1090–1107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrakantan A, McDermott DH, Tran HT, Jurewicz M, Gallon L, Gaston R, et al. Role of beta3 integrin in acute renal allograft rejection in humans. Clin J Am Soc Nephrol. 2007;2(6):1268–1273. doi: 10.2215/CJN.01380307. [DOI] [PubMed] [Google Scholar]
- 9.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O'Connor PM, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272(41):25576–25582. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 10.Madhavan SM, O'Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22(11):2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJ. The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics. 2002;79(4):539–546. doi: 10.1006/geno.2002.6729. [DOI] [PubMed] [Google Scholar]
- 12.Kanji Z, Powe CE, Wenger JB, Huang C, Ankers E, Sullivan DA, et al. Genetic Variation in APOL1 Associates with Younger Age at Hemodialysis Initiation. J Am Soc Nephrol. 2011;22(11):2091–2097. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, Snyder HJ, et al. APOL1 Variants Increase Risk for FSGS and HIVAN but Not IgA Nephropathy. J Am Soc Nephrol. 2011;22(11):1991–1996. doi: 10.1681/ASN.2011040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behar DM, Kedem E, Rosset S, Haileselassie Y, Tzur S, Kra-Oz Z, et al. Absence of APOL1 Risk Variants Protects against HIV-Associated Nephropathy in the Ethiopian Population. Am J Nephrol. 2011;34(5):452–459. doi: 10.1159/000332378. [DOI] [PubMed] [Google Scholar]
- 17.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]


