Abstract
Immunosuppressive therapies that block the CD40/CD154 costimulatory pathway have proven to be uniquely effective in preclinical xenotransplant models. Given the challenges facing clinical translation of CD40/CD154 pathway blockade, we examined the efficacy and tolerability of CD40/CD154 pathway-sparing immunomodulatory strategies in a pig-to-nonhuman primate islet xenotransplant model. Rhesus macaques were rendered diabetic with streptozocin and given an intraportal infusion of ~50,000 IEQ/kg wild-type neonatal porcine islets. Base immunosuppression for all recipients included maintenance therapy with belatacept and mycophenolate mofetil plus induction with basiliximab and LFA-1 blockade. Cohort 1 recipients (n=3) were treated with the base regimen alone; cohort 2 recipients (n=5) were additionally treated with tacrolimus induction, and cohort 3 recipients (n=5) were treated with alefacept in place of basiliximab, and more intense LFA-1 blockade. Three of 5 recipients in both cohorts 2 and 3 achieved sustained insulin-independent normoglycemia (median rejection-free survivals 60 and 111 days, respectively), compared to 0 of 3 recipients in cohort 1. These data show that CD40/CD154 pathway-sparing regimens can promote xenoislet survival. Further optimization of these strategies is warranted to aid the clinical translation of islet xenotransplantation.
Keywords: Xenotransplantation, costimulation blockade, type 1 diabetes, islets, LFA-1, LFA-3, tacrolimus, T cell memory
Introduction
Inadequate availability of human donors continues to severely limit allotransplantation in all its forms. In contrast, xenogeneic donors represent a virtually unlimited and on-demand supply of organs and tissues, making xenotransplantation an appealing strategy for use in humans. Based on current preclinical evidence, pig-to-human pancreatic islet transplantation appeabrs to be the form of xenotransplantation with the greatest potential for clinical translation in the near future (1); however, many practical and immunologic barriers must be overcome before widespread application of this approach in humans is possible. Perhaps the most formidable of these obstacles is development of an effective, tolerable, and translatable immunosuppressive strategy to overcome the potent xenospecific immune response (2).
Antibodies that block the CD40/CD154 costimulatory signaling pathway have proven uniquely successful in their ability to consistently promote xenograft survival. The efficacy of CD154-specific therapies has been demonstrated repeatedly in various models (3–5), including xenoislet transplant; in two proof-of-concept studies, CD154-specific antibodies promoted engraftment and long-term survival of porcine islets in nonhuman primates (6, 7). Likewise, various CD40-specific antibodies have been tested in clinically relevant models of allokidney (8–10), alloislet (11, 12), and recently xenoislet (13) transplantation with promising results. Despite success in preclinical models, application of CD154-specific antibodies in preliminary human trials yielded disappointing results including concerns related to an increased incidence of thromboembolic events and limited efficacy (14, 15); clinical translation of intact CD154-specific antibodies has largely been abandoned in the wake of these developments. The translational potential of CD40-specific therapies is being pursued as an alternate means of impairing the CD40/CD154 interaction while sparing CD154-specific adverse sequelae (16). However, preclinical investigation of various CD40-specific antibodies with different depletional and receptor agonism characteristics is ongoing, and the lack of consensus regarding the optimal CD40-specific approach may delay widespread clinical application. Given the limitations of both CD154- and CD40-specific therapies, investigation of alternative immunomodulatory strategies is warranted.
The ability of CD40/CD154 pathway-sparing immunosuppressive regimens to promote xenoislet engraftment and long-term survival in a clinically relevant model has not been convincingly demonstrated (17, 18); however, our group has had success targeting alternative costimulatory pathways in allotransplant models. In recently published data, the LFA-1-specific monoclonal antibody TS-1/22 and the CD2-binding fusion protein alefacept have shown efficacy in alloislet (19) and allokidney (20) applications as adjuncts to costimulation blockade-based immunosuppressive regimens. Although previously untested in a xenotransplant system, we hypothesized that these alternative agents could facilitate xenoislet survival in the absence of CD40/CD154 pathway blockade. In this study, we demonstrate the feasibility, tolerability, and efficacy of clinically appealing CD40/CD154 pathway-sparing regimens using an established nonhuman primate (NHP) islet xenotransplant model.
Methods
Nonhuman Primates: Induction of Diabetes and Recipient Care
Captive bred adolescent rhesus macaques (Macaca mulatta) were used as xenograft recipients. Diabetes was induced by streptozocin (STZ, 1250 mg/m2 IV; Zanosar, Teva Parenteral Medicines, Irvine, CA) 4 weeks prior to transplant. Post-streptozocin care consisted of measurement of weight, fasting and postprandial blood glucose levels and nutritional support. Insulin (NPH, Ultralente; Eli Lilly, Indianapolis, IN) was administered twice daily to maintain fasting blood glucose (FBG) at <130 mg/dL. Glycemic control was determined by intravenous glucose tolerance test (IVGTT) with a bolus of dextrose (500mg/kg). Glucose levels were measured prior to the bolus, and then at 10, 30, 60, and 90-minute intervals; in addition, primate c-peptide was measured from serum obtained at each IVGTT timepoint. Diabetes was confirmed by the failure of blood glucose to normalize 90 minutes after dextrose bolus in the absence of detectable primate c-peptide. All procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals (21),” and were approved by our Institutional Animal Care and Use Committee (IACUC).
Procurement and Preparation of Neonatal Porcine Islets
One- to four-day-old wild-type Duroc or Large White crossbreed neonatal pigs of either sex (total body weight 1.5–2.0 kg, Swine Research and Technology Centre, University of Alberta; Palmetto Research Swine, Reeseville, SC) were used as pancreas donors. Pancreatectomies and islet isolations were performed at either the Surgical-Medical Research Institute at the University of Alberta, Edmonton, or Emory’s Animal Cell & Tissue Laboratory (EACTL) using a previously described modified Korbutt technique (22). In brief, harvested pancreas tissue was first digested with 1.0 mg/mL collagenase (Type XI, Sigma). Free islets were then filtered and cultured in supplemented Ham’s F10 medium (Gibco, Burlington, Ontario, Canada) for a period of 7 days. Islets isolated at the University of Alberta were cultured for 5 days prior to shipment to EACTL for final culture and quantitative assessment. All islet suspensions were transplanted into NHPs on day 7 post-isolation.
Pre-Transplant Quantitative Assessment of Islet Preparations
On the day of transplantation, islets were assessed at EACTL for quantity by dithizone (Sigma-Aldrich), for viability by SYTOX® Green Fluorescent Nucleic Acid Stain (Invitrogen, Eugene, OR), for bacterial contamination by both Gram stain and culture and for in vitro function by static incubation assay as previously described (6). Functionality of islets was quantified using the glucose stimulation index (GSI), calculated by dividing the amount of insulin release at high glucose concentrations (20 mmol/L glucose) by that at low concentrations (2.8 mmol/L glucose).
Islet Transplantation
On day of transplant, the islet preparations were resuspended in 20 mL of transplant media supplemented with 200 units of heparin and etanercept 3mg/kg (Enbrel; Amgen & Wyeth, Philadelphia, PA). Following mini-laparotomy, approximately 50,000 islet equivalents (IEQ)/kg of NPIs were transplanted intraportally into each of the NHP recipients via gravity drainage of the suspension into a mesocolic vein through a 22-gauge intravenous catheter.
Post-transplantation Monitoring of Xenoislet Function
Recipient fasting and postprandial blood glucose levels were monitored daily by ear-stick (Glucometer Elite; Bayer, Elkhart, IN). Insulin was administered twice daily to maintain fasting blood glucose (FBG) at <200 mg/dL. IVGTTs were performed at monthly intervals during the post-transplant period. Porcine c-peptide (Pc-P) was measured from sera obtained at each IVGTT timepoint as well as from serial samples obtained throughout the post-transplant period, using the manufacturer’s protocol from Linco’s radioimmune assay kit (Linco Research; St. Charles, MO) as previously described (6).
Experimental Groups and Immunosuppressive Regimens
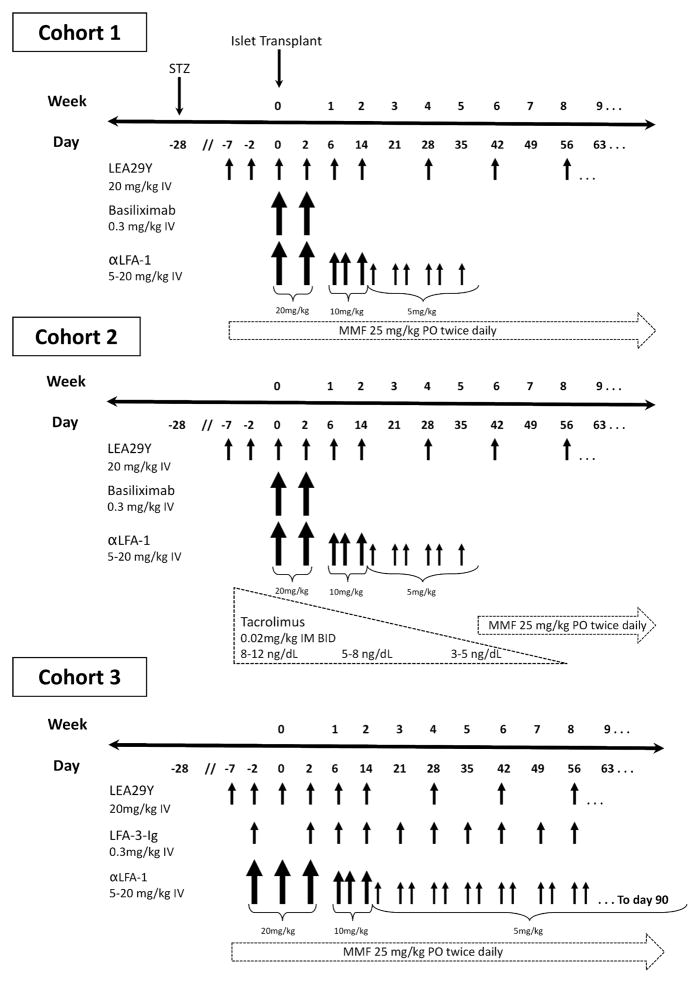
We tested a base immunosuppressive regimen which consisted of peritransplant induction immunosuppression with the high-affinity CTLA4Ig variant belatacept (LEA29Y; 20mg/kg IV, Bristol Meyers Squibb) on pre-transplant days 7 and 2, intraoperatively, and on post-transplant days 2 and 6; additional doses were given as maintenance therapy on day 14 and then every 2 weeks until experimental endpoint. Extended induction with the mouse anti-human LFA-1 monoclonal antibody TS-1/22 (Biovest International, Minneapolis, MN) was administered as an intravenous infusion starting with 20mg/kg on day of transplant and post transplant day 2. The dose was then tapered to 10mg/kg on days 6, 10, and 14, and then 5mg/kg given twice weekly until post-transplant day 35. Peritransplant induction with the IL-2 receptor-specific antibody basiliximab (Simulect; Novartis, E. Hanover, NJ; 0.3 mg/kg IV) was administered intraoperatively and on post-transplant day 2. Lastly, base immunosuppression included maintenance therapy with mycophenolate mofetil (25mg/kg PO BID; Cellcept, Roche Pharmaceuticals, Nutley, NJ) starting pre-transplant day 7 through experimental endpoint. As controls, three primates (cohort 1) received the base regimen alone.
Five primates (cohort 2) received the base regimen, but were given extended induction with tacrolimus (0.02mg/kg IM BID; Prograf, Astellas Pharma) beginning one week prior to transplant followed by mycophenolate maintenance. Tacrolimus dosing was adjusted weekly to maintain a target level of 8–12 ng/mL. This dose was gradually tapered over 8 weeks to achieve levels of 3–5 ng/mL by post-transplant day 56, at which point recipients were transitioned back to mycophenolate for the duration of their experimental course.
Five primates (cohort 3) were treated with the base regimen but were given extended induction with the CD2-specific fusion protein alefacept (0.3mg/kg IV; Amevive, Astellas Pharma, Deerfield, IL) on pre-transplant day 2, post-transplant day 2, and then weekly through post-transplant day 60 in place of basiliximab. In addition, cohort 3 recipients were given more intense TS-1/22 induction, which consisted of one additional dose on pre-transplant day 2 (20mg/kg IV), and biweekly doses of 5mg/kg through post-transplant day 90.
The belatacept used in these experiments was provided by Bristol-Myers Squibb; alefacept was provided by Astellas. All other drugs were purchased from the Emory University Hospital Pharmacy.
Immunohistochemistry
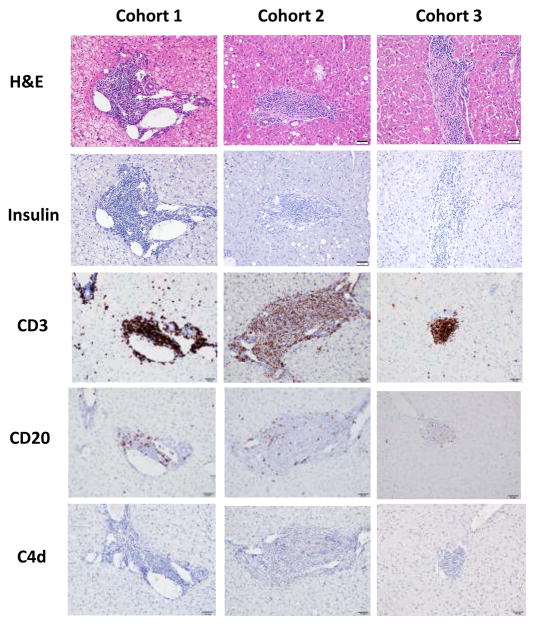
Immunohistochemical analysis of transplanted intrahepatic islets was performed at time of recipient necropsy using standard hematoxylin and eosin as well as staining for insulin (Sigma, St. Louis, MO), CD3, CD20 (Dako, Carpinteria, CA), and complement C4d (American Research Products, Waltham, MA). Islet histology demonstrating destruction of insulin-producing cells in association with a lymphocytic infiltrate was used as confirmation of rejection.
Experimental Endpoints, Determination of Islet Rejection
Primary graft dysfunction and functional rejection were the primary experimental endpoints in this study. Functional rejection was defined as the need for resumption of exogenous insulin (determined by FBG >200 for two consecutive days) following a period of normoglycemia and insulin independence in the absence of detectable porcine c-peptide. Primary graft dysfunction (failure of engraftment), the failure to achieve insulin-independent normoglycemia for any period of time, was defined as four consecutive days after post-transplant day 40 with FBGs >300mg/dL that were not associated with events that can cause hyperglycemia (i.e. infection).
Xenoantibody Detection
A flow-cytometric assay was employed as previously described (23). Immortalized pig aortic endothelial cells (PAEC) prepared from Gal+ or GTKO pigs (Immerge Biotherapeutics) were used as a source of pig antigens. The endothelial cell phenotype was assessed by expression of CD31 using mouse anti-pig CD31 antibody (Serotec # MCA 1746F). To assay for antibody binding, 50 μL of Gal+ or GTKO PAEC suspensions (2×106 cells/mL) were incubated with an equal volume of heat-inactivated serum samples diluted 1:4 in staining buffer (phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA)). Antibody binding was revealed using PE-labeled goat anti-human IgM (Fcμ specific) antibodies (Southern, Birmingham, AL), or FITC-labeled goat anti-human IgG (Fcγ specific) antibodies (Invitrogen, Carslbad, CA). All incubations were performed at 4°C. 5,000 events were acquired on a FACs Calibur and data analyzed using Cell Quest or Flow Jo software. Results were expressed as the median fluorescence intensity (MFI). A pool of normal human AB serum (Sigma) was analyzed in each assay as a positive control. Experiments performed on different days were normalized to this human pool in order to compensate for inter-assay variability.
Polychromatic Flow Cytometry
Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) was performed at regular intervals on all islet recipients. PBMCs were isolated from freshly collected recipient whole blood specimens using a Ficoll density gradient (Ficoll-Paque, GE Healthcare, Piscataway, NJ), and stained with the following fluorophore-conjugated antibodies: CD3-V450, CD4-PerCPCy5.5, CD8-PerCP, CD95-FITC, CD11a-APC, CD2-PE, CD20-PE (BD Bioscience, San Jose, CA) or CD28-PECy7 (eBioscience, San Diego, CA). After incubation for 15 minutes at 4°C, cells were washed with 2% fetal bovine serum in phosphate buffered saline and analyzed with an LSRII flow cytometer (BD Biosciences). Secondary analysis of data was performed using FlowJo software (Tree Star, San Carlos, CA). Absolute numbers of specific populations were extrapolated from complete blood counts (CBCs) performed on recipient samples at the time of flow analysis.
Results
CD40/CD154 pathway-sparing regimens promote xenoislet engraftment and survival
Islet preparations used in all cohorts were quantitatively and functionally similar in vitro; in addition, recipients in the three cohorts were similar in weight. The site of islet isolation did not appear to affect transplant outcome (Table 1).
Table 1.
Recipient and Islet Characteristics
| Cohort | ID | Weight (kg) | IEQ/kg (1×104) | GSI | Viability (%) | Isolation Site |
|---|---|---|---|---|---|---|
| 1 | Ctrl-1 | 3.1 | 5.70 | 2.68 | 83.0 | EACTL |
| Ctrl-2 | 3.5 | 5.70 | 2.68 | 83.0 | EACTL | |
| Ctrl-3 | 3.8 | 5.63 | 3.2 | 81.6 | EACTL | |
|
| ||||||
| 2 | Tac-1 | 4.8 | 5.55 | 1.53 | 77.8 | Edm |
| Tac-2 | 4.7 | 5.15 | 1.21 | 74.8 | Edm | |
| Tac-3 | 3.6 | 5.87 | 2.68 | 83.0 | EACTL | |
| Tac-4 | 4.5 | 5.41 | 2.18 | 78.6 | Edm | |
| Tac-5 | 3.8 | 5.60 | 7.13 | 81.4 | EACTL | |
|
| ||||||
| 3 | Alef-1 | 5.2 | 5.41 | 1.61 | 77.2 | EACTL |
| Alef-2 | 5.0 | 5.33 | 1.12 | 85.3 | Edm | |
| Alef-3 | 4.2 | 5.52 | 7.13 | 81.4 | EACTL | |
| Alef-4 | 4.2 | 5.47 | 2.43 | 81.0 | Edm | |
| Alef-5 | 4.5 | 5.50 | 2.69 | 77.3 | Edm | |
IEQ – Islet equivalents. GSI – glucose stimulation index. EACTL – Emory Animal Cell and Tissue Laboratory. Edm – Surgical-Medical Research Institute, University of Alberta, Edmonton.
We tested three different immunosuppressive regimens in this study (figure 1). As controls, cohort 1 received a base regimen of belatacept, TS-1/22, basilixmab, and mycophenolate only. Cohort 2 received the base regimen plus extended induction with tacrolimus with delayed initiation of mycophenolate. Cohort 3 received the base regimen plus alefacept in place of basiliximab, and more intense induction with TS-1/22.
Figure 1. Immunosupression protocols.
Protocol timelines and treatment schedules for the three experimental cohorts are depicted here. Relative doses are indicated by differences in arrow size. STZ – streptozocin. LEA29Y – belatacept. αLFA-1 – TS-1/22. MMF – mycophenolate mofetil. LFA-3-Ig – alefacept.
Primary outcomes of interest were (a) engraftment, defined as successful achievement of insulin independent normoglycemia and (b) duration of rejection-free survival (table 2). No recipient in the control group (cohort 1) successfully engrafted (supplemental figure 1), and all were sacrificed ~50 days post-transplant for non-engraftment. In cohort 2 (tacrolimus-treated recipients), 3 of 5 recipients achieved insulin independence; median rejection-free survival in this group was 60 days (range 46 – 99 days; supplemental figure 2). Similarly, in cohort 3 (alefacept-treated recipients), 3 of 5 transplanted recipients engrafted with a median rejection-free survival of 111 days (range 92 – 114 days; supplemental figure 3). Two recipients in both cohorts 2 and 3 failed to achieve normoglycemia and were sacrificed for nonengraftment.
Table 2.
Engraftment and rejection-free survival results
| Recipient ID | Treatment | Insulin Independent | Rejection-Free Survival (days) |
|---|---|---|---|
| Ctrl-1 | αLFA-1 (35 d), LEA29Y, αIL-2R, MMF | N | - |
| Ctrl-2 | αLFA-1 (35 d), LEA29Y, αIL-2R, MMF | N | - |
| Ctrl-3 | αLFA-1 (35 d), LEA29Y, αIL-2R, MMF | N | - |
|
| |||
| Tac-1 | αLFA-1 (35 d), LEA29Y, αIL-2R, Tac/MMF | Y | 99 |
| Tac-2 | αLFA-1 (35 d), LEA29Y, αIL-2R, Tac/MMF | Y | 60 |
| Tac-3 | αLFA-1 (35 d), LEA29Y, αIL-2R, Tac/MMF | Y | 46 |
| Tac-4 | αLFA-1 (35 d), LEA29Y, αIL-2R, Tac/MMF | N | - |
| Tac-5 | αLFA-1 (35 d), LEA29Y, αIL-2R, Tac/MMF | N | - |
|
| |||
| Alef-1 | αLFA-1 (90 d), LEA29Y, LFA-3Ig, MMF | Y | 114 |
| Alef-2 | αLFA-1 (90 d), LEA29Y, LFA-3Ig, MMF | Y | 92 |
| Alef-3 | αLFA-1 (90 d), LEA29Y, LFA-3Ig, MMF | Y | 111 |
| Alef-4 | αLFA-1 (90 d), LEA29Y, LFA-3Ig, MMF | N | - |
| Alef-5 | αLFA-1 (90 d), LEA29Y, LFA-3Ig, MMF | N | - |
αLFA-1 TS-1/22; LEA29Y belatacept; αIL-2R basiliximab; MMF mycophenolate mofetil; Tac – tacrolimus: LFA-3Ig – alefacept.
In all xenoislet recipients, measured porcine c-peptide (Pc-P) levels corresponded with observed xenograft function (supplemental figure 4). Recipients experiencing insulin independence had detectable Pc-P both in serial serum samples collected at regular intervals and in response to glucose stimulation. In contrast, recipients with functional rejection had Pc-P levels that approached the lower limit of detection. One recipient in cohort 3 (Alef-5) was declared to have primary graft dysfunction and sacrificed prior to the typical 40-day time point given a complete absence of detectable Pc-P and ongoing hyperglycemia.
Immunohistochemistry confirms rejection
In all cases of non-engraftment or loss of graft function after a period of insulin independence, immunohistochemical assessment of transplanted islets at the time of necropsy confirmed rejection as the cause of graft failure, demonstrating islets with a dense lymphocytic infiltrate and no insulin positivity (figure 2). This infiltrate stained heavily for CD3+ T cells, and moderately for CD20+ B cells. There was minimal to absent staining for the complement component C4d. There was no qualitative difference among cohorts, or between recipients experiencing failure of engraftment versus graft rejection, in the character of the infiltrates. All necropsies were performed no more than two weeks after experimental endpoint.
Figure 2. Rejecting islets demonstrate dense lymphocytic infiltrates with minimal insulin positivity.
Post-mortem islet histology is shown here. At time of sacrifice, intrahepatic islets from all recipients consistently demonstrated dense lymphocytic infiltrates (H&E, left column) without insulin positivity (right column), confirming rejection as the cause of graft failure both in recipients experiencing failure of engraftment and in those achieving sustained insulin independence. Representative sections are shown for each cohort.
Alternative immunosuppressive regimens are well tolerated by recipients
As a sensitive measure of overall primate health and well being following transplant, recipient weights were carefully monitored for the duration of their experimental course. While most recipients experienced some weight loss in the early post-transplant period, long-term weight loss was uncommon, affecting only one of 13 recipients without impacting graft survival (figure 3). All recipients maintained appropriate weight status through experimental endpoint, suggesting that these regimens are relatively well tolerated.
Figure 3. CD40/CD154 pathway-sparing regimens are well tolerated.
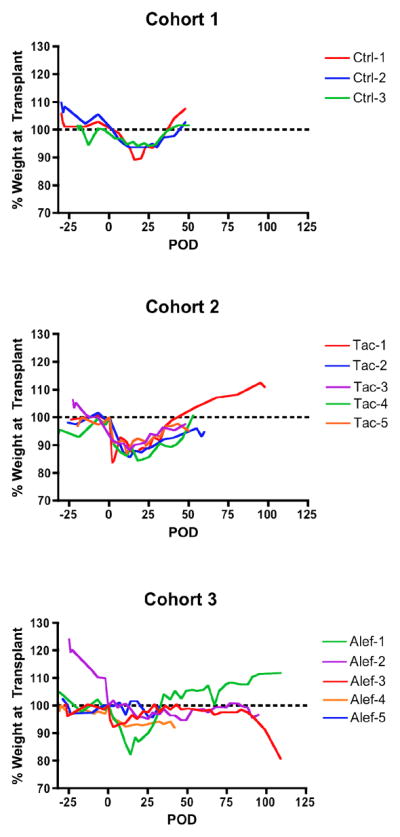
Individual recipient weights over the experimental course are depicted here. Percent change is calculated based on weight on day of transplant. While some weight loss in the immediate post-transplant period was common, most recipients successfully stabilized around post-transplant day 25. Late weight loss was uncommon, affecting only a single cohort 3 recipient (Alef-3), and did not result in early experimental termination. Dotted line represents weight baseline at transplant. POD – post-operative day.
As an indicator of the integrity of recipient protective immunity, rhesus cytomegalovirus (rhCMV) viral loads were monitored following transplant. RhCMV virema was common among recipients in all experimental cohorts (Supplementary figure 5) but responded well in all cases to treatment with ganciclovir. No evidence of complications related to rhCMV viremia were noted in any recipient over the course of treatment, or at time of necropsy.
CD40/CD154 pathway-sparing regimens permit an acquired xenoantibody response
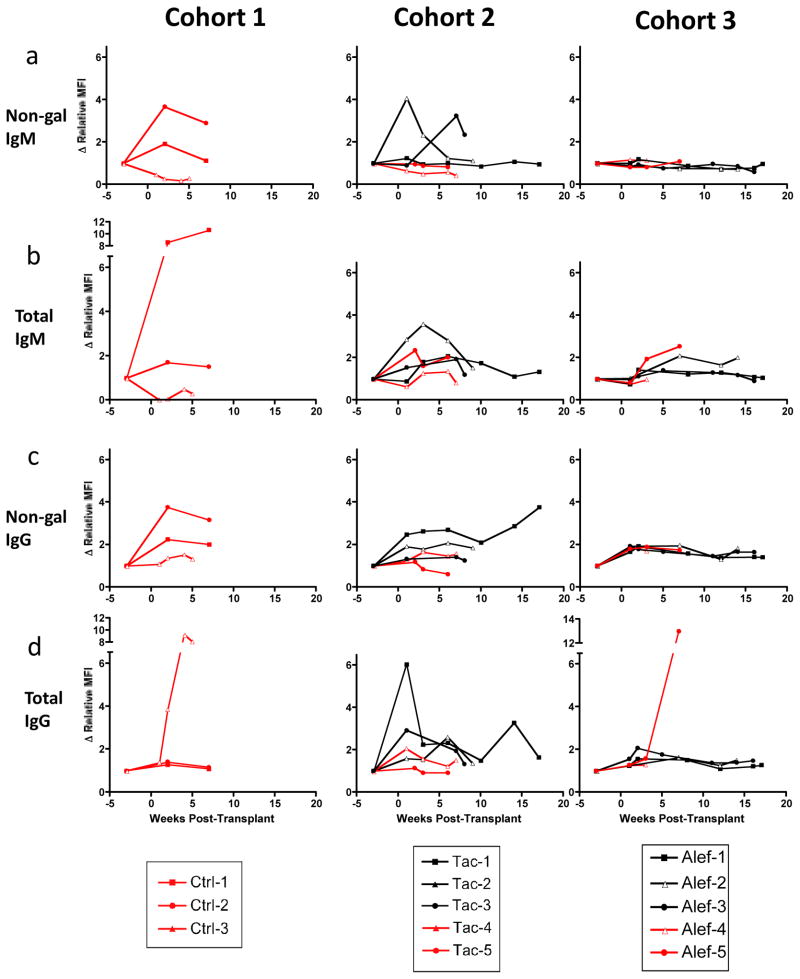
To evaluate the role of humoral immunity in the setting of CD40/CD154 pathway-sparing immunosuppressive regimens, we measured levels of total anti-pig (including anti-gal) as well as non-Gal xenoantibody at regular intervals over the course of the experiment using a validated flow cytometric assay.
Moderate increases in acquired xenoantibodies were detected among recipients in all three experimental cohorts (figure 4); overall, 7 of 13 recipients developed an increase in measured xenoantibody of 3-fold or more relative to pre-transplant levels. Large increases in acquired xenoantibody (>10-fold) were less common, affecting 2 of 3 cohort 1 recipients and one of five cohort 3 recipients.
Figure 4. CD40/CD154 pathway-sparing regimens permit an acquired xenoantibody response.
We measured levels of total and non-gal IgG and IgM in all recipients over the course of the experiment. As much as 12-fold increases in xenoantibody levels were noted in some individual recipients. In all graphs, recipients who experienced primary graft dysfunction are represented with red lines, while recipients experiencing insulin independence are represented with black lines. MFI data are normalized to standard samples and represented as fold increase from pre-transplant baseline. Δ Relative MFI – fold increase in nomralized mean fluroescence intensity.
Combined CD2 and LFA-1 blockade prevents a post-transplant increase in peripheral memory T cells
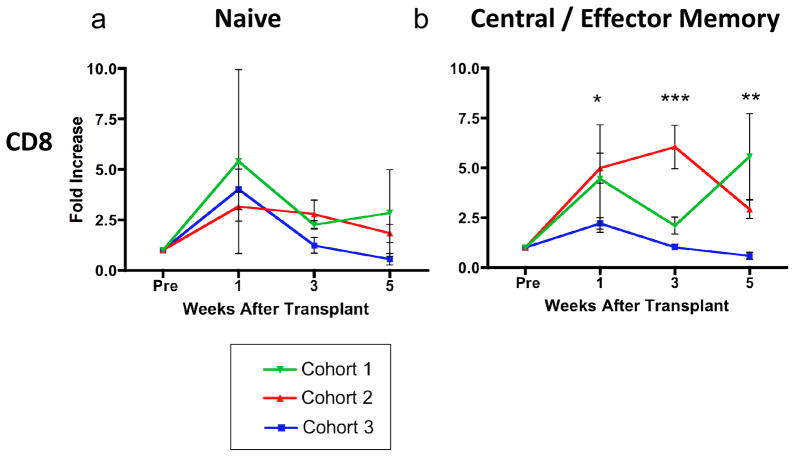
To evaluate the effects of our immunosuppressive regimens on peripheral lymphocyte populations, we monitored the composition of T cell subsets over the course of treatment using flow cytometry and established markers of nonhuman primate memory differentiation (24). Treatment with alefacept and extended TS-1/22 induction appeared to blunt a post-transplant increase in the absolute number of memory T cells seen in recipients in other treatment cohorts. Following LFA-1 adhesion molecule blockade with TS-1/22, recipients in all cohorts experienced a significant peripheral lymphocytosis, which manifested as an average 3-fold increase in the absolute number of naïve (CD28+CD95−) T cells (figure 5a) detectable in peripheral blood. However, while recipients treated with the base regimen plus tacrolimus (cohort 2) or the base regimen alone (cohort 1) experienced a significant increase in the number of both CD28+CD95+ central and CD28−CD95+ effector memory T cells by five weeks post-transplant, the size of these populations in alefacept-treated recipients remained relatively stable and significantly lower than seen with recipients in cohorts 1 and 2 (figure 5b).
Figure 5. Alefacept-containing regimen blunts the post-transplant increase in CD95+ central and effector memory T cells.
We measured the expression of the primate memory markers CD28 and CD95 on peripheral blood lymphocytes over time using flow cytometry. The fold increase in absolute number of CD8+ T cells relative to pre-transplant baseline (Pre) in the subsets of interest are depcited here, with measurements pooled for each cohort. All recipients experienced an increase in the number of CD28+ CD95− naïve memory T cells in the periphery following transplant (left graph), an expected effect of treatment with anti-LFA-1 agents. However, cohort 3 recipients treated with alefacept and more intense LFA-1 blockade experienced a much smaller increase in CD95+ central and effector memory T cells than recipients in cohorts 2 or 3. The median fold increase in memory T cells experienced by recipients in cohorts 1, 2 and 3 varied significantly at the 1, 3 and 5 week timepoints. Error bars represent standard error of the mean (SEM). Statistical analysis was performed using non-parametric ANOVA (Kruskal-Wallis test). * p < 0.05 ** p <0.01 ***p<0.001
Discussion
Previous studies have demonstrated the crucial importance of the CD40/CD154 interaction in mediating xenograft rejection; blockade of this pathway confers prolonged islet (6, 7, 13, 25–27), heart (4), and kidney (5) xenograft survival. Although the efficacy of this approach remains undisputed, recent data from our group (13) and others (28) suggest that targeting the CD40/CD154-pathway may not be an absolute necessity for xenograft function. In the current study, we sought to further explore the ability of CD40/CD154 pathway-sparing regimens to promote long-term xenograft survival. These results represent conclusive evidence that porcine islets can engraft and achieve sustained function in the absence of CD40− or CD154− specific agents.
In this study, conventional immunosuppression with tacrolimus or targeting the CD2/LFA-3 interaction with alefacept, each in combination with the anti-LFA-1 monoclonal antibody TS-1/22, resulted in prolonged insulin independence in macaque recipients transplanted with wild-type neonatal porcine islets. The median durations of xenograft survival, 60 and 111 days for recipients in cohorts 2 and 3 respectively, were comparable to results achieved with previously published xenoislet regimens targeting the CD40/CD154-pathway (6, 13, 25). While the graft survival of cohort 3 recipients was improved compared to recipients in cohort 2, this study was not designed to evaluate the relative efficacy of these two novel regimens. Instead, our purpose was to provide proof-of-concept that the use of CD40/CD154 pathway-sparing regimens in xenotransplant is feasible.
Several commonly used conventional immunosuppressive agents, though effective in promoting transplant survival, possess undesirable or dangerous toxicities that limit their clinical usefulness. In addition to adverse effects on wound healing, the mTOR inhibitor sirolimus has recently been linked to proteinuria in both kidney and islet allograft recipients (29, 30); likewise, other broadly immunosuppressive agents such as steroids and calcineurin inhibitors (CNIs) promote diabetogenesis, an obviously counterproductive side effect in patients undergoing islet transplantation (31). The immunosuppressive protocols described in this study were designed to combine several clinically appealing characteristics: in addition to exclusion of CD40/CD154-specific agents, these regimens were steroid- and rapamycin-sparing, avoiding the metabolic complications associated with each of these conventional therapies. Although cohort 2 recipients did receive CNI therapy, tacrolimus exposure was minimized through use of a tapered induction regimen. Interestingly, in our limited experience (n=2) using tacrolimus as continuous maintenance therapy with a higher target therapeutic range (8–12 ng/dL), islet recipients failed to achieve insulin independence (unpublished observation). Histologic assessment of liver sections from recipients receiving high dose tacrolimus maintenance at necropsy demonstrated intact islets with no evidence of rejection (data not shown), perhaps implicating the tendency of tacrolimus to inhibit β-cell insulin release in a dose-dependent fashion (32). Use of CNIs in future xenoislet protocols must take this highly relevant toxicity into consideration.
CD40/CD154-sparing regimens were generally well tolerated, as shown by continual weight gain in recipients in all cohorts over the course of treatment. One cohort 3 recipient did experience weight loss late in the experimental course, which was immediately preceded by loss of xenograft function; therefore, this isolated occurrence may have been related to metabolic dysregulation from insufficient insulin production. In addition, protective immunity was generally preserved; though rhCMV reactivation was common, CMV-related infectious complications (or other opportunistic infections) were not observed in any recipient; viremia cleared with ganciclovir and was not thought to be a contributing factor in xenograft rejection.
While our results provide evidence for the tolerability of these regimens, their translational potential remains questionable. Efalizumab, previously the only clinically available anti-LFA-1 monoclonal antibody, was recently voluntarily withdrawn from the market due to concerns related to progressive multifocal leukoencephalopathy (33). In addition, recent results from a phase 2 clinical trial in de novo kidney recipients failed to show non-inferiority of alefacept compared to tacrolimus (34, 35), leaving the clinical fate of alefacept for transplant indications in doubt. While the purpose of this study was to provide proof-of-concept that prolonged islet xenograft survival is possible in the absence of CD40- or CD154-specific therapies, further investigation of alternate immunomodulatory strategies will be required to determine the regimen with the optimal translational potential.
Costimulation blockade resistance among differentiated T cell populations has been suggested as a possible mechanism of graft rejection in the setting of belatacept therapy. Primed donor-reactive T cells are less dependent on CD28 costimulatory signals (36, 37) and are therefore less susceptible to immune modulation via CTLA4-Ig or its variants. Though primarily described in allotransplant models (19, 36, 37), costimulation blockade-resistant rejection may also affect xenograft outcomes, necessitating investigation of therapies that contend with this phenomenon and providing a secondary rationale for the design of our immunosuppressive regimens. By preventing T cell receptor (TCR)-mediated signal transduction, tacrolimus may circumvent the reduced costimulatory requirements of memory and effector populations (38). Likewise, LFA-1 and CD2 are both expressed to a greater extent on more differentiated, CD28-negative effector T cells; targeting these alternative costimulatory molecules with TS-1/22 and alefacept, respectively, may selectively inhibit costimulation blockade-resistant populations (19, 20, 39). Our results provide some evidence of this effect, as treatment with alefacept in combination with extended TS-1/22 therapy blunted the post-transplant increase in central and effector memory T-cells.
While these CD40/CD154 pathway-sparing regimens appear to facilitate xenoislet engraftment and function, they clearly only postpone eventual graft rejection. The mechanisms responsible for this delayed xenograft rejection appear to be primarily cellular, as demonstrated by heavy staining for CD3+ T cells infiltrating intrahepatic islets in recipients experiencing graft failure. This pattern is identical to that observed in previously published islet xenotransplant studies (6, 7, 13, 25, 40), providing further evidence for the remarkable persistence of this pathway of immune recognition and rejection. Graft failure following blockade of specific costimulatory interactions, either alone or in combination, make apparent the redundancy of alternate routes of T cell activation. The adaptability and tenacity of the T cell response may necessitate taking a different approach to achieve xenograft tolerance; for example, alternative sites for xenoislet transplant with immunomodulatory characteristics, such as bone marrow (41) or “immune privilege,” such as the testis (42) may protect islets from T cell activity by various mechanisms. Our group and others are currently investigating the optimal site for islet xenotransplantation.
The CD40/CD154 pathway is a crucial mediator of humoral immunity; ligation of CD40 by CD154 is necessary for B cell activation, proliferation and isotype switching (43, 44). As such, CD154-based regimens appeared to completely suppress pig-specific antibody production in previously successful xenoislet protocols (6, 7). In contrast, in the current study, several recipients experienced increases in total- and non-Gal acquired xenoantibody, suggesting that one consequence of sparing the CD40/CD154 interaction may be an increased effect of humoral immunity. However, increases in xenoantibody levels did not consistently correlate with decreased graft function, and immunohistochemical analysis of cellular infiltrates from rejected islets showed relatively low staining for CD20+ B cells or complement C4d. These results suggest a rather less important role for acquired humoral immunity in the delayed xenograft rejection experienced in this study, but certainly do not exclude the importance of a humoral response developing commensurate with the T cell response.
In conclusion, we have demonstrated that sustained survival and function of neonatal porcine islets is possible in the absence of CD40/CD154 pathway blockade. These alternative immunomodulatory strategies were well tolerated by recipients, but did not prevent eventual cellular rejection. Further investigation of CD40/CD154-sparing immunosuppressive therapies is warranted in order to determine the optimal regimen for clinical translation.
Supplementary Material
Post-transplant viral monitoring and prophylaxis
Supplemental Figure 1. Treatment with base regimen alone does not protect xenografts from early rejection.
Supplemental Figure 2. Tacrolimus-containing regimen promotes xenograft survival.
Supplemental Figure 3. Alefacept-containing regimen promotes xenograft survival.
Supplemental Figure 4. Measured serum porcine c-peptide values correspond with observed xenograft function.
Supplemental Figure 5. Rhesus cytomegalovirus reactivation is common but transient in treated recipients.
Acknowledgments
The authors would like to acknowledge the Juvenile Diabetes Research Foundation (Grant # 21-2006-882), the National Institute of Health (Grant # 1U01AI090956-01), and the Yerkes National Primate Center (Grant # P51RR-00065) for their funding of this project, and Sebastian Perez for assistance with statistical analysis.
Funding Source:
Juvenile Diabetes Research Foundation Grant – 21-2006-882
National Institute of Health – 1U01AI090956-01
Yerkes National Primate Center - P51RR-00065
Abbreviations
- NHP
nonhuman primate
- STZ
streptozocin
- FBG
fasting blood glucose
- IACUC
Institutional Animal Care and Use Committee
- EACTL
Emory Animal Cell & Tissue Laboratory
- GSI
glucose stimulation index
- IEQ
islet equivalent
- IVGTT
intravenous glucose tolerance test
- Pc-P
porcine c-peptide
- RhCMV
rhesus cytomegalovirus
- PBMC
peripheral blood mononuclear cell
- CBC
complete blood count
- CNI
calcineurin inhibitor
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ekser B, Cooper DK. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010;6(2):219–230. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7(7):519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 3.Appel MC, Banuelos SJ, Greiner DL, Shultz LD, Mordes JP, Rossini AA. Prolonged survival of neonatal porcine islet xenografts in mice treated with a donor-specific transfusion and anti-CD154 antibody. Transplantation. 2004;77(9):1341–1349. doi: 10.1097/01.tp.0000116771.68839.c1. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 6.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 7.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 8.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9(8):1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 9.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75(5):637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 10.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174(1):542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 12.Wantanabe MYKST, Kamachi H, Kuraya D, Koshizuka Y, Ogura M, Yoshida T, Sakai F, Miura T, Todo S. Long-Term Acceptance of Islet Allografts by ASKP1240 (4D11), a Fully Human Anti-CD40 Monoclonal Antibody in Cynomolgus Monkeys (abstract) Am J Transplant. 2011;11(Supplement s2):1. [Google Scholar]
- 13.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, et al. CD40-Specific Costimulation Blockade Enhances Neonatal Porcine Islet Survival in Nonhuman Primates. Am J Transplant. 2011;11(5):947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk AD, Knechtle SJ, Sollinger HW. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation (abstract #223) Am J Transplant. 2001;1(Suppl 1):1. [Google Scholar]
- 15.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 16.Kirk AD. 4D11: The Second Mouse? Am J Transplant. 2009;9(8):1701–1702. doi: 10.1111/j.1600-6143.2009.02749.x. [DOI] [PubMed] [Google Scholar]
- 17.Buhler L, Deng S, O’Neil J, Kitamura H, Koulmanda M, Baldi A, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11(5):396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 19.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute of Laboratory Animal Resources (U.S.) NIH publication. Bethesda, Md: U.S. Dept. of Health and Human Services, Public Health Service; Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals; p. v. [Google Scholar]
- 22.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97(9):2119–2129. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen BN, Azimzadeh AM, Schroeder C, Buddensick T, Zhang T, Laaris A, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18(2):94–107. doi: 10.1111/j.1399-3089.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7(10):2260–2268. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 26.Casu A, Bottino R, Balamurugan AN, Hara H, van der Windt DJ, Campanile N, et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia. 2008;51(1):120–129. doi: 10.1007/s00125-007-0844-4. [DOI] [PubMed] [Google Scholar]
- 27.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 28.Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci U S A. 2009;106(21):8659–8664. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephany BR, Augustine JJ, Krishnamurthi V, Goldfarb DA, Flechner SM, Braun WE, et al. Differences in proteinuria and graft function in de novo sirolimus-based vs. calcineurin inhibitor-based immunosuppression in live donor kidney transplantation. Transplantation. 2006;82(3):368–374. doi: 10.1097/01.tp.0000228921.43200.f7. [DOI] [PubMed] [Google Scholar]
- 30.Senior PA, Paty BW, Cockfield SM, Ryan EA, Shapiro AM. Proteinuria developing after clinical islet transplantation resolves with sirolimus withdrawal and increased tacrolimus dosing. Am J Transplant. 2005;5(9):2318–2323. doi: 10.1111/j.1600-6143.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56(1):23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Boots JM, van Duijnhoven EM, Christiaans MH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002;13(1):221–227. doi: 10.1681/ASN.V131221. [DOI] [PubMed] [Google Scholar]
- 33.FDA Statement on the Voluntary Withdrawal of Raptiva From the US Market. 2009 [cited 2011 May 19, 2011]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149561.htm.
- 34.Bromberg J, Cibrik D, Steinberg S, West-Thielke P, Yang H, Erdman J, et al. A Phase 2 Study to Assess the Safety and Efficacy of Alefacpt (ALEF) in De Novo Kidney Transplant Recipients (abstract) Am J Transplant. 2011;11(supplement S2):1. [Google Scholar]
- 35.Rostaing L, Mourad M, Charpentier B, Glyda M, RIgotti P, Falk F, et al. Efficacy and Safety of Alefacept in Combination with Tacrolimus, Mycophenolate Mofetil and Steroids in De Novo Kidney Transplantation (abstract) Am J Transplant. 2011;11(supplement S2):1. [Google Scholar]
- 36.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 38.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 39.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson P, Badell IR, Lowe M, Cano J, Song M, Leopardi F, et al. Islet Xenotransplantation Using Gal-Deficient Neonatal Donors Improves Engraftment and Function. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantarelli E, Melzi R, Mercalli A, Sordi V, Ferrari G, Lederer CW, et al. Bone marrow as an alternative site for islet transplantation. Blood. 2009;114(20):4566–4574. doi: 10.1182/blood-2009-03-209973. [DOI] [PubMed] [Google Scholar]
- 42.Nasr IW, Wang Y, Gao G, Deng S, Diggs L, Rothstein DM, et al. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005;174(10):6161–6168. doi: 10.4049/jimmunol.174.10.6161. [DOI] [PubMed] [Google Scholar]
- 43.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver TA, Charafeddine AH, Kirk AD. Costimulation blockade: towards clinical application. Front Biosci. 2008;13:2120–2139. doi: 10.2741/2829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Post-transplant viral monitoring and prophylaxis
Supplemental Figure 1. Treatment with base regimen alone does not protect xenografts from early rejection.
Supplemental Figure 2. Tacrolimus-containing regimen promotes xenograft survival.
Supplemental Figure 3. Alefacept-containing regimen promotes xenograft survival.
Supplemental Figure 4. Measured serum porcine c-peptide values correspond with observed xenograft function.
Supplemental Figure 5. Rhesus cytomegalovirus reactivation is common but transient in treated recipients.