Abstract
Despite high prevalence of tinnitus and its impact on quality life, there is no cure for tinnitus at present. Here, we report an effective means to temporarily suppress tinnitus by amplitude- and frequency-modulated tones. We systematically explored the interaction between subjective tinnitus and 17 external sounds in 20 chronic tinnitus sufferers. The external sounds included traditionally used unmodulated stimuli such as pure tones and white noise and dynamically modulated stimuli known to produce sustained neural synchrony in the central auditory pathway. All external sounds were presented in a random order to all subjects and at a loudness level that was just below tinnitus loudness. We found some tinnitus suppression in terms of reduced loudness by at least one of the 17 stimuli in 90% of the subjects, with the greatest suppression by amplitude-modulated tones with carrier frequencies near the tinnitus pitch for tinnitus sufferers with relatively normal loudness growth. Our results suggest that, in addition to a traditional masking approach using unmodulated pure tones and white noise, modulated sounds should be used for tinnitus suppression because they may be more effective in reducing hyperactive neural activities associated with tinnitus. The long-term effects of the modulated sounds on tinnitus and the underlying mechanisms remain to be investigated.
Keywords: subjective tinnitus, hyperacusis, sound therapy, loudness, loudness recruitment, amplitude modulation, frequency modulation, pure tones, white noise
Introduction
Tinnitus is the perception of sound in the absence of any external stimuli, affecting 15% of the general population (Lockwood et al. 2002). Reports of tinnitus continue to rise due to both widespread use of personal entertainment and communication devices, particularly in children (Widen and Erlandsson 2004; Bulbul et al. 2009; Landgrebe et al. 2009), and military-connected noise exposure in Veterans (Fausti et al. 2009). Severe tinnitus disrupts daily function from sleep to work, often leading to anxiety, depression, and other behaviors of distress (Zoger et al. 2001). Despite intensive research and development, from surgery and drugs to laser and magnetic stimulation, there is no cure for tinnitus at present. The situation is likely to change due to the recent paradigm shift in both the understanding and treatment of tinnitus.
As opposed to the traditional paradigm viewing tinnitus as an ear disease, tinnitus is now widely accepted as a brain disorder (e.g., Roberts et al. 2010). A hearing loss in the auditory periphery is likely necessary, but not sufficient, for tinnitus to occur (Cacace 2003; Lanting et al. 2009; Gu et al. 2010). Animal studies have shown that cochlear damage due to traumatizing sounds, ototoxic drugs, or other means produces significant changes in the central auditory pathway. One change is a reorganized tonotopic map, with cortical neurons in the hearing loss region being tuned to the edge of normal hearing region (Rajan and Irvine 1998; Eggermont and Komiya 2000). Although it is not clear whether this over-representation of “edge frequency” is a neural correlate of tinnitus, deviation of tinnitus frequency in the cortical tonotopic map has been demonstrated in human tinnitus sufferers, suggesting that cortical reorganization plays a significant role in tinnitus generation (Muhlnickel et al. 1998). Another change is an overall hyperactivity in the central auditory pathway from cochlear nuclei to auditory cortices, including increased spontaneous activity, increased serial synchrony or bursting firing within a nerve fiber, and increased spatial synchrony between nerve fibers (e.g., Sasaki et al. 1980; Wang et al. 1996; Kaltenbach et al. 2002; Eggermont 2007; Bauer et al. 2008). Because decreased spontaneous and driven activity is typically observed at the auditory nerve level in response to cochlear damage (Kujawa and Liberman 2006), this central hyperactivity, a hypothesized neural correlate of tinnitus, may reflect increased central gain (Jastreboff and Hazell 1993; Salvi et al. 2000; Norena 2010).
Traditional management has used external sounds, typically pure tones and white noise, to mask tinnitus (e.g., Hazell and Wood 1981; Vernon 1981). As an effect secondary to restoring audibility and speech intelligibility, hearing aids, and more recently cochlear implants, can also produce significant relief from tinnitus (e.g., Trotter and Donaldson 2008; Pan et al. 2009b; Sweetow and Sabes 2010). Furthermore, counseling and cognitive training can enhance masking and amplification benefits (e.g., Hazell et al. 1985). Guided by the improved understanding of underlying neural mechanisms, recent management has designed novel sounds in an attempt to treat the hypothesized root causes of tinnitus. For example, to promote lateral inhibition, “spectrally notched” sounds that contain no energy at the tinnitus frequency have been shown to reduce tinnitus loudness in human subjects (Okamoto et al. 2010). To reduce putative, maladaptive central gain, sounds have been selectively amplified in the hearing loss region and shown to relieve tinnitus in human subjects (Davis et al. 2008). Recent animal studies have also demonstrated the feasibility of this causal treatment approach by stimulating the vagus nerve to reverse cortical changes (Engineer et al. 2011), or preventing cortical reorganization after noise exposure by providing an enriched acoustic environment (Norena and Eggermont 2006). While these causal treatment strategies are promising, they should be viewed as studies in progress due to their methodological limitations or a lack of high-level evidence for their benefits in human tinnitus sufferers (e.g., Hoare et al. 2011; Zeng et al. 2011. F1000.com/8035957). The need remains great for not only establishing the efficacy of sound therapy in general, but also searching for novel sounds that can effectively alleviate tinnitus in particular.
Based on our previous observation that tinnitus can be temporarily abolished by low-rate electric stimulation from a cochlear implant (Zeng et al. 2011), we searched for corresponding acoustic stimulation to suppress tinnitus in subjects who do not have a cochlear implant. We concentrated on low-rate amplitude- and frequency-modulated sounds for their ability to produce sustained, robust, and highly synchronized synchronous activity in the central auditory pathway (e.g., Frisina 2001; Lu et al. 2001; Liang et al. 2002; Malone et al. 2010). We hypothesized that the externally driven synchronized neural activity generated by these low-rate modulated stimuli will reduce tinnitus-related neural hyperactivity in the central auditory pathways, thereby providing temporary suppression of tinnitus.
Methods
Subjects
The subjects included 20 adults (mean = 60; range 23–76 years) with non-transient, chronic tinnitus for more than 6 months in at least one ear. Of the 20 subjects, 16 reported tinnitus bilaterally, and four reported tinnitus unilaterally, all in the left ear. For unknown reasons, only two of the 20 subjects were women, who were still under-represented despite the fact that men are more likely to have tinnitus than women (e.g., Lockwood et al. 2002). To be eligible for the present study, subjects could not have a medically treatable form of tinnitus, recent outer or middle ear disease, be actively taking known ototoxic medications, pursuing other forms of tinnitus treatment, or experiencing severe depression. No subject had normal hearing in the tinnitus ear. On average, the subjects had a sloping hearing loss, with normal audiometric thresholds through 1,000 Hz and gradually elevating to a moderate hearing loss at 8,000 Hz. However, two subjects had only a mild hearing loss (30–35 dB HL) at one or two frequencies. In the four subjects with lateralized tinnitus, three had greater hearing loss in the tinnitus ear than the non-tinnitus ear, with an asymmetry of 20–40 dB at one or more audiometric frequencies. While the etiology of tinnitus was likely multi-factorial, otologic factors such as hearing loss due to presbycusis, noise exposure, or cerumen impaction appeared to be related to tinnitus onset in 12 of the 20 subjects. Seven subjects’ tinnitus was likely related to infectious otitis media or sinusitis or both. One subject suffered hearing loss and tinnitus related to an ototoxic drug exposure as a child. All subjects provided written consent to participate in the study following the guidelines of the University of California Irvine’s Institutional Review Board.
Stimuli
Pure tones of 500-ms in duration were used in the tinnitus matching and loudness growth experiments. The main tinnitus suppression experiment used 17 modulated and unmodulated external stimuli of 3 min in duration. In addition to a white noise control, four types of stimuli with four frequency ranges were created. These stimuli included two unmodulated sounds in pure tones and narrow-band noises and two modulated sounds with amplitude and frequency modulation. The four frequency ranges were 75–750, 750–1,500, 3,000–6,000, and 6,000–9,000 Hz. The narrow-band noises had cutoffs that were demarcated by the four frequency ranges using sixth-order Butterworth filters. Amplitude-modulated tones had a constant modulation rate of 40 Hz and modulation depth of 100%. Frequency-modulated tones had a modulation rate of 8 Hz for the 75–750 Hz frequency range and 40 Hz for all other frequency ranges, with all having a frequency modulation depth of 10%. The carrier frequency within each frequency band was chosen individually according to the measured tinnitus frequency for the pure tone, amplitude-modulated tones, and frequency-modulated tones. The carrier frequency was set to the tinnitus frequency if both were within the same frequency band. For the other bands, the carrier frequency was set by repeaterly multiplying or dividing the tinnitus frequency by 0.8 until it was the closest to the band’s geometric center frequency, which was 237, 1,061, 4,243, and 7,348 Hz for the four frequency bands, respectively. For the lowest frequency band, its carrier frequency was further divided by 2 to create at least one condition whose carrier frequency was lower than 200 Hz (Zeng et al. 2011). As a result, actual carrier frequencies were 117 ± 12 (mean ± SD), 1,103 ± 147, 4,265 ± 794, and 7,492 ± 864 Hz for the four frequency bands, respectively. All stimuli, including the 500-ms pure tones used in the tinnitus matching and loudness growth experiments as well as the 17 3-min stimuli in the tinnitus suppression experiment, were generated by a custom program (MATLAB, MathWorks, Natick, MA) and presented to the subject through Sennheiser HDA-200 headphones.
Procedure
To participate in the present study, all subjects had to fill out four surveys including, Tinnitus Severity Index (Folmer et al. 2004), Tinnitus Handicap Index (Newman et al. 1996), Beck Depression Inventory (Beck and Steer 1984), and Beck Anxiety Inventory (Beck et al. 1988). They also had to participate in a test battery including an extensive case history, otoscopy, tympanometry, an audiogram, and behavioral estimates of tinnitus. Audiometry was performed in a double-walled sound-attenuated booth for frequencies 250–8,000 Hz using a GSI 61 Clinical Audiometer and TDH-39 headphones.
In the tinnitus matching experiment, subjects listened to a 500-ms pure tone presented either contralaterally to the tinnitus ear in ipsilateral tinnitus cases or to the ear with lower audiometric thresholds in bilateral tinnitus cases. The level of the 500-ms pure tone was controlled by a double-staircase, adaptive procedure (Jesteadt 1980; Zeng and Turner 1991). In one staircase, the initial level was louder than the tinnitus, while in the other staircase the initial level was softer. Stimulus presentation from the two staircases was randomly interleaved and the stimulus level in the first staircase was controlled by a 2-down, 1-up rule and a 1-down, 2-up rule in the second staircase. The stimulus levels converged where the subject judged them to be louder or softer than the tinnitus 71% of the time. The tinnitus-matched level, or the point of subjective equality, was the average of these two levels. Equal loudness contour was obtained by matching to tinnitus loudness at frequencies from 250 to 8,000 Hz in octave steps, plus an additional 12,000 Hz. Once loudness was matched across these frequencies, the tinnitus frequency was determined by varying the frequency along the equal loudness contour using the same double-staircase, adaptive procedure. Finally, the detection threshold in decibels sound pressure level (dB SPL), as well as matched loudness in dB SPL, was determined at the tinnitus frequency. Subtracting the former measure from the latter gave tinnitus loudness in dB sensation level (SL). The double-staircase adaptive procedure also helped train the subjects to focus on loudness-related changes while ignoring other qualitative differences when reporting on their tinnitus and stimulus loudness in the main experiment.
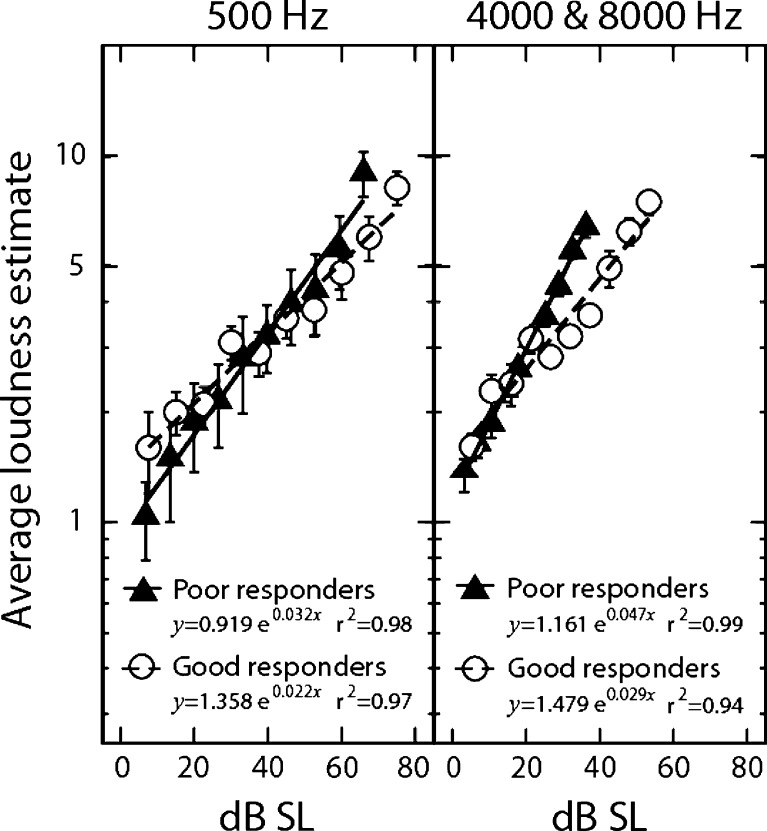
In the loudness growth experiment reported in Figure 6, subjects used a 0–10 numeric scale with 0 being inaudible and 10 being unbearably loud to estimate loudness of a 500-ms pure tone. The tone frequency was 500, 4,000, or 8,000 Hz. The tone level was selected individually to cover the entire dynamic range, starting at 5 dB SL and in 5 dB steps. The presentation order of tones was randomized. With each presentation, the subject gave a numerical value corresponding to the tone. Each tone was repeated three times, and the three estimates were averaged to produce the reported loudness.
FIG. 6.
Average loudness growth functions at low frequency (500 Hz, left panel) and high frequencies (4,000 and 8,000 Hz, right panel) between four good (open circles) and 11 poor (filled triangles) responders. Loudness growth was not measured in the remaining three good and two poor responders. Error bars represent standard error of the mean. Good fit was obtained for all four sets of loudness data (r2 ≥ 0.94). The constant (a) before the exponential function is a scaling factor while the exponent (θ) before x in the fitted equation is the slope of loudness growth function (e.g., 0.032 for poor responders at 500 Hz). Two-tailed t tests showed a significant difference in slope between good and poor responders at both low and high frequencies (p < 0.05). The dynamic range in dB (DR_dB) can be simply derived by setting loudness to the maximal value (i.e., y = 10) and solving the x value in the fitting equation: 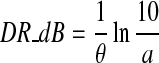
In the main experiment, subjects used the same procedure as in the loudness growth experiment, except that the subject had to estimate loudness of both tinnitus and a 3-min external stimulus. First, the subject reported the baseline loudness of tinnitus prior to any testing. Then, the subject adjusted the presentation level of the 3-min stimulus so that the stimulus was just softer than his or her tinnitus (e.g., if the tinnitus loudness estimate was 5, then the stimulus would be presented at a loudness of 3 or 4). On rare occasions (<3% trials) where the tinnitus sensation level was low, namely, 1–2 dB SL, the external stimulus was presented at the same sensation level. No subject reported that his or her tinnitus was totally masked by the external stimulus. The external stimulus was presented to the tinnitus ear in subjects with unilateral tinnitus or the ear with the louder tinnitus in subjects with bilateral tinnitus of unequal loudness; the stimulus was presented diotically in subjects with bilateral tinnitus of equal pitch and loudness. While the 3-min external stimulus was being presented, the subject reported both the loudness of the external stimulus and the loudness of the tinnitus at 30-s intervals (Tang et al. 2006). Tinnitus suppression was defined as a percent reduction in tinnitus loudness, from its baseline loudness, reported during this 3-min sound exposure. The presentation order of the 17 stimuli was randomized for each individual subject. The 20 subjects and 17 stimuli produced a total of 340 trials, allowing a systematic investigation into the interaction between subjective tinnitus and external stimulus.
Statistical analysis
Descriptive statistics were calculated for subject demographics and tinnitus characteristics. Categorical data were analyzed using the chi-square distribution while continuous data were analyzed using the independent sample t test or the non-parametric Mann–Whitney U test with p values considered significant at <0.05.
Both univariate and multivariate models were used to analyze the tinnitus suppression data from the 340 trials. In the univariate model, Type III analysis with Wald statistic was used to examine the significance of the stimulus type effect after controlling for the effects of stimulus frequency in the model, or vice versa (SAS 1999). The Type III analysis was used for its independence of the order of effects entered into the model, corresponding to the random presentation order of all stimuli, regardless of type and frequency, in the present experimental procedure. The degrees of freedom were equal to the number of parameters associated with the effect. If the main effects were significant, then post hoc comparisons with Bonferroni corrections were used to determine the level of significance between different stimulus types and frequencies.
Two thirds of the 340 trials produced 0% suppression, resulting in a highly skewed distribution that departs from the normal distribution assumed by ANOVA. We employed two additional statistical procedures to tackle this non-normal distribution problem (Duan et al. 1983). First, the effect of suppression was analyzed based on only the 101 stimuli that produced greater than 0% tinnitus suppression. The suppression data were log-transformed and normalized prior to analysis by linear regression. Second, the original percent suppression data were transformed into a binary form: A stimulus produced either suppression (>0%) or no suppression. Then logistic regression was used to fit the binary data and to estimate the probability of tinnitus suppression by each stimulus. The logistic regression coefficients were estimated by the generalized estimating equations method, which minimized possible correlations introduced by the repeated measures from the same cohort of subjects (Hanley et al. 2003).
Results
Representative tinnitus suppression results
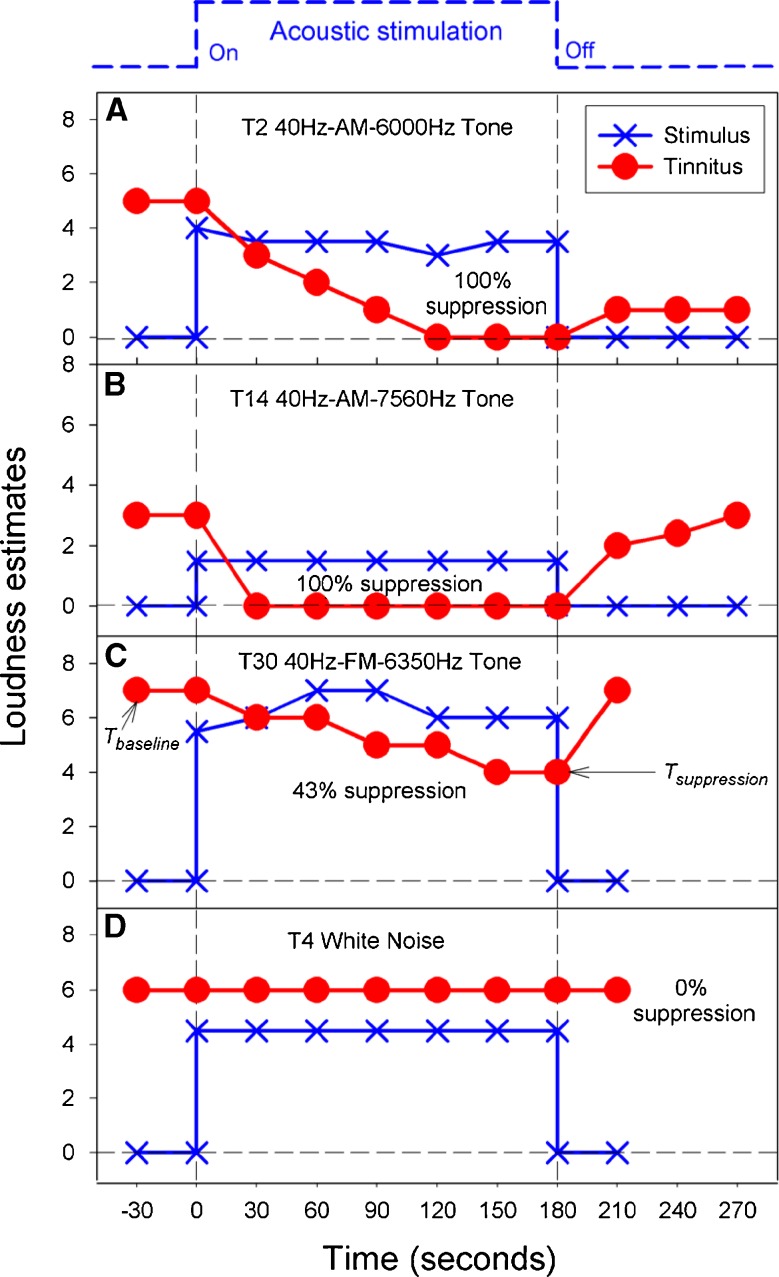
One third of the trials (101/340) produced some tinnitus suppression in 90% of the subjects (18/20). On average, tinnitus loudness was reduced by 39% (SD = 30 %; range, 0–100%). Figure 1 shows four sets of representative individual data with or without tinnitus suppression. Note first that, at the onset of the stimulus, loudness of the stimulus was estimated 1 or 2 numeric values below that of the tinnitus, validating our loudness balance and tinnitus match procedures. Second, both panels a and b show 100% suppression with the 40-Hz amplitude-modulated tones but have different time courses of suppression and recovery. In panel a, the subject needed 120 s to reach the 100% suppression level and additionally showed residual inhibition (tinnitus loudness = 1 or barely audible) for at least up to 90 s after the suppressor stimulus was turned off. In panel b, 100% suppression was achieved immediately after the onset of the suppressor (within the 30-s resolution) while little or no residual inhibition was observed. Third, panel c shows partial suppression with a long time course similar to panel a. Different from the panels a and b which showed little or no change in suppressor loudness, the subject in panel c showed an increase in the FM suppressor loudness from 5.5 (medium to medium-loud) at the onset of the stimulus to 7 (loud) 60 s after the onset. Finally, panel d shows that the white noise suppressor had no effect on tinnitus loudness. Next, we analyze both group and subgroup suppression data to answer the following fundamental questions: Which sound is the most effective? Who will likely benefit from it?
FIG. 1.
Representative tinnitus suppression data in terms of loudness estimates as a function of time. A 100% tinnitus suppression in the presence of a 40-Hz amplitude-modulated 6,000-Hz tone. B 100% tinnitus suppression in the presence of a 40-Hz amplitude-modulated 7,560-Hz tone. C 43% tinnitus suppression in the presence of a 40-Hz frequency-modulated 6,350-Hz tone. D 0% tinnitus suppression in the presence of a white noise. The solid circles represent loudness estimates of the subjective tinnitus while the Xs represent that of the external stimulus. Tinnitus suppression is defined as: [(Tbaseline – Tsuppression)/Tbaseline] × 100, where Tbaseline represents tinnitus loudness in absence of and before external stimulation, and Tsuppression represents minimal tinnitus loudness in the presence of external stimulation.
Relation to stimulus characteristics
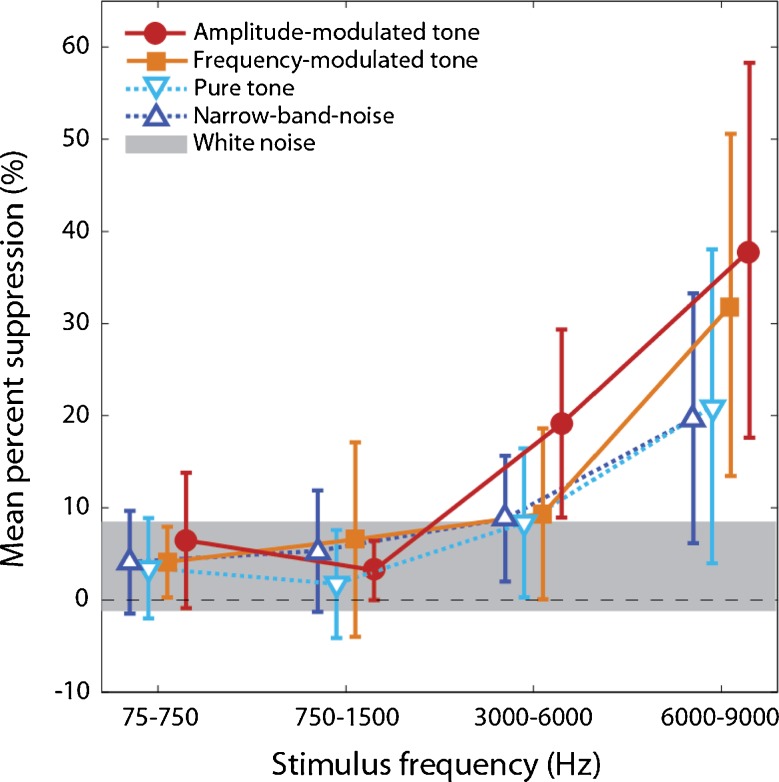
Figure 2 shows the mean percent suppression from all 340 trials as a function of stimulus frequency range and stimulus type. Both stimulus type (univariate; Type III = 29.2, df = 4, p < 0.01) and stimulus frequency (Type III = 20.4, df = 4, p < 0.01) are significant factors in achieving tinnitus suppression. Compared with white noise, the two modulated stimuli (amplitude and frequency modulation) and the two high-frequency stimuli (3,000–6,000 Hz and 6,000–9,000 Hz) produced significantly more tinnitus suppression (p < 0.01 for all conditions). Other stimulus types and frequencies did not produce any significantly more suppression than white noise (p > 0.05).
FIG. 2.
Percent tinnitus suppression as a function of stimulus frequency (X-axis) and stimulus type (symbols): amplitude-modulated tones (filled circles), frequency-modulated tones (filled squares), pure tones (open inverted-triangles), and narrow-band noise (open triangles). Solid lines represent modulated stimuli whereas dashed lines represent unmodulated stimuli. Error bars = 95% confidence interval. The gray bar plotted across the bottom represents the 95% range for the white noise control. See text for significant differences in tinnitus suppression.
To address the skewed distribution issue in the data, two additional statistical procedures were employed to confirm and quantify the above observed tinnitus suppression effect. First, linear regression was performed only on the 101 trials that produced suppression. Of the 101 trials, 31 were produced by amplitude-modulated tones, followed with 24, 23, 20, and 3 produced by frequency-modulated tones, narrow-band noises, pure tones, and white noise, respectively. Both factors were still significant when modeled together (stimulus type, Type III = 13.2; df = 3, p < 0.01; stimulus frequency, Type III = 13.6; df = 3, p < 0.01). After adjusting for stimulus frequency, all stimuli except for pure tones produced significantly greater amounts of suppression than white noise, with the amplitude-modulated tones producing the most (1.9 times more than white noise, p < 0.01). After adjusting for stimulus type, only the highest-frequency stimuli produced 1.5 times more suppression than white noise (p < 0.01).
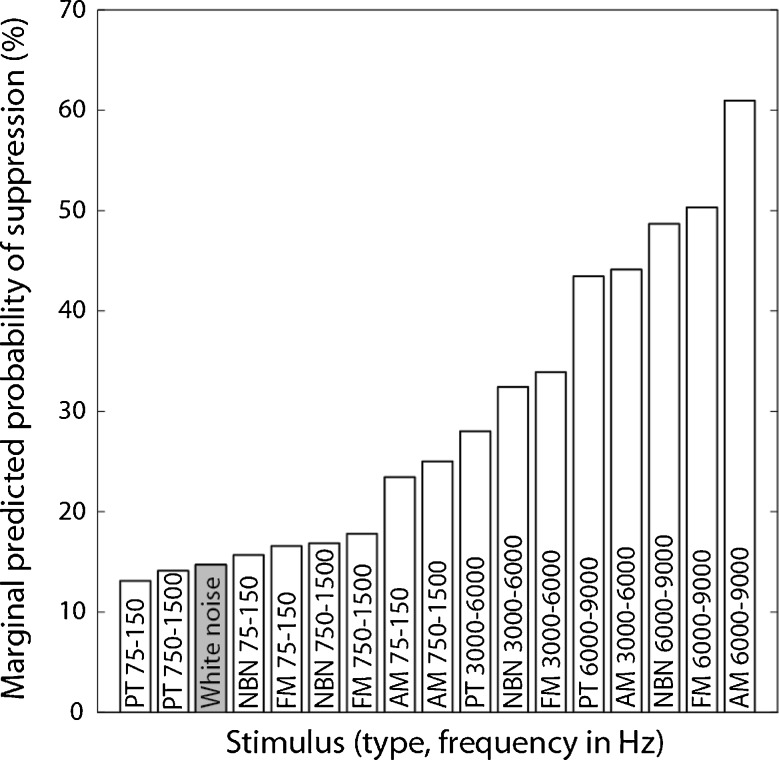
Second, a multivariate logistic regression model (n = 340) again confirmed that both factors are significant in determining the probability of tinnitus suppression occurrence (stimulus type, Type III = 8.8; df = 3, p = 0.03; stimulus frequency, Type III = 30.6, df = 3, p < 0.01). Using white noise as the referent category for comparison, the remaining 16 stimuli were ranked by their predicted probability of suppression occurrence. Figure 3 shows the ranking results that modulated high-frequency stimuli were more likely to suppress tinnitus than unmodulated low-frequency stimuli and white noise (filled bar). Compared with 13% probability for low-frequency (75–750 Hz) pure tones and 15% probability for white noise, amplitude-modulated high-frequency (6,000–9,000 Hz) tones were four times more likely to produce tinnitus suppression, or have 60% predicted probability of suppression. Experimentally, a high-frequency amplitude-modulated tone produced some suppression in 45%, or nine of the 20 subjects, including nine with 100%, one with 75%, one with 50%, and 1 with 15% suppression.
FIG. 3.
Stimulus ranking by the marginally predicted probability of tinnitus suppression back-calculated from the regression coefficients of a logistic regression model that included main effects of stimulus type and stimulus frequency. White noise (gray bar) was used as the referent category for comparison. The condition with the greatest likelihood of achieving suppression was amplitude-modulated tones in the 6,000–9,000 Hz region. In other words, one could expect 60% of tinnitus cases to be suppressed to some degree with a high-frequency, amplitude-modulated tone.
Relation to subject characteristics
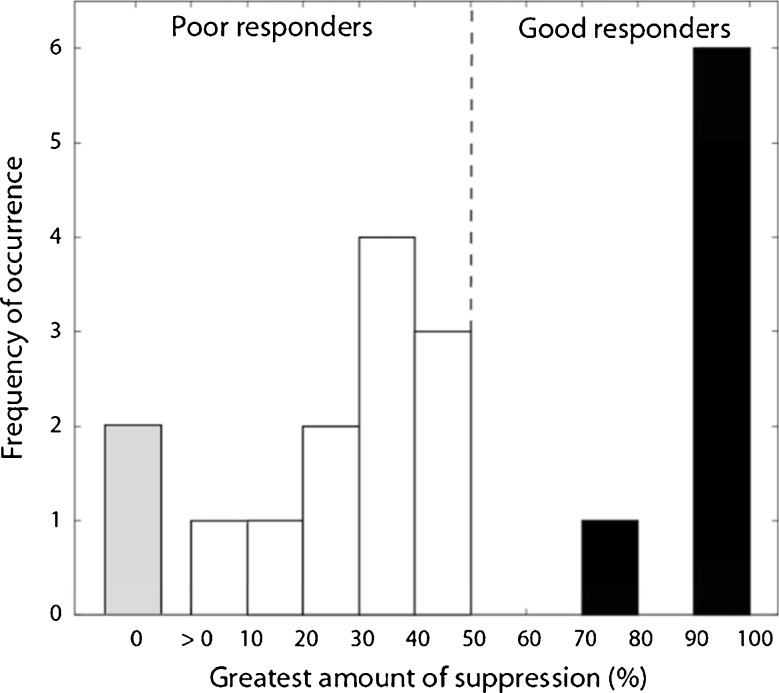
In practice, it is more useful to quantify the maximal suppression in response to a single stimulus than the mean suppression to all stimuli. Figure 4 shows a histogram plotting the number of subjects against their greatest amount of tinnitus suppression. The histogram shows a bimodal distribution (Shapiro-Wilk Test of Normality, p = 0.006), with 35% being good responders (>70% suppression; n = 7) and 65% poor responders (<50%; n = 13).
FIG. 4.
Histogram of the greatest amount of suppression, regardless of stimulus condition, in 20 tinnitus sufferers. The bimodal distribution separated subjects into seven good (>70% suppression) and 13 poor (<50% suppression) responders to the present sound therapy.
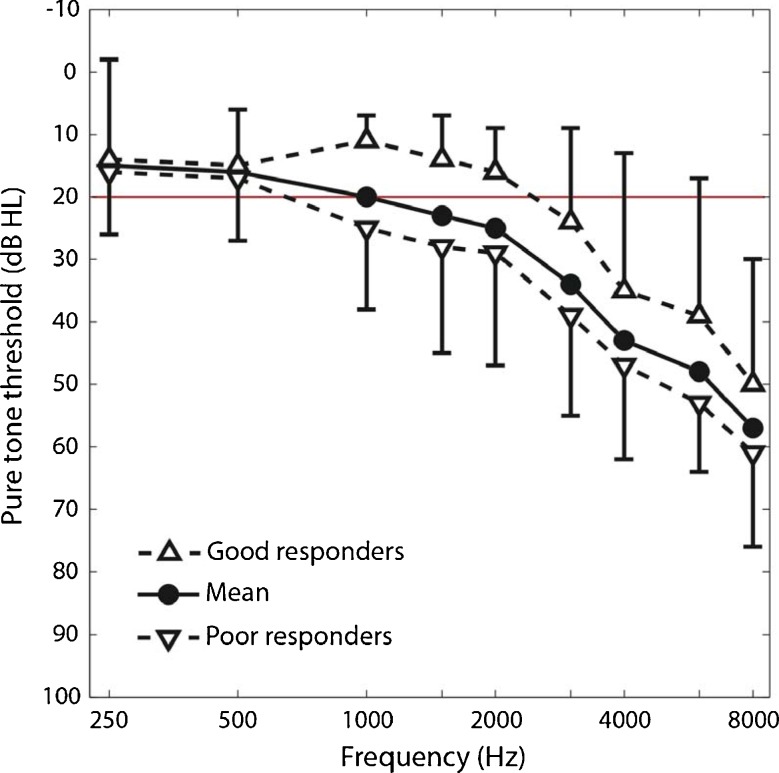
We characterized the subject demographic data by their age, noise exposure history, insomnia, depression, anxiety (top of Table 1), and pure-tone thresholds (top of Table 1 and Fig. 5) but found no significant differences between good and poor responders. We also analyzed the subject tinnitus data (bottom of Table 1). Although no significant differences were found in tinnitus location, duration, type, severity, and pitch between the good and poor responders, note that both good and poor responders matched their tinnitus frequency to about 7,000 Hz, much higher than the 500–2,500 Hz edge frequency of normal hearing (red line = 20 dB HL in Fig. 5). Interestingly, the only different factors between the good and poor responders were two loudness-related measures (bold text in Table 1). Compared with good responders, poor responders matched their tinnitus to significantly lower sensation levels (6 vs. 12 dB SL; p = 0.02) but higher loudness rankings (6 vs. 4; p = 0.03). Lower level but louder sensation usually signals the presence of either severe loudness recruitment or hyperacusis or both (Goldstein and Shulman 1996; Nelson and Chen 2004).
TABLE 1.
Subject and tinnitus characteristics (n = 20) between good (>70% suppression) and poor (<50% suppression) responders
| Good responders (n = 7) | Poor responders (n = 13) | P value | |
|---|---|---|---|
| Subject characteristics | |||
| Age (years) | 57 (16) | 62 (9) | 0.75 |
| Pure tone average threshold at 0.5, 1, 2, and 4 kHz | 42 (22) | 53 (14) | 0.19 |
| Self-reported noise exposure—no | |||
| No | 3 | 6 | 1.0 |
| Yes | 4 | 7 | |
| Self-reported insomnia—no | |||
| No | 3 | 6 | 1.0 |
| Yes | 4 | 7 | |
| Self-reported anxiety—no | |||
| No | 4 | 7 | 1.0 |
| Yes | 3 | 6 | |
| Self-reported depression—no | |||
| No | 4 | 10 | 0.61 |
| Yes | 3 | 3 | |
| Beck Depression Inventory (0–63) | 12 (9) | 7 (7) | 0.25 |
| Beck Anxiety Inventory (0–63) | 10 (10) | 9 (12) | 0.69 |
| Tinnitus characteristics | |||
| Localization of tinnitus—no | |||
| Unilateral, right | 0 | 0 | 0.23 |
| Unilateral, left | 2 | 2 | |
| Bilateral | 5 | 11 | |
| Subjective judgment of tinnitus type—no | |||
| Tonal | 1 | 4 | 0.69 |
| Non-tonal | 3 | 5 | |
| Both | 3 | 4 | |
| Tinnitus duration (years) | 18 (15) | 18 (18) | 0.69 |
| Tinnitus pitch-matched frequency or Tf (Hz) | 6929 (2090) | 7123 (1691) | 0.82 |
| Hearing threshold at Tf (dB SPL) | 58 (25) | 79 (13) | 0.07 |
| Equal loudness level at Tf (dB SPL) | 69 (23) | 84 (14) | 0.14 |
| Equal loudness level atTf(dB SL) | 12 (4) | 6 (5) | 0.02 |
| Tinnitus ranking (0–10) | 4 (2) | 6 (1) | 0.03 |
| Tinnitus Handicap Inventory (0–100) | 52 (35) | 42 (22) | 0.53 |
| Tinnitus Severity Index (0–100) | 31 (14) | 33 (8) | 0.82 |
FIG. 5.
Pure-tone audiograms or mean hearing thresholds as a function of frequency from 40 ears of the present 20 tinnitus subjects (solid black line, filled circles). Audiograms for the seven good responders (dashed line, regular triangles) and 13 poor responders (dashed line, inverted triangles). The horizontal red line represents normal audiometric thresholds. Errors bars represent 1 SD.
To explore the effect of loudness growth on tinnitus suppression, loudness growth functions were measured at low (500 Hz) and high frequencies (4,000 and 8,000 Hz). Figure 6 shows the average loudness growth functions at these low and high frequencies from 11 poor responders and four good responders. Indeed, compared with good responders, poor responders had 1.5 and 1.8 times steeper loudness growth as well as 16 and 20 dB narrower dynamic ranges at low and high frequencies, respectively.
Discussion
Comparison with previous studies
Previous sound therapies have generally used a masking approach, in which an external sound is presented to either completely or partially mask tinnitus (e.g., Feldmann 1971; Vernon 1977; Hazell and Wood 1981; Jastreboff et al. 1996; Henry et al. 2004). The term “masking” is used here to describe generally a reduction in loudness of tinnitus (an internal sound) caused by an external sound. Note that this “tinnitus masking” definition is different from the standard sound-on-sound masking definition—“the process or the amount by which the threshold of audibility for one sound is raised by the presence of another (masking) sound” (ASA 1960). First, the masking target is different between sound-on-tinnitus and sound-on-sound. Second, the defining effect is different—a loudness reduction in one case and a threshold elevation in the other. Third, the temporal pattern of effect is different: Tinnitus can be masked for several minutes or even hours after a masking sound is turned off (i.e., residual inhibition, see Feldmann 1971; Terry et al. 1983) but sound audibility is hardly affected beyond a hundred milliseconds (e.g., Jesteadt et al. 1982). Here, we use the “tinnitus masking” definition to compare the present study with previous studies.
The present study is similar in some aspects but different in other aspects from previous tinnitus masking studies. The present study is similar in that external sound is used to reduce tinnitus loudness. The present study is different from previous studies in the external sounds used and their effects on tinnitus perception. First, the present study used both modulated and unmodulated stimuli but previous studies typically used unmodulated sounds such as pure tones, narrow-band noises, or white noise. Given the long history and broad usage of white noise, it was surprising to find white noise least effective in reducing tinnitus loudness in the present experiment. Second, the present study presented sound at a fixed loudness level, namely, just softer than the tinnitus, whereas previous studies often presented sound at a fixed sensation level (e.g., Vernon 1977; Terry et al. 1983; Roberts et al. 2008). Specifically, the average sensation level was lower than 8 dB in the present study but was at 65 dB in the Roberts et al. (2008) study. Similar to total masking where the subjects heard only the external sound but not the tinnitus, 30% or six of the 20 subjects (see Fig. 4) showed total suppression of the tinnitus in the present study. Similar to partial masking in the sound-on-sound sense, 60% of the present subjects showed some degrees of tinnitus suppression, with the subjects hearing both the stimulus and the tinnitus, albeit at a reduced loudness level.
Recently, other types of sounds such as fractal tones and customized music have been used to treat tinnitus (Davis et al. 2008; Okamoto et al. 2010; Sweetow and Sabes 2010). One form of the customized music selectively amplifies the frequency region where tinnitus sufferers have hearing loss (Davis et al. 2008). On the contrary, another form of customized music removed or “notched” the energy in the frequency region surrounding the tinnitus frequency (Okamoto et al. 2010), which is likely to occur in the region of hearing loss (Norena et al. 2002). Despite opposite technical approaches, both forms of customized music reportedly lead to significant tinnitus reduction after long-term (6–12 months) and regular (2–4 hours daily) listening to the modified sounds. It is unknown whether these customized sounds in the previous studies produced immediate tinnitus suppression similar to what was observed in the present study. Because tinnitus relief has been directly linked to the use of hearing aids and cochlear implants, there is a general consensus that sound exposure is desirable, but presently there is no consensus on the types of desirable sounds and their effectiveness in tinnitus sound therapy (e.g., Trotter and Donaldson 2008; Pan et al. 2009b; Sweetow and Sabes 2010).
Physiological mechanisms
Our observations could help differentiate the neural mechanisms underlying tinnitus. First, our result is inconsistent with the “over-representation of edge frequency” and its related lateral inhibition hypothesis (e.g., Gerken 1996; Muhlnickel et al. 1998). Gerken (1996, see his Fig. 6) made a specific prediction on using a “low-intensity-shaped” narrow-band noise with center frequency just higher than the edge frequency to suppress tinnitus because it would smooth the central representation of the audiometric edge, presumably in the inferior colliculus. Gerken also noted that his “low-intensity-shaped” noise is not a masker and emphasized that the location and shape of the noise would be critical to the effectiveness of tinnitus suppression. Gerken’s lateral inhibition model predicts that the two mid-frequency bands (750–1,500 and 3,000–6,000 Hz) would have produced the most tinnitus suppression in the present study because the “edge frequency” was between 500–2,500 Hz (Fig. 5). Contradictory to this prediction, the present study showed the most suppression by the high-frequency (6,000–9,000 Hz) stimuli.
Our result is partially consistent with a central gain mechanism of tinnitus in which the gain adjusts dynamically according the loudness of any stimulating sounds (Jastreboff 1990; Schaette and Kempter 2006; Norena 2010). The inconsistent aspect is that the present stimuli, modulated or unmodulated, were all loudness-balanced, and thus would produce similar effects on tinnitus if external stimulus loudness is indeed critical to setting the central gain. The consistent aspect is that our tinnitus subjects with relatively normal loudness growth showed more suppression than those with accelerated loudness growth, a sign of increased central gain that may require long-term adaptation.
Our result is most consistent with the tinnitus mechanism based on hyperactive neural activities in terms of increased spontaneous rate and increased within- and between-fiber synchrony throughout the entire central auditory pathway (Sasaki et al. 1980; Wang et al. 1996; Kaltenbach et al. 2002; Seki and Eggermont 2003; Eggermont 2007; Bauer et al. 2008). Compared with pure tones and noises that mostly produce onset and offset auditory cortical activity, the present modulated stimuli produce robust and sustained acoustically driven activity (Lu et al. 2001; Liang et al. 2002) that may help restructure cortical firing patterns away from those that generate tinnitus. Moreover, the 40-Hz amplitude modulation should generate a strong a 40-Hz auditory steady-state response (e.g., Ross et al. 2003), enhancing the gamma rhythm to potentially disrupt thalamocortical dysrthythmia (Llinas et al. 1999) and drawing attention away from tinnitus (Weisz et al. 2007; De Ridder et al. 2011). Frequency modulation also induces strong auditory steady-state responses (Picton et al. 2003) but produces less suppression than amplitude modulation, possibly due to the shallow frequency modulation depth (10%) used in the present study. Understanding of the underlying neural mechanisms is critical to future tinnitus treatment strategies. The present study adds to the growing body of evidence for sound therapies that should be designed to treat the cause, rather than the symptom of tinnitus (Norena and Eggermont 2006; Okamoto et al. 2010; Engineer et al. 2011; Zeng et al. 2011).
Conclusions
Our study systematically examined the interactions between subjective tinnitus and external sounds that included both traditionally used unmodulated sounds and novel dynamically modulated sounds. Different from previous studies that either used a fixed sound pressure level or did not specify the level, the present study uniformly presented sounds at a loudness-controlled level that was just below tinnitus loudness and did not mask tinnitus. Our results provided evidence that modulated sounds, particularly low-rate amplitude-modulated tones with a high carrier frequency in the tinnitus pitch range, are the most effective in reducing tinnitus loudness. Additionally, our results showed that tinnitus sufferers with less loudness recruitment or hyperacusis were more likely to show tinnitus suppression than those with these clinical symptoms. Future studies are needed to delineate the mechanisms underlying the present suppression and to determine whether it will produce long-term therapeutic benefits.
Acknowledgments
The authors thank the participation and persistence of the 20 tinnitus sufferers who had to endure many boring and frustrating hours of testing. The authors also thank Tom Lu, Jerry Northern, Paul Manis, Jennifer Melcher, and four anonymous reviewers for comments on the manuscript, and Edward Wu and Essie Fine for assistance with subject recruitment and data collection. This work was supported by American Tinnitus Association and NIH Grant P30 DC008369.
Conflict of interest
Q.T. and F.G.Z. have an equity interest in, while J.A.C. is currently an employee of, SoundCure, Inc., Boston, MA, an AlliedMinds company that may potentially benefit from the research results. In addition, F.G.Z. also serves on the company's Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, Irvine, in accordance with its conflict of interest policies.
References
- ASA (1960) Acoustical terminology SI, 1–1960. In: New York: American Standards Association.
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::AID-JCLP2270400615>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bulbul SF, Muluk NB, Cakir EP, Tufan E. Subjective tinnitus and hearing problems in adolescents. Int J Pediatr Otorhinolaryngol. 2009;73:1124–1131. doi: 10.1016/j.ijporl.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Cacace AT. Expanding the biological basis of tinnitus: crossmodal origins and the role of neuroplasticity. Hear Res. 2003;175:112–132. doi: 10.1016/S0378-5955(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Davis PB, Wilde RA, Steed LG, Hanley PJ. Treatment of tinnitus with a customized acoustic neural stimulus: a controlled clinical study. Ear Nose Throat J. 2008;87:330–339. [PubMed] [Google Scholar]
- Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan N, Manning WG, Morris CN, Newhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1:115–126. doi: 10.2307/1391852. [DOI] [Google Scholar]
- Eggermont JJ. Pathophysiology of tinnitus. Prog Brain Res. 2007;166:19–35. doi: 10.1016/S0079-6123(07)66002-6. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res. 2000;142:89–101. doi: 10.1016/S0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil Res Dev. 2009;46:797–810. doi: 10.1682/JRRD.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Feldmann H. Homolateral and contralateral masking of tinnitus by noise-bands and by pure tones. Audiology. 1971;10:138–144. doi: 10.3109/00206097109072551. [DOI] [PubMed] [Google Scholar]
- Folmer RL, Martin WH, Shi Y. Tinnitus: questions to reveal the cause, answers to provide relief. J Fam Pract. 2004;53:532–540. [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001;158:1–27. doi: 10.1016/S0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Gerken GM. Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res. 1996;97:75–83. [PubMed] [Google Scholar]
- Goldstein B, Shulman A. Tinnitus–hyperacusis and the loudness discomfort level test—a preliminary report. Int Tinnitus J. 1996;2:83–89. [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR (2010) Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104:3361-3370 [DOI] [PMC free article] [PubMed]
- Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hazell JW, Wood S. Tinnitus masking—a significant contribution to tinnitus management. Br J Audiol. 1981;15:223–230. doi: 10.3109/03005368109081442. [DOI] [PubMed] [Google Scholar]
- Hazell JW, Wood SM, Cooper HR, Stephens SD, Corcoran AL, Coles RR, Baskill JL, Sheldrake JB. A clinical study of tinnitus maskers. Br J Audiol. 1985;19:65–146. doi: 10.3109/03005368509078966. [DOI] [PubMed] [Google Scholar]
- Henry JA, Rheinsburg B, Zaugg T. Comparison of custom sounds for achieving tinnitus relief. J Am Acad Audiol. 2004;15:585–598. doi: 10.3766/jaaa.15.8.6. [DOI] [PubMed] [Google Scholar]
- Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope. 2011;121:1555–1564. doi: 10.1002/lary.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 1993;27:7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17:236–240. [PubMed] [Google Scholar]
- Jesteadt W. An adaptive procedure for subjective judgments. Percept Psychophys. 1980;28:85–88. doi: 10.3758/BF03204321. [DOI] [PubMed] [Google Scholar]
- Jesteadt W, Bacon SP, Lehman JR. Forward masking as a function of frequency, masker level, and signal delay. J Acoust Soc Am. 1982;71:950–962. doi: 10.1121/1.387576. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88:699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Frick U, Hauser S, Hajak G, Langguth B. Association of tinnitus and electromagnetic hypersensitivity: hints for a shared pathophysiology? PLoS One. 2009;4:e5026. doi: 10.1371/journal.pone.0005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, Kleine E, Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 2002;87:2237–2261. doi: 10.1152/jn.2002.87.5.2237. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Temporal codes for amplitude contrast in auditory cortex. J Neurosci. 2010;30:767–784. doi: 10.1523/JNEUROSCI.4170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JJ, Chen K. The relationship of tinnitus, hyperacusis, and hearing loss. Ear Nose Throat J. 2004;83:472–476. [PubMed] [Google Scholar]
- Newman CW, Jacobson GP, Spitzer JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2010;35:1089–1109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17:559–563. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- Norena A, Micheyl C, Chery-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7:358–369. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Stracke H, Stoll W, Pantev C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci U S A. 2010;107:1207–1210. doi: 10.1073/pnas.0911268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Tyler RS, Ji H, Coelho C, Gehringer AK, Gogel SA. The relationship between tinnitus pitch and the audiogram. Int J Audiol. 2009;48:277–294. doi: 10.1080/14992020802581974. [DOI] [PubMed] [Google Scholar]
- Pan T, Tyler RS, Ji H, Coelho C, Gehringer AK, Gogel SA. Changes in the tinnitus handicap questionnaire after cochlear implantation. Am J Audiol. 2009;18:144–151. doi: 10.1044/1059-0889(2009/07-0042). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DR. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiol Neurootol. 1998;3:123–144. doi: 10.1159/000013786. [DOI] [PubMed] [Google Scholar]
- Reed GF. An audiometric study of two hundred cases of subjective tinnitus. AMA Arch Otolaryngol. 1960;71:84–94. doi: 10.1001/archotol.1960.03770010088009. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9:417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Draganova R, Picton TW, Pantev C. Frequency specificity of 40-Hz auditory steady-state responses. Hear Res. 2003;186:57–68. doi: 10.1016/S0378-5955(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/S0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- SAS I (1999) SAS/STAT® User’s guide, version 8. In: Ch. 29, pp 1414–1416. Cary, NC: SAS Institute Inc.
- Sasaki CT, Kauer JS, Babitz L. Differential [14C]2-deoxyglucose uptake after deafferentation of the mammalian auditory pathway—a model for examining tinnitus. Brain Res. 1980;194:511–516. doi: 10.1016/0006-8993(80)91233-0. [DOI] [PubMed] [Google Scholar]
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur J Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/S0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Sweetow RW, Sabes JH. Effects of acoustical stimuli delivered through hearing aids on tinnitus. J Am Acad Audiol. 2010;21:461–473. doi: 10.3766/jaaa.21.7.5. [DOI] [PubMed] [Google Scholar]
- Tang Q, Liu S, Zeng FG. Loudness adaptation in acoustic and electric hearing. J Assoc Res Otolaryngol. 2006;7:59–70. doi: 10.1007/s10162-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AM, Jones DM, Davis BR, Slater R. Parametric studies of tinnitus masking and residual inhibition. Br J Audiol. 1983;17:245–256. doi: 10.3109/03005368309081485. [DOI] [PubMed] [Google Scholar]
- Trotter MI, Donaldson I. Hearing aids and tinnitus therapy: a 25-year experience. J Laryngol Otol. 2008;122:1052–1056. doi: 10.1017/S002221510800203X. [DOI] [PubMed] [Google Scholar]
- Vernon J. Attempts to relieve tinnitus. J Am Audiol Soc. 1977;2:124–131. [PubMed] [Google Scholar]
- Vernon J (1981) The history of masking as applied to tinnitus. J Laryngol Otol Suppl:76–79 [PubMed]
- Wang J, Salvi RJ, Powers N. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. J Neurophysiol. 1996;75:171–183. doi: 10.1152/jn.1996.75.1.171. [DOI] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen SE, Erlandsson SI. Self-reported tinnitus and noise sensitivity among adolescents in Sweden. Noise Health. 2004;7:29–40. [PubMed] [Google Scholar]
- Zeng FG, Turner CW. Binaural loudness matches in unilaterally impaired listeners. Q J Exp Psychol A. 1991;43:565–583. doi: 10.1080/14640749108400987. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Tang Q, Dimitrijevicb A, Starr A, Larky J, Blevins NH (2011) Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms. Hear Res 277:61-66 [DOI] [PMC free article] [PubMed]
- Zoger S, Svedlund J, Holgers KM. Psychiatric disorders in tinnitus patients without severe hearing impairment: 24 month follow-up of patients at an audiological clinic. Audiology. 2001;40:133–140. doi: 10.3109/00206090109073108. [DOI] [PubMed] [Google Scholar]