Abstract
Rotations of the head evoke compensatory reflexive eye rotations in the orbit to stabilize images onto the fovea. In normal humans, the angular vestibulo-ocular reflex (aVOR) gain (eye/head velocity) changes depending on the head rotation plane. For pitch and yaw head rotations, the gain is near unity, but during roll head rotations, the aVOR gain is ∼0.7. The purpose of this study was to determine whether this physiological discrepancy affects dynamic visual acuity (DVA)—a functional measure of the aVOR that requires subjects to identify letters of varying acuities during head rotation. We used the scleral search coil technique to measure eye and head velocity during passive DVA testing in yaw, roll, and pitch head impulses in healthy controls and patients with unilateral vestibular hypofunction (UVH). For control subjects, the mean aVOR gain during roll impulses was significantly lower than the mean aVOR gain during yaw and pitch impulses; however, there was no difference in DVA between yaw, roll, or pitch. For subjects with UVH, only aVOR gain during head rotations toward the affected side (yaw) were asymmetric (ipsilesional, 0.32 ± 0.17, vs. contralesional, 0.95 ± 0.05), with no asymmetry during roll or pitch. Similarly, there was a large asymmetry for DVA only during yaw head rotations, with no asymmetry in roll or pitch. Interestingly, DVA during roll toward the affected ear was better than DVA during yaw toward the affected ear—even though the ipsilesional roll aVOR gain was 60 % lower. During roll, the axis of eye rotation remains nearly perpendicular to the fovea, resulting in minimal displacement between the fovea and fixation target image projected onto the back of the eye. For subjects with UVH, the DVA score during passive horizontal impulses is a better indicator of poor gaze stability than during passive roll or pitch.
Keywords: vestibulo-ocular reflex, head impulses, dynamic visual acuity, gain
Introduction
Image stability during head movements is normally maintained by slow compensatory eye movements (in the direction opposite to head motion) generated by the vestibulo-ocular reflex (VOR; e.g., Gonshor and Jones 1976). Two types of VORs generate this compensatory response: the angular VOR (aVOR), using information about angular head motion from the semicircular canals, and the translational VOR (tVOR), using information about linear head motion from the otolith organs (e.g., Ramat and Zee 2003). aVOR gain (defined as the instantaneous eye velocity divided by inverted head velocity) is useful to measure the degree to which the aVOR can generate compensatory eye movements during head movements. aVOR gain can be deconstructed into three components based upon the axes around which the globe rotates: yaw (horizontal), pitch (vertical), and roll (torsional).
When viewing an earth-fixed target during high-acceleration head impulses, the normal gain of the yaw or pitch aVOR is compensatory (gain, ∼1); however, the gain of the roll aVOR is under-compensatory at ∼0.7 (Aw et al. 1996; Migliaccio et al. 2006). An individual with normal aVOR has little difference in visual acuity during head still (static) or moving (dynamic) conditions. In contrast, an individual with uncompensated vestibular hypofunction has a marked degradation of visual acuity when the head is moving. This reduced dynamic visual acuity (DVA) occurs when images slip on the retina in excess of 2°/s (Hess et al. 1978; Grossman et al. 1989; Demer et al. 1993). During the DVA test, an individual’s visual acuity is measured using optotypes that are displayed when the head is moving above a certain angular velocity (Herdman et al. 1998; Tian et al. 2001). Significant asymmetry on the DVA test during yaw, but not pitch, head impulses has been observed in individuals with unilateral vestibular hypofunction (Herdman et al. 1998; Schubert et al. 2002). DVA symmetry during pitch head impulses may be preserved in these patients because the pitch head acceleration stimulus alternately (up vs. down) excites the contralesional vertical canals and, in the case of superior vestibular neuritis, also the ipsilesional posterior semicircular canal (Arbusow et al. 1999). During roll head impulses, the head is rotated about the naso-occipital axis. Because the roll aVOR is under-compensatory, retinal image slip will occur. This retinal slip is unique in that the fixation target image on the retina is mostly rotated and therefore only minimally displaced with respect to the fovea (Solomon et al. 2003). It is not known whether an image that mostly rotates on the fovea results in reduced dynamic visual acuity.
The objectives of this study were to: (1) determine whether the normal under-compensatory roll aVOR gain results in better (lower magnitude) DVA score during roll head impulses, compared to yaw and pitch impulses, in control subjects and (2) determine whether there is a significant difference in visual acuity between head kept stationary (static) and head moved in roll with unpredictable timing and direction (dynamic) in patients with unilateral vestibular hypofunction (UVH; these subjects have lower roll aVOR gain as a consequence of their lesion). We hypothesized that DVA score symmetry, for head impulses in patients with UVH, would significantly decrease for yaw head impulses, whereas it would remain relatively stable for pitch and roll head impulses because of, respectively, the contribution from the contralesional vertical canals and the smaller affect of torsional image slip on DVA. In contrast, the DVA symmetry scores in age-matched healthy subjects would be similar during head impulses in all planes.
Material and method
Subjects
Participation in this study was voluntary, and informed consent was obtained from all participants, in adherence with the Declaration of Helsinki with a protocol approved by the Johns Hopkins School of Medicine Institution Review Board. We studied ten healthy controls (seven men), aged 22–69 years (mean = 32 ± 16 years), and four patients with UVH (three deafferented from the removal of vestibular schwannoma, one vestibular neuritis), aged 27–66 years (mean = 51 ± 16.8 years). Vestibular hypofunction was confirmed via clinical observation of a positive head impulse test in yaw and, when appropriate, a surgical report confirming nerve section. Time from onset of vestibular hypofunction ranged from 7 to 52 weeks, with a mean of 47.7 ± 33.2 weeks. No patient underwent vestibular rehabilitation prior to entering this study.
Eye and head recordings (scleral search coil)
Angular eye and head positions were measured using the dual-axis scleral search coil technique (Skalar, Delft, the Netherlands). The coil system consisted of a 102 × 102 × 102-cm cubic frame, which generated three orthogonal magnetic fields (frequencies, 41.6, 55.5, and 83.3 kHz; intensity, 0.088 G; Straumann et al. 1995; Migliaccio et al. 2006). Participants were seated upright with their nasion positioned in the center of the magnetic field frame. The participant’s head was positioned so that the inter-pupillary line and Reid’s line were in the Earth-horizontal plane (zero reference position).
Binocular eye movements were recorded in three rotational dimensions using a dual-axis search coil placed on each eye. Head rotation was measured using a custom-fit bite block embedded with a third dual-axis search coil. Angular eye and head positions were sampled at 500 Hz with16-bit resolution. Analog (pre-sampled) signals were low-pass-filtered with a single-pole analog anti-aliasing filter that had a 3-dB cutoff at 100 Hz (Migliaccio et al. 2006). Digital (post-sampled) signals were filtered using a digital 50-tap, zero-phase, low-pass, finite impulse response filter with cutoff at 50 Hz.
To determine the extent of vestibular hypofunction, aVOR gain was measured during passive head impulses delivered in the three canal planes: yaw (horizontal), left anterior/right posterior, and right anterior/left posterior. The passive head impulses were of unpredictable timing and direction, with peak amplitude ∼20°, peak velocity ∼200°/s, and peak acceleration ∼4000°/s2 (Schubert et al. 2006).
Dynamic visual acuity test
Participants wore their prescription glasses as appropriate. Static visual acuity was measured with the search coils on the eyes while the participant’s head was fixed in the zero reference position.
Each subject was then fit with a headband and Watson rate sensor (Micromedical Technologies, Inc., Chatham, IL, USA), which measured angular head velocity. This velocity signal was used by the DVA software to trigger the flashing optotype when head velocity was between 120° and 180° per second; otherwise, the monitor was blank. DVA testing (described below) involved delivering passive head impulses about the roll, yaw, and pitch planes. For roll, the participant’s head was fixed in yaw and pitch using a bite block that only allowed roll motion about the nasion from the zero reference position, i.e., it only allowed the head to rotate about the naso-occipital axis. The participant’s head was not fixed for yaw or pitch head impulse planes. Participants then performed practice trials during passive head impulses in roll, yaw, and pitch planes (roll—rotational head impulses about the naso-occipital axis, yaw—horizontal head impulses about the cephalo-caudal axis, pitch—vertical head impulses about the inter-aural axis). Before commencing each impulse, the participant’s head was placed in the zero reference position for 5 s, allowing for the calibration of angular eye and head position. Each experimental session lasted approximately 45 min.
Single optotypes (the letter E) were presented on a 19-in. Dell PC monitor (resolution, 1,600 × 1,200) with a screen refresh rate frequency of 85 Hz. Subjects were at a viewing distance of 2 m. There were four possible orientations for the optotype: 0°, 90°, 180°, and 270°. The orientation of the optotype was randomly generated for each trial. Participants were required to identify the orientation of the optotype within three presentations, for a potential of 165 trials (11 × 5 × 3; Schubert et al. 2006). The optotype size decreased with increasing level of visual acuity—in steps equivalent to a logarithm of the minimal angle of resolution (LogMAR) of 0.1 (Ferris et al. 1982). The DVA test score was calculated by subtracting the static visual acuity LogMAR from the passive visual acuity LogMAR score (Schubert et al. 2006).
Data analysis
Angular eye and head positions were represented by rotation vectors with respect to roll, yaw, and pitch coordinates (Haslwanter 1995; Migliaccio and Todd 1999). The onset of each head impulse was identified using a polynomial curve, fitted to the head-in-space velocity vs. time. The time of head onset was defined as the point at which the magnitude of the fitted curve was >2 % of the curve’s peak magnitude (this threshold was ∼4°/s). A similar method was used to identify the onset of each eye movement response. Since the time between the onset of the head impulse and its peak velocity was <150 ms, analysis of impulse data was restricted to a period of 150 ms from the onset. Head impulse trials including eye blinks, other artifacts, and atypical velocity profiles were excluded from the analysis.
aVOR gains were determined by measuring head and eye velocity 20 ms prior to peak head velocity (in order to eliminate quick phases) per trial and then averaging across trials (Schubert et al. 2006).
Statistical analysis
Two-way ANOVA was performed, using SigmaPlotTM, to determine the factors that affected DVA score and aVOR gain. DVA score and aVOR gain were the dependent variables, for each respective ANOVA. For both ANOVAs, the independent factors were patient group (control vs. patient, a between-subject factor) and direction of head impulse (left/down/clockwise vs. right/up/counterclockwise, a within-subject factor). When the contribution of an independent variable was significant, post hoc comparisons were performed using ANOVA or pairwise comparisons. All levels of significance were assessed at alpha < 0.05. aVOR gains are presented as means ± 1 SD.
Results
aVOR gain
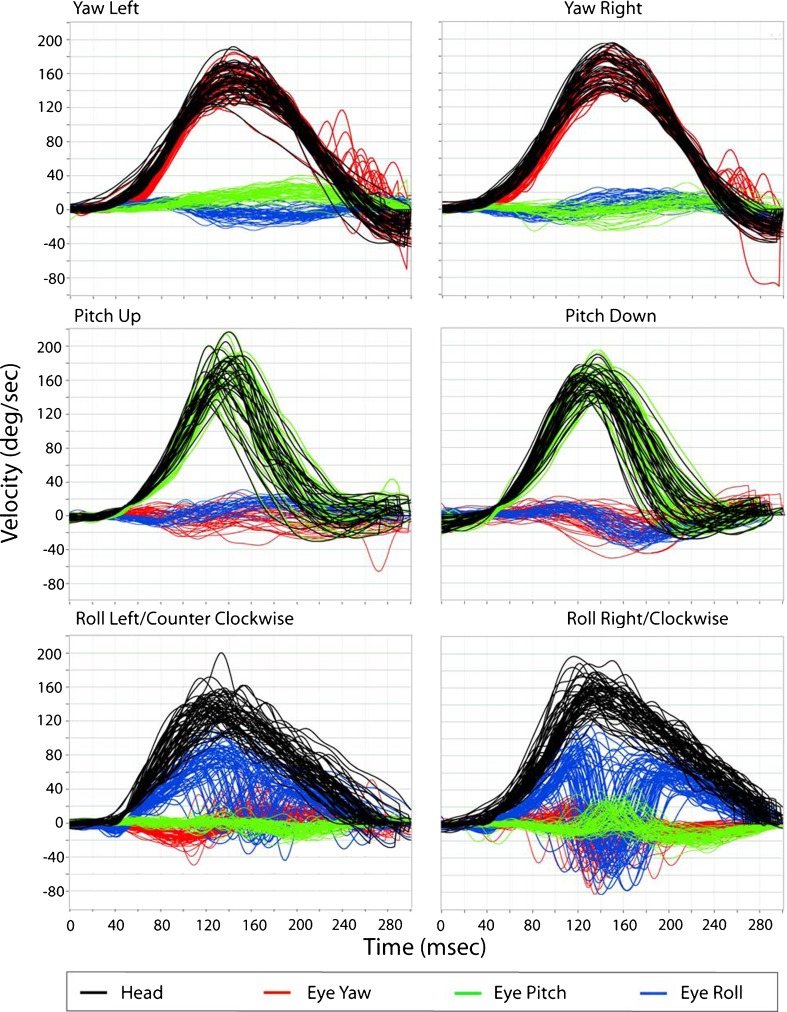
Figure 1 is an example of the aVOR during passive yaw, pitch, and roll head rotation testing for ipsilesional and contralesional head impulses in a healthy control. In this example, the inverted eye velocity traces for yaw (red, top plots) and pitch (green, middle plots) closely approximate the corresponding head velocities (black traces). The mean aVOR gains for yaw and pitch aVOR are 0.95 ± 0.03 and 0.97 ± 0.06, respectively. Note however, that the roll eye velocity (blue, bottom plots) does not as closely approximate the corresponding head velocity (black), with aVOR gain of 0.65 ± 0.12.
FIG. 1.
Eye and head velocity traces in yaw, pitch, and roll head rotations during passive DVA testing in yaw (top), pitch (middle), and roll (bottom) for a healthy control subject. Eye velocity closely approximates head velocity for yaw and pitch, less so for roll. Eye traces have been inverted only for the respected head plane of rotation (yaw eye velocity is inverted only for the yaw head rotations). Note that for roll right rotations, a consistent downward deflection occurs between 120 and 200 ms, not as apparent during roll to the left. Similarly, the pitch trace is also deflected (upwards). This represents a resetting torsional quick phase.
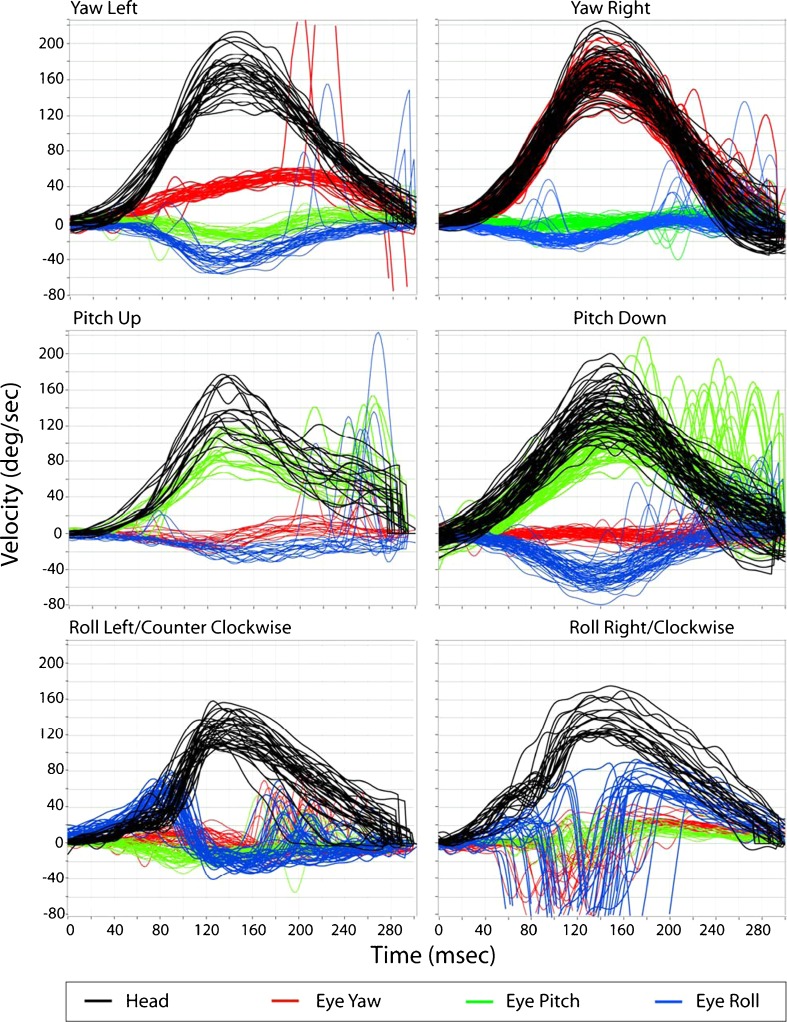
Figure 2 includes eye and head velocity data from a subject with left UVH. Note that the compensatory aVOR gain for leftward yaw (0.27 ± 0.03) and roll (0.15 ± 0.05) is reduced. In this subject, pitch aVOR gains are less affected for upward (0.72 ± 0.09) or downward (0.76 ± 0.06) head impulses. The contralesional yaw and roll aVOR gains were 1.02 ± 0.04 and 0.6 ± 0.11, respectively.
FIG. 2.
Eye and head velocity traces in yaw, pitch, and roll head rotations during passive DVA testing in yaw (top), pitch (middle), and roll (bottom) in a subject with left UVH. The aVOR gains during yaw head impulses are very asymmetric, but not during roll or pitch.
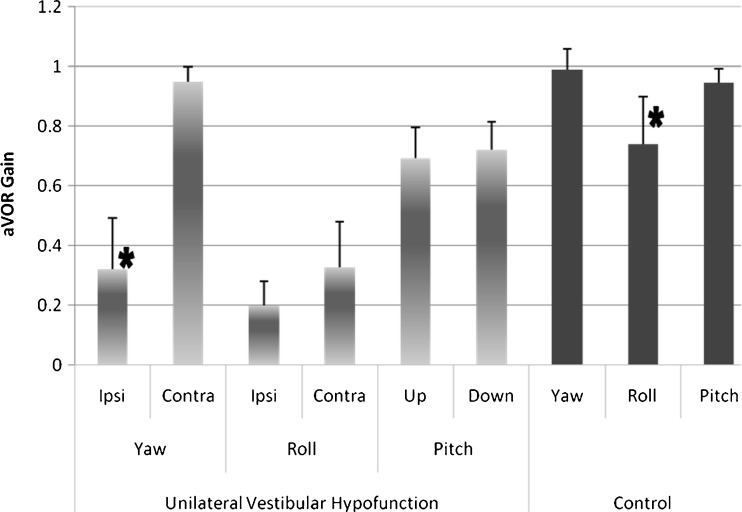
In healthy subjects, there was no aVOR gain asymmetry during yaw, pitch, or roll plane head impulses (i.e., left vs. right, up vs. down, clockwise vs. counterclockwise), and so directional data were combined (ANOVA: control group, “direction,” p = 0.80 (yaw), p = 0.18 (pitch), and p = 0.85 (roll)). However, comparing aVOR gains for each head rotation plane revealed that gains during roll (0.74 ± 0.15) were lower than those for yaw (0.99 ± 0.06) and pitch (0.95 ± 0.05) in healthy subjects (ANOVA: control group, “plane,” p < 0.00001; Fig. 3).
FIG. 3.
Mean and 1 SD aVOR gain in unilateral vestibular hypofunction and healthy controls. In the patient subjects (light bars), we found aVOR gain asymmetry only in the yaw canal plane. There was no asymmetry in aVOR gain within roll or pitch canal planes. For the controls (dark bars), there was no difference in aVOR gain within any cardinal plane head impulses; these data were combined by plane (yaw, roll, pitch). However, the roll aVOR gains in the control subjects were lower than aVOR gains during yaw and pitch. *p < 0.0001.
Each of the UVH subjects showed a significant reduction in aVOR gain during ipsilesional yaw impulses (mean = 0.32 ± 0.17) compared with contralesional impulses (0.95 ± 0.04; ANOVA: patient group, yaw plane, “direction,” p < 0.001). Although reduced, aVOR gains during ipsilesional roll impulses (0.19 ± 0.08) were similar to contralesional impulses (0.35 ± 0.15, p = 0.20). There was little difference in aVOR gain for upward (0.69 ± 0.10) vs. downward (0.71 ± 0.09) directed pitch head impulses (p = 0.71).
Dynamic visual acuity
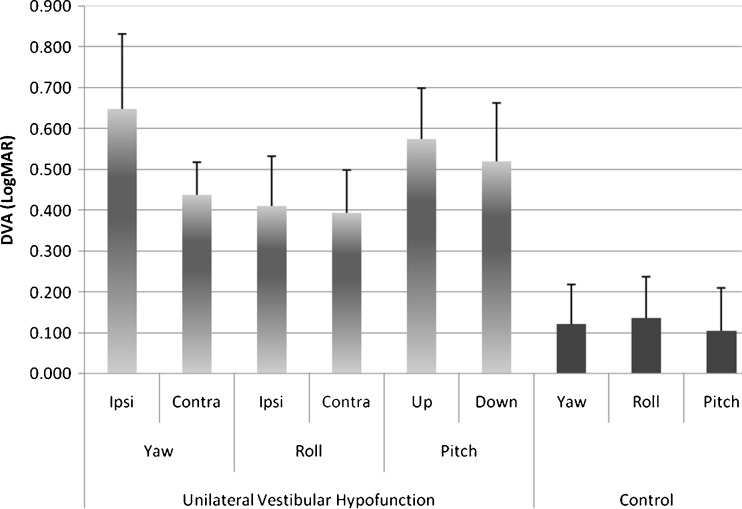
We found that DVA test scores were significantly different between the two groups (ANOVA: “group,” F = 127.9, p < 0.00001) and for the direction of the head impulse (ANOVA: “group” × “direction” interaction, F = 5.3, p = 0.02). The control subjects had significantly better DVA (lower magnitude) than the UVH patients in each of the six pairwise comparison tests (i.e., [yaw, roll, pitch] × [controls, patients]; Fig. 4). For the control group, there was no difference in DVA comparing head rotation directions within any plane (i.e., clockwise roll vs. counterclockwise roll; ANOVA: control group, “direction,” p = 0.60), and the relative difference in scores were always quite low—within 0.08 LogMAR. These LogMAR values (<0.08) equate to missing about four optotypes, or a Snellen acuity equivalent to 20/20 (Table 1).
FIG. 4.
Mean and 1 SD DVA scores in unilateral vestibular hypofunction and healthy controls. Snellen acuity of 20/20 equates to 0.000 LogMAR acuity. The DVA during all head impulse planes was worse in patient subjects with vestibular pathology than the DVA in the controls. In the patient subjects, we found DVA asymmetry for yaw head impulses only. The ipsilesional yaw DVA scores were also worse than DVA during roll to either side. DVA in controls was similar within planar head impulses (i.e., right vs. left, up vs. down) and thus combined.
TABLE 1.
DVA in control subjects
| Controls | Yaw left | Yaw right | Yaw difference | Roll left | Roll right | Roll difference | Pitch up | Pitch down | Pitch difference | Glasses? |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.198 | 0.127 | 0.071 | 0.127 | 0.127 | 0.000 | Yes | |||
| C2 | 0.056 | 0.056 | 0.000 | 0.056 | 0.056 | 0.000 | No | |||
| C3 | 0.109 | 0.109 | 0.000 | 0.125 | 0.125 | 0.000 | Yes | |||
| C4 | 0.036 | 0.036 | 0.000 | 0.018 | 0.036 | 0.018 | No | |||
| C5 | 0.038 | 0.158 | 0.120 | 0.141 | 0.079 | 0.062 | Yes | |||
| C6 | 0.127 | 0.056 | 0.071 | 0.112 | 0.000 | 0.112 | No | |||
| C7 | 0.047 | 0.064 | 0.017 | 0.189 | 0.245 | 0.056 | Yes | |||
| C8 | 0.091 | 0.283 | 0.192 | 0.211 | 0.186 | 0.025 | 0.186 | 0.123 | 0.063 | Yes |
| C9 | 0.399 | 0.271 | 0.128 | 0.250 | 0.417 | 0.167 | 0.417 | 0.382 | 0.035 | Yes |
| C10 | 0.071 | 0.087 | 0.016 | 0.020 | 0.191 | 0.171 | 0.245 | 0.103 | 0.142 | Yes |
| Mean | 0.117 | 0.125 | 0.062 | 0.125 | 0.146 | 0.061 | 0.283 | 0.203 | 0.080 | |
| SD | 0.111 | 0.088 | 0.067 | 0.078 | 0.122 | 0.067 | 0.120 | 0.156 | 0.055 |
One missed optotype equals ∼0.018 LogMAR units. There was no difference in DVA between planes (yaw, pitch) or within planes (right vs. left).
For the UVH subjects, there was no difference in DVA between ipsilesional and contralesional roll head impulses (0.410 ± 0.121 ipsilesional vs. 0.394 ± .103 contralesional, 4 % difference, p = 0.850) or pitch up vs. pitch down head impulses (0.573 ± .126 up vs. 0.520 ± .142 down, 9 % difference, p = 0.59). However, DVA during passive head impulses was significantly asymmetric in yaw (0.649 ± .182 ipsilesional vs. 0.438 ± .08 contralesional, 33 % difference, p = 0.05; Table 2). DVA during ipsilesional yaw impulses also tended to be higher than ipsilesional roll (p = 0.07) and contralesional roll (p = 0.03).
TABLE 2.
DVA in subjects with unilateral vestibular pathology
| Vestibular athology | Ipsilesional yaw | Contralesional yaw | Yaw difference | Ipsilesional roll | Contralesional roll | Roll difference | Pitch up | Pitch down | Pitch difference | Glasses? |
|---|---|---|---|---|---|---|---|---|---|---|
| Left UVH | 0.865 | 0.488 | 0.377 | 0.508 | 0.544 | 0.036 | 0.721 | 0.721 | 0.000 | Yes |
| Left UVD | 0.720 | 0.421 | 0.299 | 0.519 | 0.350 | 0.169 | 0.576 | 0.519 | 0.057 | No |
| Left UVD | 0.559 | 0.333 | 0.226 | 0.285 | 0.308 | 0.023 | 0.582 | 0.426 | 0.156 | Yes |
| Right UVD | 0.450 | 0.508 | 0.058 | 0.327 | 0.372 | 0.045 | 0.414 | 0.414 | 0.000 | Yes |
| Mean | 0.649 | 0.438 | 0.240 | 0.41 | 0.394 | 0.068 | 0.573 | 0.520 | 0.053 | |
| SD | 0.182 | 0.079 | 0.136 | 0.12 | 0.103 | 0.068 | 0.126 | 0.142 | 0.074 |
The DVA during passive head impulses is significantly different based on plane of head rotation (p = 0.04). The DVA within planar head rotations (i.e., up vs. down) is more asymmetric for yaw than DVA in either roll or pitch. Difference column shows difference in DVA within cardinal planes
UVH unilateral vestibular hypofunction, UVD unilateral vestibular deafferentation surgery due to vestibular schwannoma
Discussion
aVOR gain and DVA
During roll impulses about the naso-occipital axis, the axis of eye rotation remains nearly perpendicular to the fovea, resulting in a mostly rotational movement of the target image that stays on the fovea—even though the roll aVOR is under-compensatory. In contrast, during yaw impulses, an under-compensatory aVOR would result in the target image moving away from the fovea. Our results show that although the roll aVOR gain is ∼30 % lower than the compensatory yaw aVOR gain, there is no difference in the DVA scores between yaw and roll head impulses. This result suggests that DVA testing alone during roll head impulses may not always reveal aVOR hypofunction. Our data suggest that visual acuity is preserved as long as the target image stays on the fovea and does not depend on whether the target is rotating on the fovea. There likely exists some threshold in the magnitude of roll image motion where frontal-eyed mammals may begin to experience gaze instability.
Comparing ipsilesional and contralesional cardinal plane rotations during DVA, we found that yaw and roll aVOR gains were reduced by ∼50 %, while pitch up and down rotations were <10 % different. This is explained by the pattern of activation of the semicircular canals during a given axis of rotation. During ipsilesional roll head rotations, the contralesional vertical canals will be driven into inhibition, with the resultant roll gain reduced. In contrast, during pitch head rotations, one of the contralesional canals will be excited and the other will be inhibited, thereby sparing both acuity and VOR gain during pitch head rotations (Tusa et al. 1996).
Roll aVOR gain disparity
In our study design, we were careful to control for translation of the eyes and otolith organs during the roll stimulus. We did this by first limiting roll rotation about the nasion. Secondly, the target viewing screen was at a distance of 2 m, which limited vertical skew during roll. In prior studies, we showed that vertical skew during roll is greater while viewing near targets (1.24 m); however, even at that shorter distance, the change in eye position was <0.2° (Migliaccio et al. 2006). Finally, the horizontal and vertical eye deviations during roll head impulses are small (∼3°), indicating that the movement of the head relative to the screen was also small (Migliaccio et al. 2006).
Our data support prior studies showing that the human aVOR gain during roll is lower than it is during yaw or pitch (Aw et al. 1996). While this roll aVOR gain disparity exists in frontal-eyed mammals, e.g., humans and monkeys, this is not the case for lateral-eyed mammals. Chinchillas have recently been shown to have an isotropic aVOR gain, i.e., the gain does not change with the head rotation plane (Migliaccio et al. 2010). Aside from being isotropic, the aVOR gain in chinchillas is also much lower in all planes of head rotation, not just roll (Migliaccio et al. 2010).
Why, then, is the primate roll aVOR gain under-compensatory compared to the yaw and pitch aVOR? First, roll head movements do not displace images off the fovea when a primate views a far target near the direction of the primary gaze. If the rotation of images on the fovea affects visual stability less than when images move off the fovea, then the adaptive drive that increases yaw and pitch aVOR gains may be weaker in the case of the roll aVOR, especially in animals with good visual acuity (Migliaccio et al. 2010). Our data support this hypothesis because they show that humans exhibit similar visual acuity during yaw and roll transient head rotations despite the fact that their roll aVOR gain is typically 30 % less than their yaw aVOR gain. Second, when a frontal-eyed animal is subjected to head roll while viewing a nearby target (such as a tool held in one’s hand) outside of the primary position, a roll aVOR gain of ∼1 would interfere with stereopsis by creating a skew deviation of the eyes that would move one or both foveae off the target (Misslisch et al. 2001; Migliaccio et al. 2006). The relatively low roll aVOR gain in primates may therefore reflect not only a tolerance of image rotation on the foveae for distant targets, as shown in this study, but also a drive to maximize stereopsis during near viewing. Thirdly, the torsional oculomotor range is about half that of the horizontal or vertical range [the horizontal, vertical, and torsional oculomotor ranges are ±40°, ±35°, and ±15° in humans, respectively (Tweed and Vilis 1990; Misslisch et al. 1994), and ±30°, ±22.5°, and ±15° in rhesus monkeys (Hepp et al. 1993; Suzuki et al. 2000)]. The lower roll aVOR gain could represent a trade-off that helps the eye avoid reaching the end of its torsional range of motion.
Additionally, data from this study show that the ipsilesional yaw and roll aVOR gain during passive DVA testing of UVH patients is abnormal; this does not spontaneously recover to high-acceleration stimuli (Sadeghi et al. 2006). In contrast to the passively applied head rotations in this study, aVOR gains during high-acceleration active head rotation are close to normal as a likely result of efferent copy to assist gaze stability (Sadeghi et al. 2010). Compensation to active head rotations occurs within 1 month of unilateral labyrinthectomy in macaques and about 6 weeks in humans (Herdman et al. 2003). We therefore expect that the aVOR gains reported in our study were stable as none of our subjects were studied within these time limits.
Clinical utility
An important issue in the clinical presentation of patients with reports of dizziness and imbalance is to distinguish between functional and somatic symptoms of vestibular dysfunction (Herdman et al. 2003; Mallinson and Longridge 2005). DVA is an efficient, simple, and noninvasive test that can be routinely implemented in the clinical setting. Our results show no asymmetry in DVA for passive impulses in yaw, roll, or pitch for healthy controls. For patients with unilateral vestibular pathology, DVA during passive ipsilesional yaw rotations is worse than passive DVA toward the healthy ear and roll in either direction. Therefore, we would expect subjects with UVH to have symmetric DVA scores during pitch and roll rotations, but not yaw. Findings counter to this may suggest a component of malingering to the clinical presentation.
Limitations
Our study did not include subjects with bilateral vestibular hypofunction. Often, patients with BVH have asymmetric function, which might be distinguishable with passive DVA testing. If DVA could distinguish asymmetries within cardinal plane head impulses in subjects with BVH, this would add value to determining a valid prognosis and guide clinicians for choices in developing rehabilitation protocols (i.e., adaptation exercises with expectation for improving the lower aVOR gain vs. teaching safety precautions and choosing an assistive device).
Conclusion
Our results show that although the roll aVOR gain is under-compensatory, i.e., ∼30 % lower than the compensatory yaw aVOR gain, yet, there is no difference in the DVA scores between yaw and roll head impulses for healthy controls. This is explained by recognizing that roll head impulses cause minimal image displacement from the fovea, albeit the image does rotate on the fovea. Does target image rotation on the fovea result in reduced dynamic visual acuity? Our findings suggest that visual acuity is preserved as long as the target image stays on the fovea. Our study also shows that DVA testing during roll head impulses may not always reveal aVOR hypofunction. For subjects with UVH, DVA during passive yaw impulses is a better indicator of pathology than DVA during passive roll or passive pitch.
Acknowledgments
The authors would like to thank Mr Dale Roberts for technical assistance. MCS was supported by the NIDCD (K23-007926), AAM supported by the NHRMC (Australia, CDA568736) and DSZ was supported by the NEI (EY01849).
References
- Arbusow V, Schulz P, Strupp M, Dieterich M, Reinhardstoettner A, Rauch E, Brandt T. Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol. 1999;46(3):416–419. doi: 10.1002/1531-8249(199909)46:3<416::AID-ANA20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol. 1996;76(6):4009–4020. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- Demer JL, Oas JG, Baloh RW. Visual–vestibular interaction in humans during active and passive, vertical head movement. J Vestib Res. 1993;3(2):101–114. [PubMed] [Google Scholar]
- Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- Gonshor A, Jones GM. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976;256(2):361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Bruce EN, Huebner WP, Lanska DJ. Performance of the human vestibuloocular reflex during locomotion. J Neurophysiol. 1989;62(1):264–272. doi: 10.1152/jn.1989.62.1.264. [DOI] [PubMed] [Google Scholar]
- Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35(12):1727–1739. doi: 10.1016/0042-6989(94)00257-M. [DOI] [PubMed] [Google Scholar]
- Hepp K, Van Opstal AJ, Straumann D, Hess BJ, Henn V (1993) Monkey superior colliculus represents rapid eye movements in a two-dimensional motor map. J Neurophysiol. 69(3):965–79 [DOI] [PubMed]
- Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129(8):819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19(6):790–796. [PubMed] [Google Scholar]
- Hess K, Gresty M, Leech J. Clinical and theoretical aspects of head movement dependent oscillopsia (HMDO). A review. J Neurol. 1978;219(3):151–157. doi: 10.1007/BF00314530. [DOI] [PubMed] [Google Scholar]
- Mallinson AI, Longridge NS. A new set of criteria for evaluating malingering in work-related vestibular injury. Otol Neurotol. 2005;26(4):686–690. doi: 10.1097/01.mao.0000169639.48193.fb. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Santina CC, Carey JP, Minor LB, Zee DS. The effect of binocular eye position and head rotation plane on the human torsional vestibuloocular reflex. Vision Res. 2006;46(16):2475–2486. doi: 10.1016/j.visres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Della Santina CC. Adaptation of the vestibulo-ocular reflex for forward-eyed foveate vision. J Physiol. 2010;588(Pt 20):3855–3867. doi: 10.1113/jphysiol.2010.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Todd MJ. Real-time rotation vectors. Australas Phys Eng Sci Med. 1999;22(2):73–80. [PubMed] [Google Scholar]
- Misslisch H, Tweed D, Fetter M, Sievering D, Koenig E (1994) Rotational kinematics of the human vestibuloocular reflex. III. Listing’s law. J Neurophysiol 72(5):2490–502 [DOI] [PubMed]
- Misslisch H, Tweed D, Hess BJ. Stereopsis outweighs gravity in the control of the eyes. J Neurosci. 2001;21(3):RC126. doi: 10.1523/JNEUROSCI.21-03-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramat S, Zee DS. Ocular motor responses to abrupt interaural head translation in normal humans. J Neurophysiol. 2003;90(2):887–902. doi: 10.1152/jn.01121.2002. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res. 2006;175:471–484. doi: 10.1007/s00221-006-0567-7. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multiodal integration in the macaque vestibular system. J Neurosci. 2010;30(30):10158–10168. doi: 10.1523/JNEUROSCI.1368-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23(3):372–377. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Della Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7(4):329–338. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D, Zee DS, Straumann D. Torsional and horizontal vestibular ocular reflex adaptation: three-dimensional eye movement analysis. Exp Brain Res. 2003;152(2):150–155. doi: 10.1007/s00221-003-1460-2. [DOI] [PubMed] [Google Scholar]
- Straumann D, Zee DS, Solomon D, Lasker AG, Roberts RC. Transient torsion during and after saccades. Vision Res. 1995;35:3321–3334. doi: 10.1016/0042-6989(95)00091-R. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Straumann D, Henn V (2000) Effects of alertness on three-dimensional eye movements. Jpn J Ophthalmol 44:457–462 [DOI] [PubMed]
- Tian JR, Shubayev I, Demer JL. Dynamic visual acuity during transient and sinusoidal yaw rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2001;137(1):12–25. doi: 10.1007/s002210000640. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Grant MP, Buettner UW, Herdman SJ, Zee DS. The contribution of the vertical semicircular canals to high-velocity horizontal vestibulo-ocular reflex (VOR) in normal subjects and patients with unilateral vestibular nerve section. Acta Otolaryngol. 1996;116(4):507–512. doi: 10.3109/00016489609137881. [DOI] [PubMed] [Google Scholar]
- Tweed D, Vilis T (1990) Geometric relations of eye position and velocity vectors during saccades. Vision Res 30(1):111–27 [DOI] [PubMed]