SUMMARY
Cellular senescence suppresses cancer by arresting the proliferation of cells at risk for malignant transformation. Recently, senescent cells were shown to secrete numerous cytokines, growth factors and proteases that can alter the tissue microenvironment and may promote age-related pathology. To identify small molecules that suppress the senescence-associated secretory phenotype (SASP), we developed a screening protocol using normal human fibroblasts and a library of compounds that are approved for human use. Among the promising library constituents was the glucocorticoid corticosterone. Both corticosterone and the related glucocorticoid cortisol decreased the production and secretion of selected SASP components, including several pro-inflammatory cytokines. Importantly, the glucocorticoids suppressed the SASP without reverting the tumor suppressive growth arrest, and were efficacious whether cells were induced to senesce by ionizing radiation or strong mitogenic signals delivered by oncogenic RAS or MAP kinase kinase 6 overexpression. Suppression of the prototypical SASP component IL-6 required the glucocorticoid receptor, which, in the presence of ligand, inhibited IL-1α signaling and NF-κB transactivation activity. Accordingly, co-treatments combining glucocorticoids with the glucocorticoid antagonist RU-486 or recombinant IL-1α efficiently reestablished NF-κB transcriptional activity and IL-6 secretion. Our findings demonstrate feasibility of screening for compounds that inhibit the effects of senescent cells. They further show that glucocorticoids inhibit selected components of the SASP, and suggest that corticosterone and cortisol, two FDA-approved drugs, might exert their effects in part by suppressing senescence-associated inflammation.
Keywords: aging, cancer, inflammation, IL-6, IL-8, MMP-3
INTRODUCTION
Cellular senescence is a potent tumor suppressive mechanism that arrests the proliferation, essentially irreversibly, of cells at risk for malignant transformation (Campisi 2001; Collado & Serrano 2010). There is increasing evidence that the senescence response may be a double-edged sword, having both beneficial and deleterious effects (Campisi 2003; Adams 2009; Bartholomew et al. 2009; Coppé et al. 2010) owing to the complexity of the senescent phenotype (Campisi 2011; Rodier & Campisi 2011). This duality is consistent with the concept of evolutionary antagonistic pleiotropy (Williams 1957), which posits the existence of processes that are beneficial to young organisms but detrimental in old organisms. Thus, cellular senescence may protect organisms from cancer, especially early in life, but later in life it may promote pathologies associated with aging. This duality, and the complexity of the senescence response, suggests it may be challenging to develop drugs that selectively suppress the deleterious effects of cellular senescence, while preserving its beneficial effects.
Why might cellular senescence be antagonistically pleiotropic? The senescence growth arrest, which confers substantial protection against cancer, is clearly beneficial. However, an accumulation of growth-arrested cells can also limit tissue regeneration (Beausejour & Campisi 2006). Further, senescent cells secrete numerous cytokines, growth factors and proteases, which we term the senescence-associated secretory phenotype (SASP) (Coppe et al. 2008; Coppe et al. 2010). Depending on the physiological context, SASP components can be beneficial or deleterious. For example, SASP matrix metalloproteinases (MMPs) can limit fibrosis during tissue repair (Krizhanovsky et al. 2008; Jun & Lau 2010), but, in contrast, can disrupt normal tissue structure and function (Parrinello et al. 2005). SASP MMPs and other SASP components can also stimulate tumor growth in vivo (Krtolica et al. 2001; Liu & Hornsby 2007). Similarly, the SASP components interleukin (IL)-6 and IL-8 can reinforce the growth arrest of cells that senesce in response to activated oncogenes (Acosta et al. 2008; Kuilman et al. 2008), but these cytokines can also stimulate malignant phenotypes: epithelial-mesenchyme transitions, cell migration and invasiveness in susceptible premalignant or minimally malignant epithelial cells (Coppé et al. 2010; Laberge et al. in press).
Among the prominent SASP components are numerous proteins with pro-inflammatory activities (Davalos et al. 2010; Freund et al. 2010). Low-level, chronic inflammation is a hallmark of aging tissues, and inflammation is a major cause of, or contributor to, virtually every major age-related pathology, including cancer (Ferrucci et al. 2004; Franceschi et al. 2007; Chung et al. 2009; Davalos et al. 2010; Freund et al. 2010). Thus, senescent cells, which increase with age and at sites of age-related pathology, might stimulate local chronic inflammation and tissue remodeling, thereby fueling both the degenerative diseases of aging as well as age-related cancer. The recent demonstration that elimination of senescent cells in a progeroid mouse model prevented or significantly delayed the development of several age-related pathologies (Baker et al. 2011) strongly support the idea that cellular senescence is indeed causally implicated in generating aging phenotypes and limiting health span.
Given the potentially deleterious effects of the SASP, it might be clinically advantageous to identify means to modulate or selectively impair the SASP without affecting its beneficial effects, particularly the tumor suppressive growth arrest. Towards this end, we developed a method to screen small molecular weight compounds for abilities to selectively suppress the SASP, and identified two glucocorticoids that have this ability.
Glucocorticoids are a class of steroid hormones that includes cortisol, corticosterone, dexamethasone and related analogs, all of which have wide-ranging tissue-specific effects on metabolism and immune function (Gross & Cidlowski 2008; Zanchi et al. 2010). Accordingly, glucocorticoids are used to treat diverse medical conditions, including asthma, allergies, autoimmune diseases and certain cancers (Schlossmacher et al. 2011). Glucocorticoids have potent anti-inflammatory activities. They suppress inflammation mainly by either inducing immune cell apoptosis, or by activating or repressing genes encoding anti-inflammatory or pro-inflammatory cytokines, respectively. The latter activity is mediated by the ubiquitously expressed glucocorticoid receptor (GR), which exists in multiple isoforms and posttranslationally modified states (Zanchi et al. 2010; Oakley & Cidlowski 2011).
Here, we demonstrate that two glucocorticoids produced by the adrenal gland – cortisol, the primary glucocorticoid used by humans, and corticosterone, used primarily by rodents but produced as a steroidogenic intermediate in humans, can decrease the production and secretion of selected components of the SASP, including the pro-inflammatory prototypical SASP component IL-6. Repression of IL-6 production was due in large measure to the ability of the glucocorticoids to downregulate two important pathways that regulate the SASP: IL-1α signaling and NF-κB transcriptional activity. Our findings validate the feasibility of screening for novel and selective SASP modulators, and identify the GR as a new regulator of the phenotype.
RESULTS
Corticosterone and cortisol identified as compounds that suppress IL-6 secretion
To identify small molecules that are potential SASP modulators, we devised a screening strategy that entailed administering compounds to parallel 96-well plates containing human fibroblasts (strain HCA2) that were either quiescent or senescent (see Experimental Procedures). The compounds we tested comprised the Prestwick Chemical Library, a collection of approximately 1120 Federal Drug Administration-approved drugs. The compounds were added to duplicate wells at a single concentration (2.5 μM). After 48 h, we removed the medium from each well, and lysed the cells. We used ELISAs to assay the medium for IL-6, a major SASP factor, as an indication of whether a compound suppressed or enhanced the SASP. We assayed the cell lysates for ATP as a surrogate for cell number. The ATP assay allowed us to eliminate highly toxic compounds, or compounds that grossly altered cell number.
Of the 1120 drugs we tested, several suppressed IL-6 secretion without altering ATP levels. These drugs, then, were candidates for having the ability to suppress the SASP without causing cell toxicity or reversing the senescence growth arrest. Among these candidates were hormones of the glucocorticoid family, of which corticosterone was the most potent.
To confirm the ability of corticosterone to suppress senescence-associated IL-6 secretion, we prepared fresh HCA2 fibroblast cultures and induced senescence by X-irradiation (10 Gy). Under these conditions, cells arrest growth within 24–48 h, but require 4–5 d before SASP components are detected in the medium (Coppe et al. 2008; Rodier et al. 2009; Coppe et al. 2010; Freund et al. 2011). We added varying concentrations of corticosterone immediately after irradiation, and maintained the cells in the drug for 6 d. On the 6th day, we incubated the cells in serum-free medium with or without corticosterone, collected the conditioned medium 24 h later, and assayed the medium for IL-6 by ELISA. Corticosterone decreased IL-6 secretion in a dose-dependent manner (Fig. 1A). At 20 nM, corticosterone reduced IL-6 secretion by approximately 50%; maximal suppression (>90%) was achieved at 500 nM. The ability of corticosterone to suppress IL-6 secretion by senescent cells was not peculiar to HCA2 cells. A similar reduction was observed using another human fibroblast strain (IMR-90 from fetal lung) (Fig. S1A).
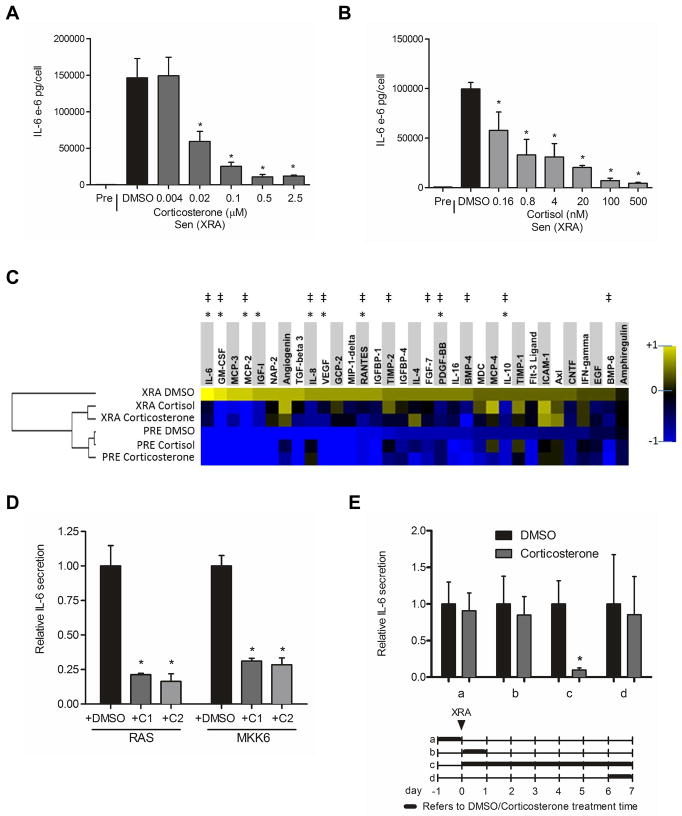
Figure 1. Corticosterone and cortisol partially suppress the SASP.
(A) Senescent (X-irradiated with 10 Gy, (Sen (XRA)) HCA2 fibroblasts were incubated in medium plus 10% serum containing the indicated concentrations of corticosterone or the highest concentration of DMSO (vehicle control). The cells were given corticosterone or DMSO immediately after irradiation and analyzed 6 d later. The cells were washed and incubated in serum-free medium without corticosterone to generate conditioned media. Conditioned media from presenescent (Pre) and control or corticosterone-treated Sen (XRA) cells were analyzed by ELISA for IL-6.
(B) Cells were treated and conditioned media were generated and analyzed as described in (A) except cortisol was used at the indicated concentrations.
(C) Conditioned media were collected from presenescent (PRE) or senescent (XRA) cells that were treated with DMSO, corticosterone (500 nM) or cortisol (100 nM) as described in (A). The conditioned media were analyzed by antibody arrays. We used the average signal from PRE and XRA DMSO cells as the baseline. Signals higher than baseline are yellow; signals lower than baseline are blue. Color intensities represent log2-fold changes from the average value. The hierarchical clustering relationship between samples is shown as a dendrogram (left). *, factors significantly (p<0.05) suppressed by cortisol. ‡, factors significantly (p<0.05) suppressed by corticosterone.
(D) Cells were infected with RAS- or MKK6-expressing lentiviruses. After selection, the cells were given DMSO-, 500 nM corticosterone (C1)- and 100 nM cortisol (C2) for 6 d. Conditioned media were generated as described above and analyzed by ELISA for IL-6. *, factors significantly different from DMSO-treated (p<0.05).
(E) Cells were treated with 500 nM corticosterone for the indicated intervals (a–d, indicated by the thick lines in the lower panel) before or after X-irradiation (XRA, indicated by the arrow). Conditioned media were prepared and analyzed by ELISA for IL-6 (upper panel). *, factors significantly different from DMSO-treated (p<0.05).
Corticosterone is the main GR Iigand in rodents and other species; however, in humans, the main GR ligand is the closely related glucocorticoid cortisol (Gross & Cidlowski 2008; Zanchi et al. 2010). We therefore tested cortisol for ability to suppress IL-6 secretion by human fibroblasts induced to senesce by X-irradiation. Cortisol decreased IL-6 secretion in a dose-dependent manner, and was more potent than corticosterone (Fig. 1B). Cortisol reduced senescence-associated IL-6 secretion by 50% at sub-nM concentrations (160–800 pM) and >90% at 100 nM.
To determine whether or to what extent corticosterone or cortisol suppressed the entire SASP, we used antibody arrays to interrogate the relative secretion of 120 cytokines and growth factors. We incubated presenescent and senescent cells with 500 nM corticosterone or 100 nM cortisol (Fig. 1C). Both glucocorticoids strongly suppressed the secretion of several pro-inflammatory cytokines and chemokines, including IL-6, IL-8, GM-CSF and MCP-2. In addition, they suppressed the secretion of several growth and angiogenic factors such as VEGF. Neither glucocorticoid suppressed all components of the SASP (Fig. 1C), and thus were selective SASP modulators.
The ability of corticosterone and cortisol to suppress senescence-associated IL-6 secretion was not limited to cells induced to senesce by X-irradiation. Both glucocorticoids were effective in cells induced to senesce by overexpression of oncogenic RAS or MKK6 (mitogen-activated protein kinase kinase 6) (Fig. 1D), which induce a growth arrest, cell enlargement, senescence-associated β-galactosidase (SA-Bgal) expression and a robust SASP (Coppe et al. 2008; Freund et al. 2011).
The suppression of IL-6 secretion by glucocorticoids required that the steroids be present for an extended period during which the SASP is being established. In irradiated cells, which induces senescence synchronously, the SASP takes 3–4 d, beginning 1–2 d after irradiation, to become established (Coppe et al. 2008; Rodier et al. 2009). Pretreating cells with corticosterone prior to inducing senescence by X-irradiation, or treating for only 24 h immediately following irradiation or after establishment of the SASP (7 d after irradiation), had no effect on IL-6 secretion (Fig. 1E). However, continuous exposure to corticosterone for 7 d after irradiation strongly suppressed IL-6 secretion (Fig. 1E).
In contrast to their effects on the SASP, corticosterone and cortisol had no effect on the fraction of cells that expressed SA-Bgal (Fig. S1B). In addition, neither glucocorticoid reversed the enlarged senescent morphology at any time during treatment, nor did either reverse the senescence growth arrest. Thus, cells made senescent by X-irradiation and treated with corticosterone or cortisol for 7 d maintained their low 24 h BrdU labeling index (Fig. S1C). Further, although the SASP depends on constitutive low level DNA damage response (DDR) signaling (Rodier et al. 2009) emanating from persistent DNA damage foci (Rodier et al. 2011), corticosterone and cortisol had no effect on the number of persistent DNA damage foci in the nuclei of cells induced to senescent by X-irradiation (Fig. S1D; S1E). Taken together, our data show that corticosterone and cortisol decrease the secretion of prominent SASP factors without affecting other prominent senescent phenotypes, including the growth arrest.
Glucocorticoids require the glucocorticoid receptor for ability to suppress the SASP
Because most SASP factors are upregulated at the level of mRNA abundance (Coppe et al. 2008; Coppe et al. 2010), we determined the effects of the glucocorticoids on the mRNA levels of several important SASP factors (IL-6, IL-8, MMP-3, IL-1α, MCP-2, MCP-3, GM-CSF). Whereas mRNA levels of the non-SASP factor IL-5 remained unchanged, all mRNAs encoding SASP factors were strongly reduced by corticosterone and cortisol (Fig. 2A), suggesting that the glucocorticoids act at the level of transcription.
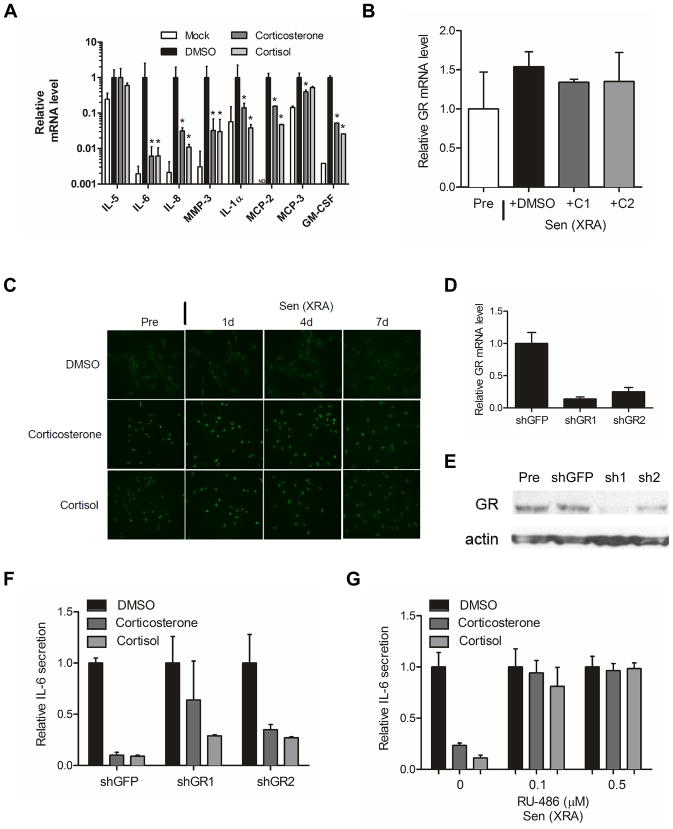
Figure 2. Effect of glucocorticoids on the SASP depends on the glucocorticoid receptor.
(A) mRNA was extracted from presenescent (Mock) or senescent X-irradiated HCA2 cells treated with DMSO, 500 nM corticosterone or 100 nM cortisol as described in the legend to Figure 1. Transcripts for IL-5, IL-6, IL-8, MMP-3, IL-1α, MCP-2, MCP-3 and GM-CSF were quantified by quantitative PCR (normalized to tubulin). *, factors significantly different from DMSO-treated (p<0.05).
(B) mRNA was extracted from Pre and Sen (XRA) cells treated with DMSO, 500 nM corticosterone (C1) or 100 nM cortisol (C2) as described above, and transcripts for the GR were quantified by PCR (normalized to tubulin). Although GR mRNA levels tended to be slightly elevated in senescent cells, the increase was not statistically significant.
(C) Pre and Sen (XRA) cells treated with DMSO, 500 nM corticosterone or 100 nM cortisol as described above were immunostained for GR 1, 4 and 7 d after X-irradiation.
(D) Cells were infected with lentiviruses expressing shRNAs against GFP (control) or GR, and selected. Seven days after selection, mRNA was extracted and transcripts for GR were quantified by PCR (normalized to tubulin).
(E) Total cell lysates were prepared from the shGFP- and shGR-expressing cells described in (D), and analyzed by western blotting for GR and actin (control).
(F) Cells infected with shGFP or shGR-expressing lentiviruses were X-irradiated and treated immediately thereafter with DMSO, 500 nM corticosterone or 100 nM cortisol. Conditioned media were collected 7 d later and analyzed by ELISA for IL-6.
(G) Cells were treated as described in (F) except for the addition of RU-486 at the indicated doses. Conditioned media were collected and analyzed by ELISA for IL-6 secretion.
Glucocorticoids are ligands for GR isoforms, which, upon ligand binding, translocate to the nucleus where they alter the transcription of numerous genes; most of the physiological effects of glucocorticoids depend on the GR (Gross & Cidlowski 2008; Zanchi et al. 2010; Oakley & Cidlowski 2011). We first asked whether GR expression changed as a consequence of senescence or addition of corticosterone or cortisol (Fig. 2B). GR mRNA levels appeared to slightly increase in senescent cells relative to presenescent cells (although these changes were not statistically significant), and were unaffected by glucocorticoid addition. The GR was largely cytoplasmic in presenescent cells, and remained cytoplasmic up to 7 d after the cells were induced to senesce by X-irradiation (Fig. 2C). However, the GR translocated into the nucleus in response to either corticosterone or cortisol (Fig. 2C), indicating that both these glucocorticoids can activate the GR. In contrast, the related mineralocorticoid receptor, which also binds cortisol and can physically interact with the GR, remained cytoplasmic after corticosterone or cortisol addition (Fig. S2A). Thus, corticosterone and cortisol each specifically induce GR nuclear localization in senescent HCA2 cells.
To test the idea that the ability of corticosterone and cortisol to suppress the expression of selected SASP components was mediated by the GR, we used RNA interference (RNAi) and lentiviruses that express short hairpin (sh) RNAs designed to deplete cells of the GR. Quantitative PCR and western blotting confirmed that two distinct shRNAs reduced GR mRNA and protein levels (Fig. 2D; 2E). GR depletion partially rescued the suppression of IL-6 secretion by corticosterone and cortisol (Fig. 2F). This partial rescue may be due to incomplete GR depletion by the shRNAs (Fig. 2D; 2E). Consistent with these results, co-treatment of senescent cells with corticosterone or cortisol plus the glucocorticoid antagonist RU-486 (Cadepond et al. 1997) rescued the senescence-associate IL-6 secretion that was suppressed by the glucocorticoids (Fig. 2G). RU-486 blocked this glucocorticoid activity without affecting GR nuclear translocalization (Fig. S2B).
Taken together, these results show that both corticosterone and cortisol induced GR nuclear translocalization in senescent cells. Moreover, because genetic or pharmacological inhibition of the GR rescued the suppression of senescence-associated IL-6 secretion by glucocorticoids, the results suggest the GR is required for the suppressive effects of glucocorticoids in senescent cells.
Glucocorticoids suppress the expression of IL-1α, an upstream SASP regulator
We previously showed that IL-1α is a critical upstream regulator of the SASP. IL-1α establishes and maintains the SASP by activating the transcription factor nuclear factor-kappa B (NF-κB) (Orjalo et al. 2009; Freund et al. 2011), which further stimulates IL-1α transcription, thereby establishing a positive feedback loop (Freund et al. 2010). We therefore asked whether glucocorticoids suppressed the SASP by interfering with IL-1α expression.
IL-1α mRNA rose rapidly after cells were induced to senesce by X-irradiation (Fig. 3A). When added at the time of irradiation, both corticosterone and cortisol delayed this rise, as well as the later rise in IL-6 mRNA (Fig. 3A; 3B). Further, the glucocorticoids continued to suppress IL-1α and IL-6 mRNA levels (<10% of control) for at least 7 d after irradiation, at which time the SASP is normally fully developed (Coppe et al. 2008; Rodier et al. 2009).
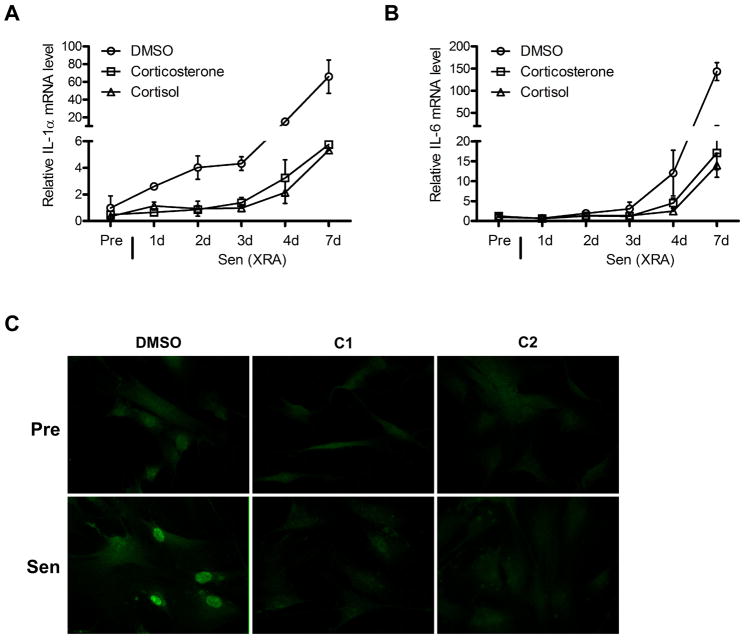
Figure 3. Glucocorticoids repress IL-1α expression.
(A) Presenescent (Pre) HCA2 cells were treated with DMSO, 500 nM corticosterone or 100 nM cortisol for 24 h, or were induced to senesce by X-irradiation (Sen (XRA)) and given DMSO, corticosterone or cortisol immediately thereafter. mRNA was extracted after the indicated intervals and transcripts for IL-1α were quantified by PCR (normalized to tubulin).
(B) mRNA extracted from cells described in (A) was used to quantify transcripts for IL-6 (normalized to tubulin).
(C) Pre and Sen (XRA) cells, prepared as described in (A), were immunostained for IL-1α. Sen (XRA) cells were immunostained 7 d after irradiation.
IL-1α localizes to both the plasma membrane and the nucleus (Werman et al. 2004; Orjalo et al. 2009). Consistent with the suppression of IL-1α mRNA levels, corticosterone and cortisol also suppressed expression of IL-1α protein, which was visible as strong nuclear staining in control, but not glucocorticoid-treated, senescent cells (Fig. 3C).
Glucocorticoids impair the IL-1α/NF-κB pathway
To determine whether glucocorticoids suppress the SASP by suppressing IL-1α signaling, we measured the abundance of interleukin-1 receptor-associated kinase 1 (IRAK1) and IκBα, an inhibitor of NF-κB. Both these proteins are key components of IL-1α/IL-1 receptor (IL-1R) signaling (Perkins 2007; Gottipati et al. 2008), and are rapidly degraded after the IL-1R is engaged by IL-1α (Perkins 2007; Gottipati et al. 2008; Orjalo et al. 2009). IRAK1 and IκBα were much less abundant in senescent, compared to presenescent, cells, indicating active IL-1R signaling in senescent cells (Fig. 4A). The abundance of RelA, an NF-κB subunit, was unchanged. Consistent with the suppression of IL-1α production and blockade of IL-1R signaling, corticosterone and cortisol restored IRAK1 and IκBα proteins to near-presenescent levels (Fig. 4A).
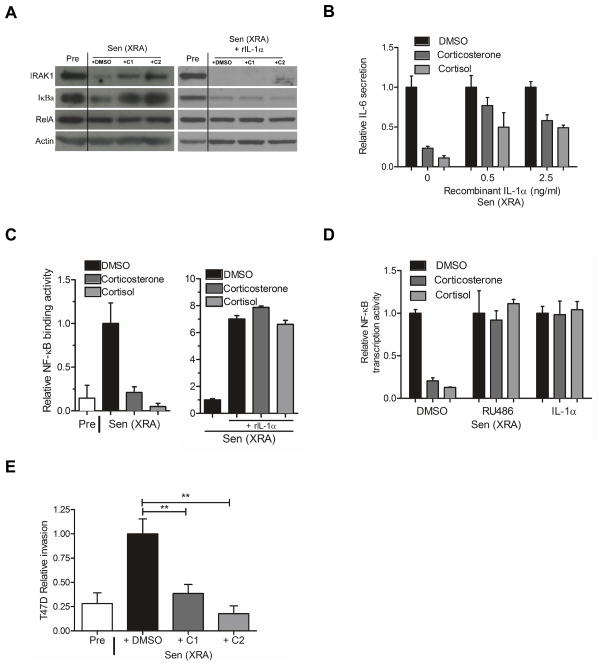
Figure 4. Glucocorticoids impair the IL-1α/NF-κB pathway and suppress the ability of the SASP to induce tumor cell invasion.
(A) Total HCA2 cell lysates were prepared from presenescent (Pre) cells, or senescent cells (Sen (XRA)) cells treated with DMSO-, 500 nM corticosterone (C1)-, or 100 nM cortisol (C2) in the absence (left panel) or presence (right panel) of recombinant IL-1α protein (rIL-1α). The lysates were analyzed by western blotting for IRAK1, IkBα, RelA and actin (control).
(B) After irradiation, Sen (XRA) cells were given DMSO, 500 nM corticosterone or 100 nM cortisol. Six d later, the cells were given recombinant IL-1α protein at the indicated doses in the presence of the glucocorticoids in serum free media. Conditioned media were collected 24 h later and analyzed by ELISA for IL-6.
(C) Nuclear extracts were prepared from Pre cells, and Sen (XRA) cells treated with DMSO, 500 nM corticosterone or 100 nM cortisol in the absence (left panel) or presence (right panel) of recombinant IL-1α protein (rIL-1α), and analyzed for NF-κB DNA binding activity.
(D) Cells were infected with a lentivirus carrying an NF-κB-luciferase reporter construct, irradiated, and allowed to senesce. Immediately after irradiation, cells were treated with DMSO, 500 nM corticosterone or 100 nM cortisol, plus 0.5 μM RU-486 or 2.5 ng/ml IL-1α, as indicated. Seven d after irradiation, cells were trypsinized, counted, lysed and assayed for luciferase activity, which was normalized to cell number.
(E) We prepared conditioned media from presenescent (Pre) cells or senescent cells (Sen (XRA) that had been treated with corticosterone (C1) or cortisol (C2) as described in the legend to Figure 1. The conditioned media were then assayed for ability to stimulate T47D human breast cancer cells to invade a basement membrane, as described in the Experimental Procedures.
However, addition of recombinant IL-1α (rIL-1α), which can rescue, at least partially, IL-6 secretion in glucocorticoid-treated cells (shown below), triggered degradation of IRAK1 and IkBα in senescent cells, confirming active IL-1R signaling. Moreover, the glucocorticoids had no effect on IκBα mRNA levels (Fig. S3), suggesting they acted indirectly to reduce SASP protein levels and consistent with their effect on IL-1α mRNA levels.
Recombinant IL-1α indeed rescued the suppression of IL-6 secretion by corticosterone and cortisol (Fig. 4B), consistent with the idea that glucocorticoids suppress SASP components such as IL-6 by targeting IL-1α/IL-1R signaling. Because GRs are known to modulate NF-κB activity, one potential mechanism by which glucocorticoids might act in this regard is by inhibiting NF-κB activity. In support of this model, corticosterone and cortisol significantly decreased both NF-κB DNA binding and transactivation activity in senescent cells (Fig. 4C; 4D). Addition of rIL-1α significantly increased NF-κB binding activity in glucocorticoid-treated senescent cells (Fig. 4C), with NF-κB showing binding activity comparable to DMSO-treated senescent cells. Further, co-treatment of senescent cells with either of the glucocorticoids plus RU-486 or recombinant IL-1α rescued NF-κB transactivation activity (Fig. 4D). Thus, glucocorticoids appear to suppress the SASP at least in part by preventing establishment of the IL-1α/NF-κB positive feedback loop that ultimately drives the expression and secretion of SASP components.
Glucocorticoids suppress the ability of the SASP to stimulate tumor cell invasion
Senescent cells secrete factors that can stimulate aggressive cancer-associated phenotypes in premalignant or malignant cells (Krtolica et al. 2001; Liu & Hornsby 2007; Coppe et al. 2008; Bartholomew et al. 2009; Coppe et al. 2010). Among these factors are the important interleukins IL-6 and IL-8 (Coppe et al. 2008). We therefore asked whether glucocorticoids suppressed the ability of the SASP to stimulate non-aggressive human breast cancer cells (T47D) to invade a basement membrane in Boyden chambers. Conditioned media prepared from presenescent cells stimulated minimal invasion by T47D cells, whereas media from senescent cells stimulated 4-fold more invasion (Fig. 4E), as expected. To test effects of glucocorticoids, we treated senescent cells with the drugs for 10 d, thoroughly washed the cells, then isolated conditioned media over the next 24 h. Both corticosterone and cortisol treatment reduced the ability of senescent conditioned media to stimulate T47D invasiveness to near-presenescent levels. Thus, in addition to suppressing the secretion of multiple SASP factors, the glucocorticoids suppressed an important biological property of the SASP.
DISCUSSION
Our results demonstrate the feasibility of screening for compounds that selectively reduce the secretion of proteins secreted by senescent cells, including the secretion of pro-inflammatory cytokines. The dual approach of assaying cellular ATP levels to detect substantial cell loss or gain coupled to ELISAs for the prototypical SASP protein IL-6 allowed us identify compounds with potential SASP-suppressing activity, but without gross toxicity or, equally importantly, the ability to reverse the senescence growth arrest.
From the library we screened, we identified two glucocorticoids, corticosterone and cortisol, which were active at doses that could be achieved therapeutically. Both glucocorticoids induce nuclear translocation of the GR, which suppressed IL-1α signaling by inhibiting NF-κB DNA binding and transactivation activity. The glucocorticoid antagonist RU-486 (Cadepond et al. 1997), which competes with corticosterone and cortisol for binding to the GR, blocked the effects of corticosterone and cortisol. Further, addition of recombinant IL-1α to cells rescued the repressive effects of corticosterone and cortisol on NF-κB activities, and consequently IL-6 secretion, thus establishing IL-1α as an important target of glucocorticoid action in senescent cells.
Glucocorticoid-mediated suppression of secretion of the interleukins IL-6 and IL-8 was insufficient to bypass the senescence growth arrest. These results are in agreement with our previous findings (Coppe et al. 2008; Freund et al. 2011), although they differ from results reported by other groups (Acosta et al. 2008; Kuilman et al. 2008). This discrepancy can be explained by the fact that the other groups used a growth factor signaling oncogene, specifically the downstream mediators of RAS signaling BRAF and MEK, to induce senescence, whereas we used X-irradiation and RAS expression to induce senescence. Both X-irradiation and RAS expression generate persistent DNA damage (Di Micco et al. 2006; Rodier et al. 2009), in particular DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS), which are required to maintain the DNA damage response (DDR) signaling that maintains the senescence growth arrest (Rodier et al. 2011). BRAF and MEK may not lock the senescence state similarly because the amount of DNA-damage is too low (Rodier et al. 2011; Tu et al. 2011). Thus, IL-6 and IL-8 suppression may not be able to bypass all forms of senescence.
Our findings are consistent with our previous results which identified IL-1α as an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network (Orjalo et al. 2009). The senescence-induced increase in IL-1α expression causes the membrane-bound form of IL-1α to activate its receptor IL-1R in a juxtacrine fashion, triggering NF-κB to produce more IL-1α. This positive feedback loop leads to an increase in NF-κB activation and, consequently, the transcription of several SASP factors. Our findings here show that glucocorticoids, acting via the GR, prevent the establishment of this positive feedback loop by impairing IL-1α expression, thereby decreasing numerous components of the SASP. Once established, however, the feedback loop appears to be unaffected by glucocorticoids. Thus, the transcriptional landscape that allows establishment of the SASP may differ from the transcriptional landscape that maintains it.
Notable features of the glucocorticoids were their ability to prevent some, but not all, of the factors that comprise the SASP. We cannot rule out the possibility that, despite washing the glucocorticoid-treated cells prior to collecting conditioned media, some of the drugs leached from the washed cells into the conditioned media to exert independent effects on cancer cell invasiveness. However, our prior results identified IL-6 and IL-8 as critical mediators of the effects of senescent conditioned media on cancer cell phenotypes (Coppe et al. 2008), and both these SASP factors were suppressed by the glucocorticoids.
Given that the SASP can have beneficial or deleterious effects, depending on the physiological context, corticosterone and cortisol may exemplify a class of drug that might be clinically useful for conditions under which the SASP is thought to be harmful. For example, DNA damaging radio- and chemo-therapies can induce a SASP in vivo (Coppe et al. 2008), which can have deleterious systemic effects, as well as the ability to stimulate the re-growth of tumor cells that were not eradicated by the anti-cancer therapy. While corticosterone and cortisol per se have their own side effects (Moghadam-Kia & Werth 2010), the use of similar compounds immediately or shortly following the radio- or chemo-therapy might alleviate some of the undesirable systemic effects and possibly increase cancer-free survival rates after treatment without compromising other vital processes such as tissue repair.
EXPERIMENTAL PROCEDURES
Cell cultures and reagents
HCA2 human neonatal foreskin, IMR-90 human fetal lung fibroblasts and T47D human breast cancer cells were obtained and cultured in 3% O2 and 10% CO2 as previously described (Coppe et al. 2008; Rodier et al. 2009; Coppe et al. 2010). Cells were induced to senesce by X-irradiation (10 Gy) or lentiviral expression of oncogenic RAS or MAP kinase kinase 6 (MKK6), as described (Coppe et al. 2008; Rodier et al. 2009; Freund et al. 2011). Presenescent and senescent cells had 24-h BrdU labeling indices of >75% and <10% respectively (Rodier et al. 2009); <10% and >70% respectively stained positive for senescence-associated beta-galactosidase activity (Dimri et al. 1995) (Biovision senescence detection kit). HEK293FT packaging cells (Invitrogen) were used to generate lentiviruses. Corticosterone, cortisol and RU-486 were from Sigma-Aldrich.
Viral vectors and infection
Lentiviruses encoding oncogenic RAS and MKK6 were described (Coppe et al. 2008; Freund et al. 2011). Lentiviruses encoding shRNAs against GFP (control) and the GR were purchased from Open Biosystems. The lentiviral NF-κB reporter-luciferase construct was purchased from SA Biosciences. Lentiviruses were produced and used as described (Coppe et al. 2008; Freund et al. 2011). To limit side effects of infection, viral titers were adjusted to infect 90% of cells, and cultures were subsequently selected in 1 μg/ml puromycin for 3 d.
Initial drug screening
The initial drug screen was performed in a 96-well format using automated liquid handling with a Biomek FX (Beckman Coulter, CA). Senescent cells were plated 24 h after X-irradiation at 7,500 cells per well in 96-well plates. Six days after plating the senescent cells, the presenescent cells were plated at 7,500 cells per well in 96-well plates. Twenty-four hours after presenescent plating, both presenescent and senescent cells were washed and incubated in low (0.2%) serum for 48 h to arrest cell proliferation of the presenescent cells. Drugs from the Prestwick Chemical Library, which contains 1120 bio-available compounds in DMSO, were given to the cells at 2.5 μM in media containing 0.2% serum. Forty-eight hours after compound addition, the medium in each well was removed and frozen for assay by ELISA to quantitate the levels of IL-6. The cells, which remained in the wells after the medium was removed, were lysed and ATP levels were measured (ATPlite 1-step assay, Perkin Elmer, MA) to exclude compounds that lowered IL-6 through toxicity (cell death). Experimental wells in each plate were normalized to plate mean or same-plate DMSO controls for the ELISA and ATP assays, respectively.
Subsequent treatments with glucocorticoids
To validate glucocorticoids as SASP regulators, we added them within 15 min after irradiation (unless otherwise indicated). For cells induced to senesce by MKK6 or RAS overexpression, glucocorticoid treatment started 16 h after infection. Glucocorticoids were re-added in fresh media every other day. Six days after irradiation or selection, cells were given serum-free DMEM with or without glucocorticoid for 24 h; the conditioned media were collected and frozen for ELISAs.
Real-time quantitative PCR
Cells (7,500/well) in 96-well plates were lysed and reverse transcribed using the Cells-To-Ct kit (Ambion). Quantitative PCR was performed using the Roche Universal ProbeLibrary (UPL) and following primer-probe combinations: Tubulin-A (Probe 58; F:5′CTTCGTCTCCGCCATCAG3′, R:5′TTGCCAATCTGGACACCA3′), IL-6 (Probe 45; F:5′GCCCAGCTATGAACTCCTTCT, R:5′GAAGGCAGCAGGCAACAC), IL-8 (Probe 72; F:5′AGACAGCAGAGCACACAAGC3′, R:5′ATGGTTCCTTCCGGTGGT3′), MMP-3 (Probe 36; F:5′CAAAACATATTTCTTTGTAGAGGA CAA, R:5′TTCAGCTATTTGCTTGGGAAA3′), GR (Probe 34; F:5′GAAAGCCACGCTCCCTTC3′, R:5′AGACTTAGGTGAAACTGGAATTGCT3′), IL-1α (Probe 6; F:5′GGTTGAGTTTAAGCCAATC CA3′, R:5′TGCTGACCTAGGCTTGATGA3′), IκBα (Probe 86; F:5′GGTGCTGATGTCAATGC TCA3′, R:5′ACACCAGGTCAGGATTTTGC3′).
Western blotting
Cells were lysed in RIPA buffer. Lysates were sonicated (10 sec), followed by centrifugation. Samples were incubated at 70° C for 10 min, loaded on 4–15% gradient tris-glycine SDS-polyacrylamide gels (Invitrogen) and separated by electrophoresis. Proteins were transferred to PVDF membranes, blocked in TBST 5% milk for 1 h at room temperature, and probed overnight at 4° C with primary antibodies in blocking buffer. Membranes were washed in TBST, and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Blots were developed using Western detection substrate (GE Healthcare).
Immunofluorescence
Cells were cultured in 8-well chamber slides, fixed in 4% formaldehyde (Sigma) for 10 min at 4° C and permeabilized in PBS-0.5% Triton for 10 min in 4° C. Slides were blocked for 30 min in 4% goat serum (Invitrogen). Primary antibodies were diluted in blocking buffer and incubated with cells for 1 h at room temperature. Cells were washed, incubated with secondary antibodies for 30 min at room temperature, washed and mounted with slow-fade gold (Molecular Probes). Images were acquired using an Olympus BX20 fluorescence microscope with the spotfire software (Diagnostics Instruments) and processed with Photoshop CS (Adobe).
Antibodies
Primary antibodies and dilutions were: anti-GR (SC-8992, Santa Cruz; 1:500), anti-actin (ab6276, Abcam; 1:50000), anti-MCR (SC-11412, Santa Cruz; 1:500), anti-IRAK1 (SC-5288, Santa Cruz; 1:500), anti-IκBα (#9247, Cell Signaling; 1:500), anti-RelA (SC-109, Santa Cruz; 1:500), and anti-53BP1 (A300-272A, Bethyl; 1:500). Secondary antibodies used for western analysis were: goat anti-mouse IgG HRP conjugate (#170-5047, BioRad; 1:5000), and goat anti-rabbit IgG HRP conjugate (#166-2408, BioRad; 1:5000). Secondary antibody used for immunostaining was Alexa Fluor 488 goat anti-rabbit IgG (#A11008, Invitrogen; 1:750).
NF-κB binding activity and transactivation assays
We prepared nuclear extracts using the nuclear extract kit (Active Motif), and determined NF-κB DNA binding using the TransAM NF-κB p65 kit (Active Motif). For transactivation assays, cells infected with the NF-κB reporter-luciferase lentivirus were lysed in buffer (Promega), and luciferase activity was normalized to cell number, as described (Freund et al. 2011).
Antibody arrays
Cultures were washed and incubated in serum-free DMEM for 24 h and the conditioned media were diluted to equivalent cell numbers using DMEM. Antibody arrays from Raybiotech (AAH-CYT-G1000-8) were used according to the manufacturer’s instructions. Arrays were scanned using a GenePix 4200A Professional microarray scanner. Signal intensities were quantitated using LI-COR Odyssey software and normalized to positive controls for each sample, which were then normalized across all samples, as previously described (Freund et al. 2011).
ELISA
Conditioned media were filtered and stored at −80° C. Cell numbers were determined in every experiment. ELISAs were performed using kits and procedures from PerkinElmer (IL-6 AL223F). Data were normalized and expressed as pg/ml/cell/24h.
Invasion assay
T47D human breast cancer cells (120,000 cells/well) were plated atop a layer of Matrigel in the upper chambers of Transwells (BD Biosciences). The lower chambers were filled with conditioned media (lacking glucocorticoids) from presenescent or senescent HCA2 fibroblasts previously treated with corticosterone or cortisol for 10 d. After 18 h, cells that migrated to the underside of the upper chamber filter were stained and counted, as described (Coppe et al. 2008; Coppe et al. 2010).
Statistical analysis
Error bars on all graphs represent the standard deviation of at least 3 independent measurements. For the antibody array, statistical significance between distributions of signals was evaluated using a two-tailed Student’s t-test and assumption of equal variance with three conditioned medium samples per condition.
Supplementary Material
(A) IMR-90 fibroblasts were induced to senesce by X-irradiation (10 Gy; Sen (XRA)) and treated immediately after irradiation with the indicated concentrations of corticosterone or the highest concentration of DMSO (vehicle control) for 7 d. Conditioned media from presenescent (Pre) and the control and glucocorticoid-treated Sen (XRA) cells were analyzed by ELISA for IL-6.
(B) Sen (XRA) HCA2 cells were treated with DMSO, 500 nM corticosterone (C1) or 100 nM cortisol (C2) for 7 d. The percentage of presenescent (Pre) and Sen (XRA) cells that express SA-Bgal were scored (upper panel). A representative field corresponding to each condition is also shown (bottom panels).
(C) The Pre and Sen (XRA) HCA2 cells described in (B) were given BrdU for 24 h, fixed and immunostained for nuclear BrdU staining, and then analyzed for the percentage of BrdU-positive cells.
(D) The Pre and Sen (XRA) HCA2 cells described in (B) were immunostained for 53BP1. The percentage of cells with >2 53BP1 nuclear foci was determined using CellProfiler software. At least 200 cells were analyzed per condition.
(E) The average number of 53BP1 foci from (D) was determined using the CellProfiler software.
(A) Presenescent (A) or Sen (XRA) HCA2 cells were immunostained for the mineralocorticoid receptor. Sen (XRA) cells were given DMSO, 500 nM corticosterone or 100 nM cortisol immediately after irradiation, and immunostained 1 or 7 d thereafter. (B) Sen (XRA) HCA2 cells were treated with DMSO, 500 nM corticosterone, or 100 nM cortisol in the presence or not (−) of RU486, and immunostained for the GR.
We extracted mRNA from Pre HCA2 cells treated with DMSO, 500 nM corticosterone or 100 nM cortisol for 24 h, and Sen (XRA) HCA2 cells treated with these compounds for 7 d starting immediately after X-irradiation. We analyzed the extracts for IκBα transcripts by quantitative PCR (normalized to tubulin). The level of IκBα mRNA in DMSO-treated Pre cells was arbitrarily assigned a value of 1.
Acknowledgments
This work was supported by funds from the Buck Institute for Research on Aging (to REH), and grants from the Dutch Cancer Society (to PLJK), the Ministry of Science and Technology of China (2012CB911203 to YSC) and the US National Institutes of Health (AG025901 to PK and JC, and AG09909 and AG017242 to JC).
Footnotes
The authors report no financial or other conflict of interest relevant to the subject of this article.
AUTHOR CONTRIBUTIONS
RML, LZ, MRS, FR and AF designed and performed the experiments, analyzed the data and wrote the manuscript; PLJK, SL and MD performed the experiments and analyzed the data; YSC, PK and PYD analyzed the data and wrote the manuscript; REH and JC designed the experiments, analyzed and interpreted the data and wrote the manuscript.
References
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Furnagalli M, DaCosta M, Brown C, Popov N, Takastu, Yabuta N, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Adams PD. Healing and hurting: molecular mechanisms, functions and pathologies of cellular senescence. Molec Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–2886. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends in Cell Biology. 2001;11:27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: Rival demons? Nature Rev Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour C, Parrinello S, Hodgson G, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz D, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell non-automous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d’Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith OM, Peacocke M, Campisi J. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004;16:240–243. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo A, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Molec Med. 2010;16:238–248. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil PK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipati S, Rao NL, Fung-Leung WP. IRAK1: a critical signaling mediator of innate immunity. Cell Signal. 2008;20:269–276. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: a family affair. Trends Endocrinol Metab. 2008;19:331–339. doi: 10.1016/j.tem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, AAL, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Laberge RM, Awad P, Campisi J, Desprez PY. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. doi: 10.1007/s12307-011-0069-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Moghadam-Kia S, Werth VP. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. 2010;49:239–248. doi: 10.1111/j.1365-4632.2009.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nature Rev Molec Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour C, Kim SH, Davalos AR, Campisi J. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher G, Stevens A, White A. Glucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cells. J Endocrinol. 2011;211:17–25. doi: 10.1530/JOE-11-0135. [DOI] [PubMed] [Google Scholar]
- Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, Yen TJ, Zhang R. Oncogenic Ras Regulates BRIP1 Expression to Induce Dissociation of BRCA1 from Chromatin, Inhibit DNA Repair, and Promote Senescence. Dev Cell. 2011;21:1077–1091. doi: 10.1016/j.devcel.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH. Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol. 2010;224:311–315. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) IMR-90 fibroblasts were induced to senesce by X-irradiation (10 Gy; Sen (XRA)) and treated immediately after irradiation with the indicated concentrations of corticosterone or the highest concentration of DMSO (vehicle control) for 7 d. Conditioned media from presenescent (Pre) and the control and glucocorticoid-treated Sen (XRA) cells were analyzed by ELISA for IL-6.
(B) Sen (XRA) HCA2 cells were treated with DMSO, 500 nM corticosterone (C1) or 100 nM cortisol (C2) for 7 d. The percentage of presenescent (Pre) and Sen (XRA) cells that express SA-Bgal were scored (upper panel). A representative field corresponding to each condition is also shown (bottom panels).
(C) The Pre and Sen (XRA) HCA2 cells described in (B) were given BrdU for 24 h, fixed and immunostained for nuclear BrdU staining, and then analyzed for the percentage of BrdU-positive cells.
(D) The Pre and Sen (XRA) HCA2 cells described in (B) were immunostained for 53BP1. The percentage of cells with >2 53BP1 nuclear foci was determined using CellProfiler software. At least 200 cells were analyzed per condition.
(E) The average number of 53BP1 foci from (D) was determined using the CellProfiler software.
(A) Presenescent (A) or Sen (XRA) HCA2 cells were immunostained for the mineralocorticoid receptor. Sen (XRA) cells were given DMSO, 500 nM corticosterone or 100 nM cortisol immediately after irradiation, and immunostained 1 or 7 d thereafter. (B) Sen (XRA) HCA2 cells were treated with DMSO, 500 nM corticosterone, or 100 nM cortisol in the presence or not (−) of RU486, and immunostained for the GR.
We extracted mRNA from Pre HCA2 cells treated with DMSO, 500 nM corticosterone or 100 nM cortisol for 24 h, and Sen (XRA) HCA2 cells treated with these compounds for 7 d starting immediately after X-irradiation. We analyzed the extracts for IκBα transcripts by quantitative PCR (normalized to tubulin). The level of IκBα mRNA in DMSO-treated Pre cells was arbitrarily assigned a value of 1.