Abstract
Background
Eczema prevention is now an active area of dermatologic and allergic research. Defining an incident case is therefore a prerequisite for such as study.
Objective
We sought to examine how an incident case of atopic dermatitis was defined in previous atopic dermatitis prevention studies in order to make recommendations on a standard definition of new atopic dermatitis cases for use in future prevention trials.
Methods
We conducted a systematic review of controlled interventional atopic dermatitis prevention studies using searches of Medline and Cochrane databases from 1980 to the end of January 2011. Studies that included atopic dermatitis as a secondary outcome, such as asthma prevention trials, were included.
Results
One hundred and two (102) studies were included in the final analysis, of which 27 (26.5%) did not describe any criteria for defining an incident case of atopic dermatitis. Of the remaining 75 studies with reported disease criteria, the Hanifin-Rajka criteria were the most commonly used (28 studies). A disease definition unique to that particular study (21 studies) was the second most commonly used disease definition, although the sources for such novel definitions were not cited.
Conclusions
The results from this systematic review highlight the need for improved reporting and standardization of the definition used for an incident case in atopic dermatitis prevention studies. Most prevention studies have used disease definitions such as the Hanifin-Rajka criteria that include disease chronicity. While acceptable for cumulative incidence outcomes, inclusion of disease chronicity precludes the precise measurement of disease onset. We propose a definition based on existing scientific studies that could be used in future prospective studies.
Keywords: outcomes, atopic dermatitis, eczema, systematic review, definition, incident case, disease criteria
INTRODUCTION
Atopic dermatitis (AD) has a world-wide distribution with a prevalence as high as 22% in developed countries.1 In many areas of the developing world, eczema represents a growing public health problem, especially in younger populations. The development of AD increases one’s risk for the development of food allergies, allergic rhinitis, and asthma as a child grows. Because of the high disease prevalence and the link with these other allergic diseases, AD prevention strategies could yield substantial public health benefits. Despite decades of research, primarily in allergen avoidance, no prevention strategy for AD has proven consistently effective.2,3 New discoveries such as identifying genes, which predispose to the dry skin and impaired barrier associated with AD, opens up the possibility of new prevention strategies that need to be evaluated through rigorous randomized controlled prevention trials.
Research into the prevention of AD has been hampered by inconsistency in study methodology, especially with regards to methods for defining high-risk populations and disease outcomes.4-6 Although there are well-established validated definitions for diagnosing established AD, there are no standardized definitions for defining an incident case as is needed for longitudinal birth cohort studies or interventional prevention studies.
The problem is not a straightforward one since many definitions of AD include chronicity as a diagnostic criterion – clearly unsuitable for defining an incident case. While many prevention studies measure cumulative incidence rates at one or two years, a more precise determination of the date of onset of AD is especially important when evaluating prevention strategies that may only delay the onset of disease. Identifying strategies that even delay the disease onset would still have a significant public health impact, given the high prevalence of AD and the finding that the earlier onset disease predicts a more severe disease course.7 We sought to conduct a systematic review of how an incident case of AD has been defined in previous primary prevention studies of AD using systematic review methodology.
METHODS
Included studies
Prospective, interventional, prevention trials published after 1980, which specified AD as an outcome, were eligible. Studies whose primary outcome was not AD, such as asthma prevention trials, but included AD as a secondary outcome, were included. There were no age or language restrictions. Observational cohort studies were excluded as these studies most often use cumulative incidence as an outcome. Current validated definitions for the diagnosis of AD are suitable for the measurement of cumulative incidence with a maximum precision of one year. We aimed to investigate incident case definitions that would capture cases as they occur in a primary prevention trial enabling a time of disease onset to be determined.
Information sources
Studies were identified by searching electronic databases, scanning reference lists of articles and AD reviews, and consultation with experts in the field. The search was applied to Medline (1980-current), Cochrane (1980-current) and the last database search was run on January 5, 2011. Multiple articles were identified through hand searching, predominantly of reviews identified in search results, reviewing the literature, and from the NHS Evidence mapping exercise of systematic reviews for AD prevention.8
Search String
See Figure 1.
Figure 1.

Search Strings for Medline and Cochrane databases
Study selection
All database search results were entered into RefWorks (“Refwork Co,” U.S.), where duplicates were removed. Screening for eligibility was performed independently in a standardized manner by two reviewers (ELS and LEK) based upon titles and abstracts. Discrepancies between reviewers were resolved by consensus. Full-text articles for all studies past screening were obtained. Full-text copies of studies with seemingly eligible titles but without abstracts were automatically included and scrutinized further for eligibility. All full-text articles were assessed for eligibility by one reviewer prior to data collection. Of note, if multiple publications of a larger longitudinal trial were identified, they were counted as only one definition unless the definitions significantly differed between publications, in which case they were kept separate.
Data collection process / items
Data collection was performed by one reviewer using a data extraction database created for the study. Information extracted from each trial included the type of intervention and the AD definition used. One author (LEK) extracted the above data from the included studies and a second reviewer (ELS) double-checked all verbatim definitions that were considered to lack a true definition of AD. Disagreements were resolved by discussion between the two reviews and consultation with a third arbitrating author.
Summary measures
The primary outcome measured was the presence of any specified definition for AD.
RESULTS
Study selection
The study selection procedure is summarized in the flowchart (Figure 2). A total of 108 articles met the criteria for inclusion. Seven of these were multiple publications from the same longitudinal study, so this definition was only counted once, resulting in 102 distinct studies for inclusion in the analysis.
Figure 2.
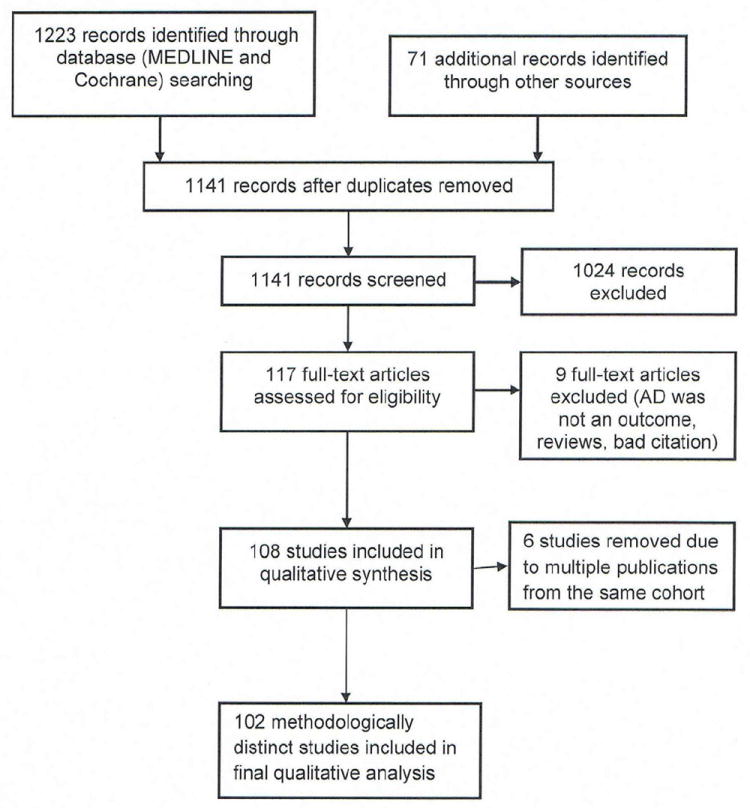
Study flow chart
Greater than 80% of the included 102 studies evaluated a dietary intervention on either the mother or the infant, with infant formula as the most common AD prevention strategy used. The other interventions were non-dietary allergen avoidance, vaccination, and an emollient intervention (Figure 3).
Figure 3.
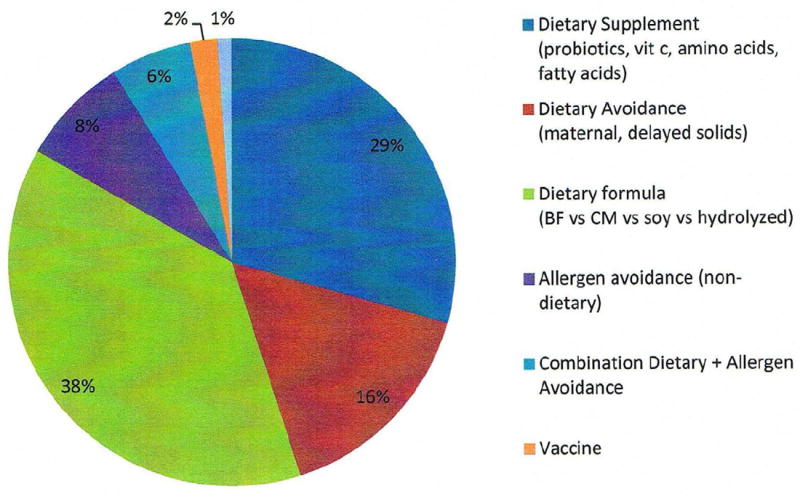
Interventions
Study result – definitions
Of those 102 studies selected for further analysis of AD definition, only 75 (73.5 %) included some form of a description of the criteria used to diagnose AD. The other 27 articles mentioned either a diagnosis made by a questionnaire that was unavailable for review (1 study), a general morphological description of eczema (4 studies), a physician or investigator diagnosis of “eczema” without further elaboration of specified criteria (20 studies), or no description at all (2 studies).
Of the 75 studies with reported disease criteria, the Hanifin-Rajka criteria were the most commonly used disease criteria in 28 studies (Table 1). Of those studies that used the Hanifin-Rajka criteria (which includes chronic or chronically relapsing dermatitis as one of its four major diagnostic criteria), only two studies specified how they dealt with the anomaly of disease chronicity in relation to definition a new case.9,10 The study by Laitinen and colleagues required visible eczema to be present for at least four weeks at the 6- and 12-month visits and for at least eight weeks at the 24- and 48-month visits. Arslanoglu and colleagues required “symptoms” to be present for at least four weeks to meet criteria for AD.10
Table 1.
Disease definitions cited in atopic dermatitis prevention studies
| Definition | Number (n=102) | Percent of studies | Definition | Reference No. |
|---|---|---|---|---|
|
| ||||
| Hanifin-Rajka | 28 | 27% | Must have 3 or more basic features: | 9,10,18-43 |
| Pruritus, Typical morphology and distribution, chronic or chronically-relapsing, personal or family history of atopy plus 3 minor criteria. See reference for full definition17 | ||||
|
| ||||
| Unique Definition | 21 | 21% | Variable | 44-64 |
|
| ||||
| ISAAC | 4 | 4% | Has your child had this itchy rash at any time in the last 12 months? | 65-68 |
| Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes? | ||||
|
| ||||
| U.K Working Party | 7 | 7% | Must have: An itchy skin condition in the last 12 months | 69-74 |
| Plus 3 or more of: | ||||
| Onset below age 2 | ||||
| History of flexural involvement | ||||
| History of generally dry skin | ||||
| Personal history of other atopic disease | ||||
| Visible flexural dermatitis as per photographic protocol | ||||
| *not used in children under 4 years | ||||
| ** in children aged under 4 years, history of atopic disease in a first degree relative may be included | ||||
|
| ||||
| Hanifin-Lobitz | 5 | 5% | Must Have Each of the Following:
|
76-80 |
Plus Two or More of the Following Features:
| ||||
| Or Ffur or more of the following features75 | ||||
|
| ||||
| Seymour | 4 | 4% | The criteria used in selecting infants with atopic dermatitis were the presence of at least two major, or one major and one minor, feature from the following lists. | 81-84 |
Major features. Major features included:
| ||||
Minor features. Minor features included:
| ||||
|
| ||||
| Halken | 4 | 4% | atopic eczema was diagnosed if physical examination revealed areas of scaly, erythematous, and itchy eczematous rash, primarily of the face, the scalp, and the flexural folds. Only eczema with at least two locations in typical areas relapsing with a duration of at least three months was recorded. | 85-88 |
|
| ||||
| Moore | 2 | 2% | Eczematous skin lesions were classified into one of four grades: 0=normal skin; 1=dry skin, cradle cap, and mild perioral erythema; 2=some or all of these features with, in addition, an area of skin, usually on the face or behind the ears, that was red, scaly, cracked, or weeping; and 3=as 2 but more extensive lesions, usually on the face, trunk, and limbs. Grades 2 and 3 were regarded as eczema, but grades 0 and 1 were not. | 89,90 |
|
| ||||
| No Reported Definition |
27 | 26% | 91-117 | |
A disease definition that was unique to that particular study (21 studies) was the second most commonly used disease definition. They did not cite a specific source and none described a scientific method or even more detailed empirical reasoning for justifying their choice of a novel definition of an incident case.
Closer examination of the 21 definitions that were unique to an individual study, most definitions included pruritus, the presence of visible eczema, and disease distribution requirements. Only 50% of these definitions had a time requirement, which ranged from requiring “chronic disease” to four weeks of eczema needing to be present (data not shown).
DISCUSSION
Main findings
This review found a large degree of variability in the methods used to define an incident case of AD in relevant prevention studies, with one quarter of studies failing to report any form of definition whatsoever. Of the studies reporting some form of definition of an incident case, many used a definition that was unique to that particular study, rendering comparisons between studies very difficult. Additionally, most studies used AD incident case definitions without strict time requirements. Combined, these data demonstrate an urgent need for a standardized, valid and repeatable definition of an incident case of AD in order to improve the ability to compare outcomes between studies and to allow more informative meta-analyses of prevention studies. While no previous studies have examined incident case definitions for AD, our results are consistent with the larger problem of the lack of standardized disease outcome measures in AD research. For example, Schmitt and colleagues found 20 different scoring systems for measuring the severity of AD.11 Mancini and colleagues found 30 different definitions for defining an individual as high risk for developing AD.4 Recently an international group called the Harmonizing Outcome Measures for Eczema (HOME) initiative began the process of creating an accepted core group of outcome measures for AD research.12
Problems with existing definitions
The two most commonly used validated criteria found in our review were the U.K. Working Party refinement of the Hanifin-Rajka criteria and the original Hanifin-Rajka criteria themselves. Despite the potential usefulness of these criteria in reliably identifying cases of established AD, as would be used for determining cumulative incidence over time in cohort studies,13 they were not designed for defining an incident case in prospective prevention studies. By defining an incident case in prevention trials, as opposed to a cumulative incidence, incidence rates can be more accurately calculated and a more accurate date of disease onset can be established. In order to qualify as a case of AD using the Hanifin-Rajka criteria, at least three out of four of the following major criteria need to be fulfilled: 1) the presence of eczema, 2) typical distribution, 3) pruritus, and 4) a relapsing and remitting course. The definition of “relapsing and remitting” is not further defined and is left to the discretion of the investigator. The U.K. criteria state that a child must have an itchy skin condition in the past 12 months. These time requirements, while appropriate for diagnosing established cases of AD, become problematic when diagnosing new onset AD during the course of a prospective study.
Defining an incident case of AD is not simply a question of noting the first time an eczematous rash appears in an infant because previous studies have shown that many forms of transient eczematous rashes occur often in infants, even in those children who do not eventually develop true AD.14 There is thus an urgent need for a standardized definition of an incident case of AD that offers a satisfactory trade-off between over-inclusion of transient eczematous eruptions of irritant and other etiologies and over-exclusion of genuine milder short-lived forms of AD that still represent a health care problem.
Proposed solution
Until more sophisticated validation studies can be performed, we suggest a modification of the U.K. Working Party criteria for AD, adapted for prospective observational or interventional studies. This modification specifies a time frame that the eczema must be present in order to be considered as a case of AD, and allows for a diagnosis to be made even if the rash is treated early in its course. We propose the following definition based on empirical reasoning considering the requirements of such a definition and informed by previous studies that signal the best markers of true AD.14
A history of an itchy skin condition which is either continuous or intermittent lasting at least four weeks plus three or more of the following:
A history of a rash in the skin creases (folds of elbows, behind the knees, fronts of ankles or around the neck), or on the extensor aspects of the forearms or lower legs
A personal history of asthma or hay fever or a history of atopic disease in a first-degree relative
A history of a generally dry skin since birth
Visible flexural dermatitis and/or visible dermatitis on the forearms or lower legs with absence of axillary involvement as defined by our online photographic protocol.15,16
Visual confirmation of eczema diagnosis by a clinician, dermatology nurse or a research nurse suitably trained in recognising the symptoms of eczema is recommended.
Clearly it is important to add the proviso that any infant fulfilling these criteria but who, on examination by a suitably trained health professional, are deemed to have a different skin disease, will be classified as not having eczema.
There are several benefits of our proposed definition, the strongest being that it is based on a current AD definition that has undergone extensive scientific development that has assessed validity and repeatability and applicability. The only change added to the U.K. Working Party definition is the addition of a specified time requirement of four weeks. This time requirement should exclude most transient eczematous rashes that are typically irritant in nature and usually of little medical consequence. The use of an established AD definition for incident AD which is derived from one used of prevalent AD allows for consistency in defining the public health burden of disease when assessed using different study designs. Another benefit of this proposed definition is that it does not allow a definition of AD to be made based on the presence of facial eczema alone. Halkjaer and colleagues found that 40% of children with facial eczema do not eventuate into chronic AD.14 Finally, this definition allows for early treatment intervention during the course of a prospective study and does not require the disease to be untreated for a full four weeks if anti-inflammatory therapy is needed. Therefore, if a child develops significant eczema in the classic locations, it would be unethical to withhold treatment. Treatment can begin immediately if needed, and provided some degree of symptoms last for a four-week period, a diagnosis of AD will still be captured using this definition.
Very mild cases of new eczema treated immediately resulting in complete clearance will not be captured by such an approach, although it is debatable how often such cases truly have AD and it is probably wiser to treat such new very mild cases with emollients alone until the disease declares itself – a situation somewhat analogous to the avoidance of labelling an infant who has one episode of wheezing as having asthma.
Other strengths of the current study include its systematic approach to the review of the literature and extensive searching of reference lists for prevention studies not found on the initial search. It is also a timely study in that there is a renewed interest in AD prevention research and prevention has become a focus for the National Eczema Association in the United States (http://www.nationaleczema.org/research/grants/, accessed 11/1/2011). It is possible that we could have missed some important studies in our searches, and we could have missed some unpublished well-developed definitions for incident cases if we had corresponded with all study authors. Our proposed AD definition is also a limitation at this stage, as it has not undergone extensive validity testing in prospective studies in the field. This limitation has to be tempered with the alternative practice to date, which has been to use unsuitable definitions or an array of poorly defined or completely undefined definitions. Our proposed definition is meant to be a starting point and we encourage those undertaking or designing new prospective studies of AD to include it along with their preferred definitions so that knowledge of its utility and validity can be built up.
Clinical Implications.
Our results show that atopic dermatitis is often poorly defined in primary prevention trials. A standardized incident case definition is needed that can accurately measure clinically relevant disease and establish a date of onset.
Acknowledgments
The authors wish to thank Christine E. Carocci for editorial assistance.
This project was supported by Oregon Clinical and Translational Research Institute (OCTRI) and Award Number K23AR057486 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Hywel Williams and Joanne Chalmers contributed to this article as part of a UK National Institute for Health Research (NIHR) Programme Grant for Applied Research funding scheme (RP-PG-0407-10177). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health in England.
Abbreviations
- AD
atopic dermatitis
Footnotes
The authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124(6):1251–1258. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Simpson EL. Atopic dermatitis prevention. Dermatologic Therapy. 2006;19(2):108–117. doi: 10.1111/j.1529-8019.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 3.Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evidence-Based Child Health. 2011;6(5):1322–1339. doi: 10.1002/ebch.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamlin SL, Kaulback K, Mancini AJ. What is “high risk?” A systematic review of atopy risk and implications for primary prevention. Pediatr Dermatol. 2009;26(3):247–256. doi: 10.1111/j.1525-1470.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams HC. Prevention of atopic eczema: a dream not so far away? Arch Dermatol. 2002;138(3):391–392. doi: 10.1001/archderm.138.3.391. [DOI] [PubMed] [Google Scholar]
- 6.Williams HC. Two “positive” studies of probiotics for atopic dermatitis: or are they? Arch Dermatol. 2006;142(9):1201–1203. doi: 10.1001/archderm.142.9.1201. [DOI] [PubMed] [Google Scholar]
- 7.Rystedt I. Prognostic factors in atopic dermatitis. Acta Derm Venereol. 1985;65(3):206–213. [PubMed] [Google Scholar]
- 8.Shams K, Grindlay DJ, Williams HC. What’s new in atopic eczema? An analysis of systematic reviews published in 2009-2010. Review. Clin Exp Dermatol. 2011;36(6):573–577. doi: 10.1111/j.1365-2230.2011.04078.x. quiz 577-578. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen K, Kalliomaki M, Poussa T, Lagström H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow-up study from birth to 4 years. Br J Nutr. 2005;94:565–574. doi: 10.1079/bjn20051503. [DOI] [PubMed] [Google Scholar]
- 10.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138(6):1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt J, Langan S, Williams HC European Dermato-Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol. 2007;120(6):1389–1398. doi: 10.1016/j.jaci.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt J, Williams H HOME Development Group. Harmonising Outcome Measures for Eczema (HOME) Br J Dermatol; Report from the First International Consensus Meeting (HOME 1); 24 July 2010; Munich, Germany. Dec, 2010. pp. 1166–1168. [DOI] [PubMed] [Google Scholar]
- 13.Brenninkmeijer EEA, Schram ME, Leeflang MM, Bos JD, spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008;158(4):754–765. doi: 10.1111/j.1365-2133.2007.08412.x. [DOI] [PubMed] [Google Scholar]
- 14.Halkjaer LB, Loland L, Buchvald FF, Agner T, Skov L, strand M, bisgaard H. Development of atopic dermatitis during the first 3 years of life: the Copenhagen prospective study on asthma in childhood cohort study in high-risk children. Arch Dermatol. 2006;142(5):561–566. doi: 10.1001/archderm.142.5.561. [DOI] [PubMed] [Google Scholar]
- 15.Williams HC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. A protocol for recording the sign of visible flexural dermatitis. Br J Dermatol. 1995;133:941–949. doi: 10.1111/j.1365-2133.1995.tb06930.x. [DOI] [PubMed] [Google Scholar]
- 16.url: http://www.nottingham.ac.uk/dermatology/eczema/Section3-2.html
- 17.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatovener (Suppl) 1980;92:44–47. [Google Scholar]
- 18.West CE, Hammarstrom ML, Hernell O. Probiotics during weaning reduce the incidence of eczema. Pediatr Allergy Immunol. 2009;20:430–437. doi: 10.1111/j.1399-3038.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 19.Bruno G, Milita O, Ferrara M, Nisini R, Cantani A, Buscinco L. Prevention of atopic diseases in high risk babies (long-term follow-up) Allergy Proc. 1993;14:181–186. doi: 10.2500/108854193778878682. [DOI] [PubMed] [Google Scholar]
- 20.Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Sehmi R. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr Res. 2005;57:276–281. doi: 10.1203/01.PDR.0000148279.72611.1D. [DOI] [PubMed] [Google Scholar]
- 21.Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, Prescott SL. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomised, controlled trial. J Allergy Clin Immunol. 2003;112(6):1178–1184. doi: 10.1016/j.jaci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen-reduced dietary intervention programme; the ZUFF-STUDY-PROGRAMME. Part II: Infant growth and health status up tp age 6 months. ZUg-Frauen Feld. Eur J Nutr. 2000;39:145–156. doi: 10.1007/s003940070018. [DOI] [PubMed] [Google Scholar]
- 23.Fälth-Magnusson K, Kjellman N-IM. Allergy prevention by maternal elimination diet during late pregnancy. J Allergy Clin Immunl. 1992;89(3):709–713. doi: 10.1016/0091-6749(92)90378-f. [DOI] [PubMed] [Google Scholar]
- 24.Hoppu U, Rinne M, Salo-Väänänen P, Lampi AM, Piironen V, Isolauri E. Vitamin C in breast milk may reduce the risk of atopy in the infant. Eur J Clin Nutr. 2005;59:123–128. doi: 10.1038/sj.ejcn.1602048. [DOI] [PubMed] [Google Scholar]
- 25.Kajosaari M. Atopy prophylaxis in high-risk infants. Prospective 5-year follow-up study of children with six months exclusive breastfeeding and solid food elimination. Adv Exp Med Biol. 1991;310:453–458. [PubMed] [Google Scholar]
- 26.Matejek N, Schwamberger H, Böhles H. The influence of breast feeding on the development of atopic dermatitis. Exclusive breast feeding versus initial short-term feeding of a partial hydrolysate followed by breast milk. Nut Res. 1998;18(8):1389–1393. [Google Scholar]
- 27.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91(10):814–819. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poysa L, Korppi M, Remes K, Juntunen-Backman K. Atopy in childhood and diet in infancy. A nine-year follow-up study. I. Clinical manifestations. Allergy Proc. 1991;12:107–111. doi: 10.2500/108854191779011800. [DOI] [PubMed] [Google Scholar]
- 29.Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow’s milk, and fish during lactation: Effect on allergic manifestations, skin-prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics. 1992;89(4 Pt 2):735–739. [PubMed] [Google Scholar]
- 30.Taylor A, Dunstan J, Prescott S. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitisation in high-risk children: a randomised controlled trial. J Allergy Clin Immunol. 2007;119(1):184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 31.Chandra RK. Five-year follow-up of high-risk infants with family history of allergy who were exclusively breast-fed or fed partial whey hydrolysate, soy, and conventional cow’s milk formulas. J Pediatr Gastroenterol Nutr. 1997;24(4):380–388. doi: 10.1097/00005176-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 32.de Jong MH, Scharp-van der Linden VT, Aalberse RC, Oosting J, Tijssen JG, de Groot CJ, et al. Randomised controlled trial of brief neonatal exposure to cows’ milk on the development of atopy. Arch Dis Child. 1998;79(2):126–130. doi: 10.1136/adc.79.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Berg A, Koletzko S, Grübl A, Filipak-Pittroff B, Wichmann HE, Bauer CP. The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol. 2003;111(3):533–540. doi: 10.1067/mai.2003.101. [DOI] [PubMed] [Google Scholar]
- 34.Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatr. 1999;88(1):7–12. [PubMed] [Google Scholar]
- 35.Grüber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126(4):791–797. doi: 10.1016/j.jaci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J am Acad Dermatol. 2010;63(4):587–593. doi: 10.1016/j.jaad.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e386–393. doi: 10.1111/j.1399-3038.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 38.Kitz R, Rose MA, Schonborn H, Zielen S, Böhles HJ. Impact of early dietary gamma-linolenic acid supplementation on atopic eczema in infancy. Pediatr Allergy Immunol. 2006;17(2):112–117. doi: 10.1111/j.1399-3038.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- 39.Chirico G, Gasparoni A, Ciardelli L, DeAmici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow’s milk infant formula. Allergy. 1997;52(1):82–88. doi: 10.1111/j.1398-9995.1997.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 40.de Seta L, Siani P, Cirillo G, DiGruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow-up at 24 months. The preliminary results. Pediatr Med Chir. 1994;16(3):251–254. [PubMed] [Google Scholar]
- 41.Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy. 2008;38(8):1342–1348. doi: 10.1111/j.1365-2222.2008.03008.x. [DOI] [PubMed] [Google Scholar]
- 42.Niers L, Martin R, Rijkers G, Sengers F, Timmerman H, van Uden N, et al. The effects of selected probiotic strains on the development of eczema (the PandA study) Allergy. 2009;64(9):1349–1358. doi: 10.1111/j.1398-9995.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 43.Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, Duchén K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta paediatrica. 2009;98(9):1461–1467. doi: 10.1111/j.1651-2227.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 44.Almqvist C, Garden F, Xuan W, Mihrshahi S, Leeder SR, Oddy W, et al. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J Allergy Clin Immunol. 2007;119(6):1438–1444. doi: 10.1016/j.jaci.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 45.Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet. 1992;339(8808):1493–1497. doi: 10.1016/0140-6736(92)91260-f. [DOI] [PubMed] [Google Scholar]
- 46.Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. J Paediatr Child Health. 2002;38(1):84–8. doi: 10.1046/j.1440-1754.2002.00725.x. [DOI] [PubMed] [Google Scholar]
- 47.Chandra RK, Puri S, Hamed A. Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. Br Med J. 1989;299(6693):228–230. doi: 10.1136/bmj.299.6693.228. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Chandra RK, Puri S, Cheema PS. Predictive value of cord blood IgE in the development of atopic disease and role of breastfeeding in its prevention. Clin Allergy. 1985;15(6):517–522. doi: 10.1111/j.1365-2222.1985.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 49.Chandra RK, Puri S, Suraiya C, Cheema PS. Influence of maternal food antigen avoidance during pregnancy and lactation on incidence of atopic eczema in infants. Clin Allergy. 1986;16(6):563–569. doi: 10.1111/j.1365-2222.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 50.Herrmann ME, Dannemann A, Gruters A, Radisch B, Dudenhausen JW, Bergmann R. Prospective study of the atopy preventive effect of maternal avoidance of milk and eggs during pregnancy and lactation. Eur J Pediatr. 1996;155(9):770–774. doi: 10.1007/BF02002904. [DOI] [PubMed] [Google Scholar]
- 51.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 52.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C. Prevention of asthma during the first 5 years of life: A randomized controlled trial. J Allergy Clin Immunol. 2006;118(1):53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Rautava S, Kalliömaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109(1):119–121. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 54.Schoetzau A, Filipiak-Pittroff B, Franke K, Koletzko S, Von Berg A, Gruebl A. Effect of exclusive breast-feeding and early solid food avoidance on the incidence of atopic dermatitis in high-risk infants at 1 year of age. Pediatr Allergy Immunol. 2002;13(4):234–242. doi: 10.1034/j.1399-3038.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 55.Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double-blind trial. Acta Paediatr. 2004;93(9):1159–1165. [PubMed] [Google Scholar]
- 56.van den Berg A, van Zwol A, Moll HA, Fetter WP, van Elburg RM. Glutamine-enriched enteral nutrition in very low-birth-weight infants: effect on the incidence of allergic and infectious diseases in the first year of life. Arch Pediatr Adolesc Med. 2007;161(11):1095–1101. doi: 10.1001/archpedi.161.11.1095. [DOI] [PubMed] [Google Scholar]
- 57.Businco L, Cantani A, Meglio P, Bruno G. Prevention of atopy: Results of a long-term (7 months to 8 years) follow-up. Ann Allergy. 1987;59(5 Pt 2):183–186. [PubMed] [Google Scholar]
- 58.Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Ann Allergy. 1989;63(2):102–106. [PubMed] [Google Scholar]
- 59.de Jong MH, Scharp-Van Der Linden VT, Aalberse R, Heymans HS, Brunekreef B. The effect of brief neonatal exposure to cows’ milk on atopic symptoms up to age 5. Arch Dis Child. 2002;86(5):365–369. doi: 10.1136/adc.86.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filipiak B, Zutavern A, Koletzko S, von Berg A, Brockow I, Grübl A, et al. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151(4):352–358. doi: 10.1016/j.jpeds.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Kalliömaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 62.Vandenplas Y, Hauser B, Van den Borre C, Sacre L, Dab I. Effect of a whey hydrolysate prophylaxis of atopic disease. Ann Allergy. 1992;68(5):419–424. [PubMed] [Google Scholar]
- 63.Lucas A, Brooke OG, Morley R, Cole TJ, Bamford MF. Early diet of preterm infants and development of allergic or atopic disease: Randomised prospective study. Br Med J. 1990;300(6728):837–840. doi: 10.1136/bmj.300.6728.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willems R, Duchateau J, Magrez P, Denis R, Casimir G. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic diseases. Ann Allergy. 1993;71(2):147–150. [PubMed] [Google Scholar]
- 65.Dunder T, Tapiainen T, Pokka T, Uhari M. Infections in child day care centers and later development of asthma, allergic rhinitis, and atopic dermatitis: Prospective follow-up survey 12 years after controlled randomized hygiene intervention. Arch Pediatr Adolesc Med. 2007;161(10):972–977. doi: 10.1001/archpedi.161.10.972. [DOI] [PubMed] [Google Scholar]
- 66.Koopman LP, van Strien RT, Kerkhof M, Wijga A, Smit HA, de Jongste JC, et al. Placebo-controlled trial of house dust mite-impermeable mattress covers: effect on symptoms in early childhood. Am J Respir Crit Care Med. 2002;166(3):307–313. doi: 10.1164/rccm.2106026. [DOI] [PubMed] [Google Scholar]
- 67.Laubereau B, Brockow I, Zirngibl A, Koletzko S, Gruebl A, von Berg A, et al. Effect of breast-feeding on the development of atopic dermatitis during the first 3 years of life – results from the GINI-birth cohort study. J Pediatr. 2004;144(5):602–607. doi: 10.1016/j.jpeds.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 68.Nilsson L, Kjellman N-IM, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Arch Pediatr Adolesc Med. 1998;152(8):734–738. doi: 10.1001/archpedi.152.8.734. [DOI] [PubMed] [Google Scholar]
- 69.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of lactobacillus GG supplementation. Pediatrics. 2008;121(4):e850–856. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 70.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(1):192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 71.van Gool CJ, Thijs C, Henquet CJ, van Houwelingan AC, Dagnelie PC, Schrander J. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis – a randomized controlled trial in infants at high familial risk. Am J Clin Nutr. 2003;77(4):943–951. doi: 10.1093/ajcn/77.4.943. [DOI] [PubMed] [Google Scholar]
- 72.Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122(4):788–794. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 73.Dotterud CK, Storrø O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. 2010;163(3):613–623. doi: 10.1111/j.1365-2133.2010.09889.x. [DOI] [PubMed] [Google Scholar]
- 74.Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in Caesarean-delivered children but not total cohort. J Allergy Clin Immunol. 2009;123(2):335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Hanifin JM, Lobitz WC., Jr Newer concepts of atopic dermatitis. Arch Dermatol. 1977;113(5):663–670. [PubMed] [Google Scholar]
- 76.Fälth-Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy – a randomized study. J Allergy Clin Immunol. 1987;80(6):868–875. doi: 10.1016/s0091-6749(87)80279-8. [DOI] [PubMed] [Google Scholar]
- 77.Shohet L, Shahar E, Davidson S. Breast feeding as prophylaxis for atopic eczema: A controlled study of 368 cases. Acta Paediatr Hung. 1985;26(1):35–39. [PubMed] [Google Scholar]
- 78.Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. J Allergy Clin Immunol. 1993;91(3):723–734. doi: 10.1016/0091-6749(93)90191-h. [DOI] [PubMed] [Google Scholar]
- 79.Hattevig G, Kjellman B, Sigurs N, Björkstén B, Kjellman NI. Effect of maternal avoidance of eggs, cow’s milk and fish during lactation upon allergic manifestations in infants. Clin Exp Allergy. 1989;19(1):27–32. doi: 10.1111/j.1365-2222.1989.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 80.Zeiger RS, Heller S, Mellon MH, Forsythe AB, O’Connor RD, Hamburger RN, Schatz M. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J Allergy Clin Immunol. 1989;84(1):72–89. doi: 10.1016/0091-6749(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 81.Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm C, Björkstén B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(5):1174–1180. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow’s milk-based formula for weaning at about 6 months of age in high allergy-risk infants: effects on atopic disease and sensitization. Allergy. 1996;51(3):192–195. [PubMed] [Google Scholar]
- 83.Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YP. Probiotic supplementation in the first 6 months of life in at-risk Asian infants: effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy. 2009;39(4):571–578. doi: 10.1111/j.1365-2222.2008.03133.x. [DOI] [PubMed] [Google Scholar]
- 84.Böttcher MF, Abrahamsson TR, Fredriksson M, Jakobsson T, Björkstén B, et al. Low breast milk TGF-beta2 is induced by Lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy. Ped Allergy Imunol. 2008;19(6):497–504. doi: 10.1111/j.1399-3038.2007.00687.x. [DOI] [PubMed] [Google Scholar]
- 85.Halken S, Hansen KS, Jacobsen HP, Estmann P, Faeling Ae, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol. 2000;11(3):149–161. doi: 10.1034/j.1399-3038.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 86.Halken S, Høst A, Hansen LG, Osterballe O. Effect of an allergy prevention programme on incidence of atopic symptoms in infancy. A prospective study of 159 “high-risk” infants. Allergy. 1992;47(5):545–553. doi: 10.1111/j.1398-9995.1992.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 87.Halken S, Høst A, Hansen LG, Osterballe O. Preventive effect of feeding high-risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatr Allergy Immunol. 1993;4(4):173–181. doi: 10.1111/j.1399-3038.1993.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 88.Nentwich I, Michkova E, Nevoral J, Urbanek R, Szépfalusi Z. Cow’s milk-specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy. 2001;56(12):1144–1156. doi: 10.1111/j.1398-9995.2001x.00926.x. [DOI] [PubMed] [Google Scholar]
- 89.Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: Three years’ follow-up. Acta Paediatr Suppl. 1996;414:1–21. doi: 10.1111/j.1651-2227.1996.tb14267.x. [DOI] [PubMed] [Google Scholar]
- 90.Moore WJ, Midwinter RE, Morris AF, Colley JR, Soothill JF. Infant feeding and subsequent risk of atopic eczema. Arch Dis Child. 1985;60(8):722–726. doi: 10.1136/adc.60.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van den Berg A, van Elburg RM, Twisk JW, Fetter WP. Glutamine-enriched enteral nutrition in very low birth weight infants: design of a double-blind randomized controlled trial. BMC Pediatr. 2004;4:17. doi: 10.1186/1471-2431-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D’Agata A, Betta P, Sciacca P, Morano c, Praticò G, Curreri R, et al. Role of dietary prevention in newborns at risk for atopy. Results of a follow-up study. Pediatr Med Chir. 1996;18(5):469–472. [PubMed] [Google Scholar]
- 93.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A NAC Manchester Asthma and Allergy Study Group. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001;358(9277):188–193. doi: 10.1016/S0140-6736(01)05406-X. [DOI] [PubMed] [Google Scholar]
- 94.Horak F, Jr, Matthews S, Ihorst G, Arshad SH, Frischer T, Kueher J, et al. Effect of mite-impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth-cohort study -- 24 months results of the study of prevention of allergy in children in Europe. Clin Experimental Allergy. 2004;34(8):1220–1225. doi: 10.1111/j.1365-2222.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 95.Kjellman NI, Johansson SG. Soy versus cow’s milk in infants with a biparental history of atopic disease: development of atopic disease and immunoglobulins from birth to 4 yrs of age. Clin Allergy. 1979;9(4):347–358. doi: 10.1111/j.1365-2222.1979.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 96.Lilja G, Dannaeus A, Foucard T, Graff-Lonnevig V, Johansson SG, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic diseases in infants up to 18 months of age – in-vivo results. Clin Exp Allergy. 1989;19(4):473–479. doi: 10.1111/j.1365-2222.1989.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 97.Lindfors ATB, Danielson L, Enocksson E, Johansson SG, Westin S. Allergic symptoms up to 4-6 years in children given cow milk neonatally. A prospective study Allergy. 1992;47(3):207–211. doi: 10.1111/j.1398-9995.1992.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 98.Lovegrove JA, Hampton SM, Morgan JB. The immunologic and long-term atopic outcome of infants born to women following a milk-free diet during pregnancy and lactation: a pilot study. Br J Nut. 1994;71(2):223–238. doi: 10.1079/bjn19940129. [DOI] [PubMed] [Google Scholar]
- 99.Tsai YT, Chou CC, Hsieh KH. The effect of hypoallergenic formula on the occurrence of allergic diseases in high risk infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1991;32(3):137–144. [PubMed] [Google Scholar]
- 100.Merrett TG, Burr ML, Butland BK, Merrett J, Miskelly FG, Vaughan-Williams E. Infant feeding and allergy: 12-month prospective study of 500 babies born into allergic families. Ann Allergy. 1988;61(6 Pt 2):13–20. [PubMed] [Google Scholar]
- 101.Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nut Res. 1998;18(8):1413–1424. [Google Scholar]
- 102.Schmitz J, Digeon B, Chastang C, Dupouy D, Leroux B, Robillard P, Strobel S. Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. J Pediatr. 1992;121(5 Pt 2):S85–S89. doi: 10.1016/s0022-3476(05)81413-1. [DOI] [PubMed] [Google Scholar]
- 103.Steenhuis TJ, van Aalderen WM, Bloksma N, Nijkamp FP, van der Loveren H, Rijkers Gt. Bacille-calmette-guerin vaccination and the development of allergic disease in children: A randomized, prospective, single-blind study. Clin Experimental Allergy. 2008;38(1):79–85. doi: 10.1111/j.1365-2222.2007.02859.x. [DOI] [PubMed] [Google Scholar]
- 104.Vandenplas Y. The use of hydrolysates in allergy prevention programmes. Eur J Clin Nutr. 1995;49(Suppl. 1):S84–S91. [PubMed] [Google Scholar]
- 105.Businco L, Marchetti F, Pellegrini G, Cantani A, Perlini R. Prevention of atopic disease in “at-risk newborn” by prolonged breast-feeding. Ann Allergy. 1983;51(2 Pt 2):296–299. [PubMed] [Google Scholar]
- 106.Burr ML, Limb ES, Maguire MJ, Amarah L, Eldridge BA, Layzell JC, Merrett TG. Infant feeding, wheezing, and allergy: a prospective study. Arch Dis Child. 1993;68(6):724–728. doi: 10.1136/adc.68.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burr ML, Miskelly FG, Butland BK, Merrett TG, Vaughan-Williams E. Environmental factors and symptoms in infants at high risk of allergy. J Epidemiol Community Health. 1989;43(2):125–132. doi: 10.1136/jech.43.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miskelly FG, Burr ML, Vaughan-Williams E, Fehily AM, Butland BK, Merrett TG. Infant feeding and allergy. Arch Dis Child. 1988;63(4):388–393. doi: 10.1136/adc.63.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.von Berg A, Koletzko S, Filipiak-Pittroff B, Laubereau B, Grübl A, Wichmann HE, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention study. J Allergy Clin Immunol. 2007;119(3):718–725. doi: 10.1016/j.jaci.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 110.Hide DW, Matthews S, Taiq SM, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy. 1996;51(2):89–93. [PubMed] [Google Scholar]
- 111.Hide DW, Matthews S, Matthews L, Steven M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy Clin Immunol. 1994;93(5):842–846. doi: 10.1016/0091-6749(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 112.Vandenplas Y, Hauser B, Van den Borre C, Clybouw C, Mahler T, Hachimi-Idrissi R, et al. The long-term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. Eur J Pediatr. 1995;154(6):488–494. doi: 10.1007/BF02029362. [DOI] [PubMed] [Google Scholar]
- 113.Shao J, Sheng J, Dong W, Li YZ, Yu SC. Effects of feeding intervention on development of eczema in atopy high-risk infants: an 18-month follow-up study. Zhonghua Er Ke Za Zhi. 2006;44(9):684–687. [PubMed] [Google Scholar]
- 114.Lauritzen L, Kjaer TMR, Fruekilde MB, Michaelsen KF, Frøkiaer H. Fish oil supplementation of lactating mothers affects cytokine production in 2-1/2-year-old children. Lipids. 2005;40(7):669–676. doi: 10.1007/s11745-005-1429-6. [DOI] [PubMed] [Google Scholar]
- 115.Mallet E, Henocq A. Long-term prevention of allergic diseases by using protein hydrolysate formula in at-risk infants. J Pediatr. 1992;121(5 Pt 2):S95–S100. doi: 10.1016/s0022-3476(05)81415-5. [DOI] [PubMed] [Google Scholar]
- 116.Oldaeus G, Anjou K, Björkstén B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Arch Dis Child. 1997;77(1):4–10. doi: 10.1136/adc.77.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saarinen KM, Juntunen-Backman K, Jarvenpaa AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: a prospective study of 6209 infants. J Allergy Clin Immunol. 1999;104(2 Pt 1):457–461. doi: 10.1016/s0091-6749(99)70393-3. [DOI] [PubMed] [Google Scholar]


