Abstract
While diving, seals are exposed to apnea-induced hypoxemia and repetitive cycles of ischemia/reperfusion. While on land, seals experience sleep apnea, as well as prolonged periods of food and water deprivation. Prolonged fasting, sleep apnea, hypoxemia and ischemia/reperfusion increase oxidant production and oxidative stress in terrestrial mammals. In seals, however, neither prolonged fasting nor apnea-induced hypoxemia or ischemia/reperfusion increase systemic or local oxidative damage. The strategies seals evolved to cope with increased oxidant production are reviewed in the present manuscript. Among these strategies, high antioxidant capacity and the oxidant-mediated activation of hormetic responses against hypoxia and oxidative stress are discussed. In addition to expanding our knowledge of the evolution of antioxidant defenses and adaptive responses to oxidative stress, understanding the mechanisms that allow adapted mammals to avoid oxidative damage has the potential to advance our knowledge of oxidative stress-induced pathologies and to enhance the translative value of biomedical therapies in the long term.
Keywords: aging, antioxidants, fasting, ischemia/reperfusion, oxidative stress, seals
Introduction
Oxidant formation and oxidative stress
Oxygen consumption by animal cells is essential for the production of the energy needed to maintain cellular functions and metabolic activity. Oxygen-mediated ATP production via the electron transport chain is, however, accompanied by the production of oxidants (Forman and Kennedy 1974; Loschen et al. 1974). Under basal metabolic conditions, ~0.1% of the oxygen consumed undergoes an univalent reduction producing superoxide radical (O2•−) (Fridovich 2004). O2•− can spontaneously react with nitric oxide (NO•) generating peroxynitrite (ONOO−) (Beckman et al. 1990; Blough and Zafiriou 1985; Radi et al. 1991) or be converted by superoxide dismutases (SOD) into oxygen and hydrogen peroxide (H2O2) (McCord and Fridovich 1969). H2O2 can diffuse across biological membranes or be decomposed to water and oxygen by catalase, glutathione peroxidases (GPx) and peroxiredoxins (Prx), in a series of reactions that prevent the formation of the highly reactive hydroxyl radical (HO•) (Boveris and Chance 1973; Kim et al. 1988; Paglia and Valentine 1967).
Oxidants are not only formed as a by-product of oxygen metabolism in the electron transport chain. NAPDH oxidases (Nox), microsomal monooxygenases (cytochromes P450), xanthine oxidase (XO), nitric oxide synthases (NOS), lipoxygenases and cyclooxygenases produce O2•−, H2O2, NO• or hydroperoxides under physiological conditions. The autoxodiation of many biologically important molecules and the electron delocalization that takes place in the reactions of hemo-proteins, result in the production of oxidants as well (Halliwell and Gutteridge 2007). Under several pathological conditions (e.g. cardiovascular and metabolic diseases) oxidant production increases altering the balance between oxidants and antioxidants and thus promoting oxidative stress (Sies 1985). Oxidative stress causes the formation of oxidatively-modified lipids, proteins, and nucleic acids and the disruption of redox signaling and control (Jones 2006).
Potential sources of oxidative stress in seals
Apnea-induced hypoxemia and ischemia/reperfusion
Seals are routinely exposed to breath-holding (apnea) bouts while diving and sleeping (Elsner and Gooden 1983; Kooyman 1989; Ridgway et al. 1975). Apnea in seals is characterized by cardiovascular adjustments (reduction in cardiac output, bradycardia and peripheral vasoconstriction) that allow the maximum utilization of the oxygen stores, but simultaneously result in the depletion of blood oxygen content and in the redistribution of blood flow towards obligatory oxygen-dependent tissues, exposing seals to ischemia and hypoxemia (Castellini et al. 1994; Elsner 1999; Kooyman and Ponganis 1998; Meir et al. 2009; Stockard et al. 2007). At the end of an apnea bout, perfusion of ischemic tissues can potentially increase oxidant production and oxidative stress (Elsner et al. 1998; Zenteno-Savín et al. 2002). In terrestrial mammals, hypoxemia increases electron leak from complexes I and III of the respiratory chain, resulting oxidant production (Hoffman et al. 2007; Stowe and Camara 2009). Perfusion of ischemic tissues exacerbates oxidant production and oxidative damage promoting reperfusion injury (Corral-Debrinski et al. 1991; McCord 1985). During ischemia, XO is activated and the ATP degradation product, hypoxanthine (HX), accumulates. During reperfusion, XO hydroxylases HX generating xanthine, O2•− and H2O2 (Parks et al. 1983). Seal tissues do not possess higher levels of oxidative damage than terrestrial mammal tissues despite being chronically exposed to apnea-induced hypoxemia and ischemia/reperfusion (Vázquez-Medina et al. 2007; Wilhelm Filho et al. 2002; Zenteno-Savín et al. 2002). These observations suggest that seals either avoid apnea-induced oxidant generation or that seals can efficiently cope with increases in oxidant production without experiencing oxidative damage.
Prolonged food and water deprivation
Along with being exposed to intermittent hypoxemia and chronic cycles of ischemia/reperfusion, phocid seals also experience prolonged periods of absolute food and water deprivation (fasting). Spontaneous long-term fasting is an integral part of the life history of phocid seals. Seals undergo prolonged fasting annually while breeding, molting and weaning (Castellini and Rea 1992). Prolonged fasting activates the hypothalamic–pituitary–adrenal axis (HPA) leading to alterations in fluid balance and cardio-respiratory function (Munck et al. 1984; Sapolsky et al. 2000). In the northern elephant seal, along with activating the HPA axis, prolonged fasting stimulates the renin-angiotensin system (RAS) and promotes insulin resistance (Ortiz et al. 2002; Ortiz et al. 2000; Viscarra et al. 2011a; Viscarra et al. 2011b; Ortiz et al. 2006; Fowler et al. 2008). In humans, rats and mice, prolonged fasting, chronic HPA and RAS activation, and insulin resistance, increase oxidative damage by activating Nox proteins, increasing mitochondrial oxidant generation and depleting antioxidants (Ceriello and Motz 2004; Costantini et al. 2011; Evans et al. 2003; Romero and Reckelhoff 1999; Sorensen et al. 2006; Souza Rocha et al. 2008; Sowers 2002; Szkudelski et al. 2004). The fact that prolonged fasting does not increase local or systemic oxidative damage in elephant seals suggests that seals are adapted to tolerate fasting-induced oxidant production (Vázquez-Medina et al. 2010; Vázquez-Medina et al. 2011c).
Aging and postnatal maturation
Oxidant production and oxidative damage accumulation increase with age contributing to senescence and physiological aging (Finkel and Holbrook 2000; Sohal and Weindruch 1996). Old Weddell seals experience muscular senescence but maintain muscle contractile ability and foraging capacity suggesting either that seals efficiently cope with age-associated oxidant production or that senescence in seals is not mediated by increased oxidative stress (Hindle et al. 2011; Hindle et al. 2009; Hindle and Horning 2010). The transition from a terrestrial to an aquatic environment during post-natal development also increases oxidant production without increasing oxidative damage in seals (Vázquez-Medina et al. 2011a). Maturation-related increases in antioxidant capacity likely help seals to counteract age-related increases in oxidant production avoiding oxidative damage (Vázquez-Medina et al. 2011b). The link between age- and dive-associated oxidant production and oxidative stress has only recently been explored (Hindle et al. 2009; Hindle et al. 2010; Vázquez-Medina et al. 2011a; Vázquez-Medina et al. 2011b), but undoubtedly warrants further investigation.
Diving vs non-diving endotherms. Insights from comparative and in vitro studies
Oxidant production
The real-time in vivo measurement of oxidants is challenging in whole-animal vertebrate systems because most oxidant species are highly reactive and have a short half-life. Studies using in vitro approaches have demonstrated that seal heart and kidney accumulate HX after experimental ischemia (Elsner et al. 1998; Elsner et al. 1995). Moreover, the tissue capacity to produce O2•− is higher in seal than in pig heart, kidney and skeletal muscle under basal conditions, and in response to an oxidant-generating system (xanthine + XO) (Zenteno-Savín et al. 2002). The production of O2•− is also higher, under basal conditions, in the liver and muscle of emperor penguins, another diving, endothermic vertebrate, than in those tissues of chickens and several non-diving marine birds (brown noddies, petrels, frigate birds, red-billed tropic birds, boobies and shearwaters) (Zenteno-Savín et al. 2010). These findings suggest that avoiding oxidant production is not the main mechanism used by diving, endothermic vertebrates to cope with ischemia/reperfusion (Zenteno-Savín et al. 2002; Zenteno-Savín et al. 2010).
Oxidative damage
Despite chronic exposure to prolonged fasting, hypoxemia and ischemia/reperfusion, seal tissues do not have higher levels of lipid peroxidation or protein oxidation products (TBARS, protein carbonyls) than pig tissues (Vázquez-Medina et al. 2007; Zenteno-Savín et al. 2002). The intracellular content of TBARS is also lower in the red blood cells (RBCs) of a group of marine mammals (elephant seals, manatees, minke whales, and stripped and franciscana dolphins) than in the RBCs of wild, terrestrial mammals (raccoons, deer, anteaters, monkeys, and ferrets), and in the liver and muscle of emperor penguins than in the liver or muscle of chickens and non-diving, marine birds (Wilhelm Filho et al. 2002; Zenteno-Savín et al. 2010). Although TBARS measurements alone are not enough to conclusively determine the absence of oxidative damage in penguin tissues and marine mammal RBCs (Halliwell and Whiteman 2004), taken together, the available data suggest that diving birds and mammals have the ability to cope with increased oxidant production without experiencing oxidative damage.
Antioxidant defenses
An enhanced antioxidant capacity appears to be a mechanism by which diving birds and mammals cope with increased oxidant production (Elsner et al. 1998; Zenteno-Savín et al. 2002; Loshchagin et al. 2002; Cantú-Medellín et al. 2011; Hindle et al. 2010; Corsolini et al. 2001). High constitutive activity and content of endogenous antioxidants has also been found in other animal species chronically exposed to variations in oxygen availability to their tissues due to factors such as environmental oxygen lack, extracellular freezing, or apneic breathing patterns in arrested metabolic states (Hermes-Lima and Zenteno-Savín 2002; Storey 1996). Penguins and seals possess higher concentrations and activities of enzymatic and non-enzymatic antioxidants than terrestrial birds and mammals. Plasma glutathione (GSH) levels are 2–3-fold higher in Weddell and harbor seals than in humans (Murphy and Hochachka 1981). Intracellular GSH content in RBCs is 2-fold higher in marine mammals than in wild, terrestrial mammals (Wilhelm Filho et al. 2002). Comparing ringed seals to pigs, the concentrations of GSH are 20-, 6-, 2- and 3-fold higher in heart, skeletal muscle, kidneys and lungs, respectively (Table 1) (Vázquez-Medina et al. 2007). The concentrations of several exogenous low molecular weight antioxidants (vitamins, carotenoids) are also higher in diving than in non-diving mammals and birds. Plasma content of -tocopherol is higher in dolphins than in dogs or cows (Kasamatsu et al. 2009). Plasma scavenging capacity against peroxyl radical is higher in emperor and Adélie penguins than in polar skuas or snow petrels (Corsolini et al. 2001). Similarly, the activities of the antioxidant enzymes SOD, catalase, GPx, glutathione S-transferase (GST) and glutathione disulphide reductase (GR) are higher in RBCs of marine than of terrestrial mammals (Wilhelm Filho et al. 2002) while catalase, GPx and GST activities are higher in the liver and muscle of emperor penguins than of chickens and non-diving, marine birds (Zenteno-Savín et al. 2010). The activities of SOD and GST are higher in heart and lung of seals than of pigs whereas catalase activity is higher in the liver of seals than of pigs (Table 1) (Vázquez-Medina et al. 2006). The activity of GPx is also higher in heart, lung and skeletal muscle of seals than of pigs while the activities of GR and glucose-6-phosphate dehydrogenase (G6PDH), two key enzymes that maintain intracellular GSH, are higher in heart, kidney, liver, lung and skeletal muscle of seals than of pigs (Table 1) (Vázquez-Medina et al. 2006, 2007). Collectively, these findings suggest that possessing increased antioxidant protection is essential for diving vertebrates.
Table 1.
Antioxidant enzyme activities and glutathione content in ringed seal (Phoca hispida) and domestic pig (Sus scrofa) tissues.
| SOD (U mg protein) | Catalase (U mg protein) | GPx (mU mg protein) | GST (mU mg protein) | GR (mU mg protein) | G6PDH (mU mg protein) | GSH-Eq (nmol g wt tissue) | ||
|---|---|---|---|---|---|---|---|---|
| Seal | Heart | 77 ± 25* | 1,956 ± 581* | 15 ± 1* | 13 ± 3* | 7 ± 2* | 15 ± 3* | 2,918 ± 227* |
| Kidney | 55 ± 10 | 8,245 ± 960 | 17 ± 2 | 7 ± 1 | 5 ± 1* | 121 ± 42* | 1,121 ± 19* | |
| Liver | 71 ± 36 | 19,196 ± 4,52* | 33 ± 8 | 35 ± 5* | 3 ± 0.6* | 134 ± 27* | 327 ± 4 | |
| Lung | 78 ± 20* | 1,614 ± 289* | 47 ±10* | 3 ± 1 | 6 ± 2* | 67 ± 17* | 830 ± 5* | |
| Muscle | 14 ± 3 | 891 ± 268* | 4 ± 0.8* | 0.9 ± 0.2 | 4 ± 1* | 71 ± 29* | 742 ± 67* | |
|
| ||||||||
| Pig | Heart | 26 ± 8 | 7,154 ± 1,900 | 8 ± 0.9 | 4 ± 1 | 0.6 ± 0.1 | 2 ± 0.7 | 117 ± 0.04 |
| Kidney | 38 ± 7 | 7,717 ± 1,176 | 36 ± 13 | 5 ± 0.8 | 0.5 ± 0.1 | 3 ± 0.5 | 490 ± 142 | |
| Liver | 24 ± 3 | 11,004 ± 3,585 | 25 ± 3 | 70 ± 12 | 0.5 ± 0.1 | 2 ± 0.4 | 451 ± 33 | |
| Lung | 8 ± 2 | 3,644 ± 630 | 16 ± 2 | 2 ± 0.7 | 0.8 ± 0.3 | 1 ± 0.5 | 262 ± 65 | |
| Muscle | 15 ± 4 | 3,462 ± 848 | 2 ± 0.26 | 0.9 ± 0.2 | 0.4 ± 0.1 | 4 ± 0.5 | 124 ± 7 | |
SOD = superoxide dismutase, GPx = glutathione peroxidase, GST = glutathione S-transferase, GR = glutathione disulphide reductase, G6PDH = glucose-6-phosphate dehydrogenase, GSH-Eq = total glutathione. Data are presented as mean ± s.e.m.
significantly different from pigs (p < 0.05). Data from (Vázquez-Medina et al. 2006, 2007).
Hypoxic and redox signaling
While oxidant production can be potentially damaging, it is also needed for the regulation of several adaptive processes (Suzuki et al. 1997). H2O2 is a second messengers that participates in redox reactions to regulate signal transduction by stimulating calcium-dependent pathways, protein phosphorylation and transcription factor activation (Suzuki et al. 1997; Forman et al. 2010). Similarly, oxygen sensing and redox signaling are essential for mediating the physiological and pathophysiological responses to hypoxia (Bunn and Poyton 1996). In vitro and ex vivo studies comparing seals and non-diving mammals have demonstrated that the seal brain possesses hypoxia tolerance and antioxidant protection due to an increased content and unique localization of neuroglobin in tissue (Folkow et al. 2008; Ramirez et al. 2011; Mitz et al. 2009; Williams et al. 2008). Neuroglobin enhances oxygen extraction and intracellular diffusion and protects against oxidative stress by activating phosphoinositide-3 kinase and by opening the mitochondrial KATP channel (Antao et al. 2010; Li et al. 2008; Sun et al. 2001). Another primary regulator of the adaptive response to hypoxia is the Hypoxia-Inducible Factor 1 (HIF-1) (Semenza 1999, 2000a). HIF-1 genes in the ringed seal are similar to those in terrestrial mammals, but in contrast to what has been observed in terrestrial mammals (Ivan et al. 2001), HIF-1 proteins are constitutively expressed in several tissues of the ringed seal, and their levels are correlated to reduced levels of protein oxidation suggesting that HIF-1 may potentially mediate hypoxia and antioxidant protection in seal tissues (Johnson et al. 2004, 2005).
In vivo studies
Adaptive responses to apnea in seals
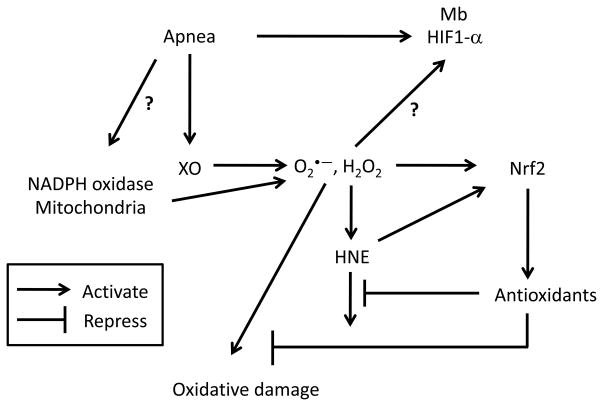
Several studies have been conducted to address the central question of how seals cope with apnea-induced oxidant production. The role of GSH, a primary antioxidant with a key role in redox signaling (for a recent review see (Forman et al. 2009), in the protection against apnea-induced oxidant production, was first discovered in a study showing that plasma GSH levels in Weddell seals decreased during forced diving and rose above resting levels at the end of the submersions (Murphy and Hochachka 1981). In another study, NO• was not detected in the exhaled gas of Weddell seals breathing through an isolated hole after freely diving under the sea ice (Falke et al. 2008). This finding suggests that the absence of NO• may be an adaptive strategy to avoid ONOO− and HO• formation after an event of ischemia/reperfusion that can potentially increase O2•− production (Granger 1988; Beckman et al. 1990; Blough and Zafiriou 1985; Radi et al. 1991). More recently, several aspects of oxidant and antioxidant metabolism were evaluated in elephant seals exposed to rest- and voluntary submersion-associated apneas. Plasma XO activity, xanthine and HX levels, but not systemic oxidative damage (F2-isoprostanes, nitrotyrosine, 4-hydroxyonenal or protein carbonyls), increased in response to apnea (Vázquez-Medina et al. 2011d). Moreover, XO protein expression increased in the skeletal muscle after repetitive apnea bouts, along with an increase in the nuclear content of NF-E2-related factor 2 (Nrf2) and HIF-1 and the protein expression of Cu,ZnSOD, catalase and myoglobin (Mb) (Vázquez-Medina et al. 2011d). These findings suggest that H2O2 produced by XO can potentially mediate the adaptive response to oxidative stress during apnea in seals since Nrf2, the redox sensitive transcription factor that regulates antioxidant gene expression, translocates into the nucleus in response to increased intracellular H2O2 production and XO produces mainly H2O2 (Jaiswal 2004; Kobayashi et al. 2006; Kelley et al. 2010). These findings also suggest that repetitive apneas stimulate the adaptive response to hypoxia in seals and that HIF-1 and Mb potentially contribute to seal’s tolerance to hypoxia (Johnson et al. 2004, 2005; Kanatous and Mammen 2010; Noren et al. 2005; Vázquez-Medina et al. 2011d). Collectively, these findings support the idea that apnea-induced oxidant production mediates the preconditioning of seal tissues (Zenteno-Savín et al. 2002) since oxidants are required for the activation of protective pathways against reperfusion injury (Figure 1) (Baines et al. 1997; Bergeron et al. 2000; Furuichi et al. 2005; Grimm et al. 2005; Leonard et al. 2006; Semenza 2000b; Yuan et al. 2010; Zhu et al. 2011).
Figure 1. Apnea stimulates adaptive responses to hypoxia and oxidative stress in seals.
Schematic representation of the proposed mechanisms that activate protective responses against oxidative stress and hypoxia in the skeletal muscle of elephant seals. H2O2 = hydrogen peroxide. HIF-1 = hypoxia inducible factor 1 HNE = 4-hydroxynonenal. Mb = myoglobin. Nrf2 = NF-E2-related factor 2. O2•− = superoxide radical. XO = xanthine oxidase.
Oxidant and antioxidant metabolism during prolonged fasting in elephant seals
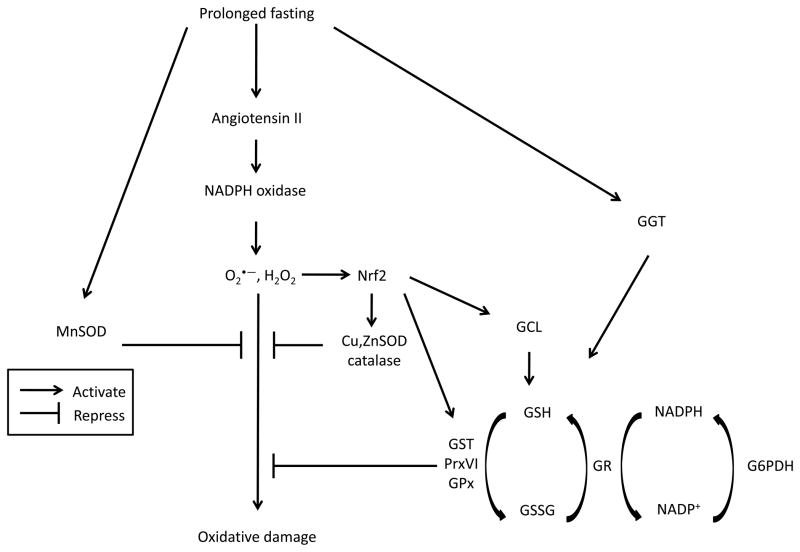
Oxidant and antioxidant metabolism has been studied in northern elephant seals during their natural fasting periods associated with breeding, molting and weaning. Prolonged fasting in the northern elephant seal promotes insulin resistance (Viscarra et al. 2011a; Viscarra et al. 2011b; Fowler et al. 2008; Houser et al. 2007), activates RAS (Ortiz et al. 2006; Ortiz et al. 2000) and the HPA axis (Ortiz et al. 2003b; Ortiz et al. 2001; Ortiz et al. 2003a), and increases NADPH oxidase 4 (Nox4) and XO activity and protein expression (Vázquez-Medina et al. 2010; Soñanez-Organis et al. In press). Prolonged fasting, however, is not associated with increased oxidative damage in this species. Systemic (F2-isoprostanes, plasma nitrotyrosine, C-reactive protein) and muscle markers of oxidative damage (TBARS, 4-hydroxynonenal, protein carbonyls, nitrotyrosine) remain unchanged after two months of absolute fasting in weaned pups (Vázquez-Medina et al. 2010). Increased activity and protein expression of several antioxidant enzymes (SOD, catalase, GPx, Prx, GST, GR, G6PDH, glutamate-cysteine ligase =GCL, -glutamyl-transpeptidase: GTT), as well as increased GSH, likely contribute to the prevention of fasting-associated oxidative damage in elephant seal pups (Vázquez-Medina et al. 2011c; Vázquez-Medina et al. 2010). Increased plasma content of water-soluble vitamins in pups and lactating females (Boaz et al. 2012), and the maintenance of elevated levels of high-density lipoproteins in breeding and molting adult males (Tift et al. 2011), may also contribute to counteract fasting-induced oxidant production in elephant seals. The understanding of how the antioxidant system of the northern elephant seal is up-regulated in response to prolonged fasting remains elusive, but preliminary studies from our laboratories, along with the present findings, suggest that angiotensin II stimulates Nox4 by increasing transforming growth factor (TGF and that Nox4 may mediate an hormetic response by activating Nrf2 (Figure 2). Hormesis is defined as an adaptive response to a moderate stress (Mattson 2008). Since H2O2 activates Nrf2 (Kobayashi et al. 2006; Umemura et al. 2008), and Nox4 constitutively produces H2O2 (Takac et al. 2011), it is possible that an increase in Nox4 expression mediates an adaptive response to fasting-induced oxidant production in elephant seals.
Figure 2. Prolonged fasting up-regulates the antioxidant system of the northern elephant seal.
Schematic representation of proposed mechanisms leading to the up-regulation of the antioxidant system in response to prolonged fasting in elephant seals. Cu,ZnSOD = copper and zinc-dependent superoxide dismutase. G6PDH = glucose-6-phosphate dehydrogenase. GCL = glutamate-cysteine ligase. GGT = γ-glutamyl transpeptidase. GSH = glutathione. GSSG = glutathione disulphide. GR = glutathione disulphide reductase. GPx = glutathione peroxidase. GST = glutathione S-transferase. H2O2 = hydrogen peroxide. MnSOD = manganese-dependent superoxide dismutase. Nrf2 = NF-E2-related factor 2. O2•− = superoxide radical. PrxVI = 1-cys peroxiredoxin.
Aging, maturation and oxidative stress in Weddell and hooded seals
According to the free radical theories of aging, the production of oxidants and the accumulation of oxidative damage are factors that mediate senescence and physiological aging (Finkel and Holbrook 2000; Sohal and Weindruch 1996; Beckman and Ames 1998; Harman 1956). Based on those theories, diving vertebrates may be particularly susceptible to oxidative stress, cellular dysfunction and senescence due to chronic exposure to diving-induced hypoxemia, ischemia/reperfusion and exercise during foraging. Unfortunately, aging or senescence studies in seals or any other diving, endothermic vertebrate are scant. Muscular senescence has been documented in Weddell seals in which collagen content is higher in longissimus dorsi and pectoralis muscles of old seals (+ 17 years old) compared to young adults (9–16 yr). In addition, a shift of the collagen isoform profile from Type III to the stiffer Type I occur with age in both muscles indicating that old seals experience muscular senescence (Hindle et al. 2009). Consistent dive durations throughout adulthood, however, imply unchanged swimming and foraging capacity, suggesting either that seals evolved mechanisms to cope with age-derived oxidant production or that senescence and physiological aging in seals are not mediated by oxidative stress (Hindle et al. 2011; Hindle et al. 2009; Hindle and Horning 2010; Lawler and Hindle 2011; Hindle et al. 2010).
Post-natal maturation is another potential source of oxidative stress in phocid seals due to their transition from a terrestrial to an aquatic environment and the concomitant beginning of their diving lifestyle. Tissue capacity to produce O2•−, but not lipid peroxidation (TBARS), protein carbonyls or oxidatively-modified DNA (8-oxo-7,8-dihydro-2′-deoxyguanosine) levels are higher in the skeletal muscle (longissimus dorsi) of adult hooded seals than of newborn or weaned pups (Vázquez-Medina et al. 2011a). Maturation in hooded seals also increases SOD, GPx and thioredoxin 1 activities, MnSOD, PrxVI and glutaredoxin 1 protein expression, as well as GSH levels, suggesting that the antioxidant system of the hooded seal develops with age progression (Vázquez-Medina et al. 2011b; Vázquez-Medina et al. 2011a). Interestingly, neither Nrf2 mRNA nor protein expression (in whole extracts or nuclear fractions) are increased in adults compared to pups suggesting that maturation in seals does not induce an acute adaptive response to oxidative stress. The later also suggests that age-related increases in oxidant production are efficiently counteracted by appropriately elevated antioxidant levels in seals and that Nrf2 activation may only increase in response to a particular extended diving episode or repetitive apneas in diving mammals (Vázquez-Medina et al. 2011d).
Conclusions and future directions
The life history of seals is characterized by extreme behaviors that expose them to potential increases in oxidant production and oxidative stress. Seals, however, have evolved mechanisms that allow them cope with prolonged fasting, hypoxemia and ischemia/reperfusion without experiencing oxidative damage. Elevated levels of endogenous antioxidants likely help seals to counteract increases in fasting-, apnea- and age-derived oxidant production. The control and regulation of the adaptive responses to oxidative stress in seals remain elusive, but initial studies have shown that ischemic preconditioning and physiological oxidant production mediate hormetic responses that stimulate the antioxidant system of developing, fasting and diving seals. More thorough investigations along these lines not only can enhance our appreciation for the evolution of such mechanisms, but also have the potential to provide valuable insight to the contribution of oxidative stress to a number of human pathologies.
Acknowledgments
We thank three anonymous reviewers for their valuable comments that helped us to improve this manuscript. JPV-M is supported by fellowships form Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACYT), The University of California Institute for Mexico and the United States (UC MEXUS) and The University of California Miguel Velez Scholarship Fund. RMO is supported by a NIH NHLBI Career Development Award (K02HL103787).
References
- Antao ST, Duong TTH, Aran R, Witting PK. Neuroglobin Overexpression in Cultured Human Neuronal Cells Protects Against Hydrogen Peroxide Insult via Activating Phosphoinositide-3 Kinase and Opening the Mitochondrial KATP Channel. Antioxid Redox Signal. 2010;13 (6):769–781. doi: 10.1089/ars.2009.2977. [DOI] [PubMed] [Google Scholar]
- Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29 (1):207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87 (4):1620. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78 (2):547. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia inducible factor 1 in hypoxia induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48 (3):285–296. doi: 10.1002/1531-8249(200009)48:3<285::AID-ANA2>3.0.CO;2–8. [DOI] [PubMed] [Google Scholar]
- Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg Chem. 1985;24 (22):3502–3504. doi: 10.1021/ic00216a003. [DOI] [Google Scholar]
- Boaz SM, Champagne CD, Fowler MA, Houser DH, Crocker DE. Water-soluble vitamin homeostasis in fasting northern elephant seals (Mirounga angustirostris) measured by metabolomics analysis and standard methods. Comp Biochem Physiol A Mol Integr Physiol. 2012;161 (2):114–121. doi: 10.1016/j.cbpa.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134 (3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76 (3):839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Cantú-Medellín N, Byrd B, Hohn A, Vázquez-Medina JP, Zenteno-Savín T. Differential antioxidant protection in tissues from marine mammals with distinct diving capacities. Shallow/short vs. deep/long divers. Comp Biochem Physiol A Mol Integr Physiol. 2011;158 (4):438–443. doi: 10.1016/j.cbpa.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Castellini M, Rea L. The biochemistry of natural fasting at its limits. Experientia. 1992;48 (6):575–582. doi: 10.1007/BF01920242. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Milsom WK, Berger RJ, Costa DP, Jones DR, Castellini JM, Rea LD, Bharma S, Harris M. Patterns of respiration and heart rate during wakefulness and sleep in elephant seal pups. Am J Physiol Regul Integr Comp Physiol. 1994;266 (3):R863–R869. doi: 10.1152/ajpregu.1994.266.3.R863. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24 (5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Stepien G, Shoffner JM, Lott MT, Kanter K, Wallace DC. Hypoxemia is associated with mitochondrial DNA damage and gene induction. JAMA. 1991;266 (13):1812–1816. doi: 10.1001/jama.1991.03470130092035. [DOI] [PubMed] [Google Scholar]
- Corsolini SC, Nigro MN, Olmastroni SO, Focardi SF, Regoli FR. Susceptibility to oxidative stress in Adélie and emperor penguin. Polar Biol. 2001;24 (5):365–368. doi: 10.1007/s003000000220. [DOI] [Google Scholar]
- Costantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011:1–10. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- Elsner R. Living in water: solutions to physiological problems. In: Reynolds JEI, Rommel SA, editors. Biology of Marine Mammals. Smithsonian Institution Press; Washington, DC: 1999. pp. 73–116. [Google Scholar]
- Elsner R, Gooden B. Monogr Physiol Soc. Vol. 40. Cambridge University Press; 1983. Diving and asphyxia: a comparative study of animals and man. [PubMed] [Google Scholar]
- Elsner R, Øyasæter S, Almaas R, Saugstad OD. Diving seals, ischemia-reperfusion and oxygen radicals. Comp Biochem Physiol A Mol Integr Physiol. 1998;119 (4):975–980. doi: 10.1016/S1095-6433(98)00012-9. [DOI] [PubMed] [Google Scholar]
- Elsner R, Øyasæter S, Saugstad OD, Blix AS. Seal adaptations for long dives: recent studies of ischemia and oxygen radicals. In: Arnoldus Schytte Blix LW, Øyvind U, editors. Developments in Marine Biology. Vol. 4. Elsevier Science; 1995. pp. 371–376. [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are Oxidative Stress- Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes. 2003;52 (1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Falke KJ, Busch T, Hoffmann O, Liggins GC, Liggins J, Mohnhaupt R, Roberts JD, Jr, Stanek K, Zapol WM. Breathing pattern, CO2 elimination and the absence of exhaled NO in freely diving Weddell seals. Respir Physiol Neurobiol. 2008;162 (1):85–92. doi: 10.1016/j.resp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Folkow LP, Ramirez J-M, Ludvigsen S, Ramirez N, Blix AS. Remarkable neuronal hypoxia tolerance in the deep-diving adult hooded seal (Cystophora cristata) Neurosci Lett. 2008;446 (2–3):147–150. doi: 10.1016/j.neulet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Kennedy JA. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem Biophys Res Commun. 1974;60 (3):1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. Signaling Functions of Reactive Oxygen Species. Biochemistry. 2010;49 (5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30 (1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Champagne CD, Houser DS, Crocker DE. Hormonal regulation of glucose clearance in lactating northern elephant seals (Mirounga angustirostris) J Exp Biol. 2008;211 (18):2943–2949. doi: 10.1242/jeb.018176. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3 (1):13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79 (6):816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 1988;255 (6):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Grimm C, Hermann D, Bogdanova A, Hotop S, Kilic U, Wenzel A, Kilic E, Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16 (4–5):531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Biosciences Oxford. 4. Oxford University Press; New York: 2007. Free radicals in biology and medicine. [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142 (2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11 (3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Zenteno-Savín T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133 (4):537–556. doi: 10.1016/S1532-0456(02)00080-7. [DOI] [PubMed] [Google Scholar]
- Hindle AG, Horning M. Energetics of breath-hold hunting: Modeling the effects of aging on foraging success in the Weddell seal. J Theor Biol. 2010;264 (3):673–682. doi: 10.1016/j.jtbi.2010.03.045. [DOI] [PubMed] [Google Scholar]
- Hindle AG, Horning M, Mellish JAE, Lawler JM. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii) J Exp Biol. 2009;212 (6):790. doi: 10.1242/jeb.025387. [DOI] [PubMed] [Google Scholar]
- Hindle AG, Lawler JM, Campbell KL, Horning M. Muscle aging and oxidative stress in wild-caught shrews. Comp Biochem Physiol B Biochem Mol Biol. 2010;155 (4):427–434. doi: 10.1016/j.cbpb.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle AG, Mellish JAE, Horning M. Aerobic dive limit does not decline in an aging pinniped. J Exp Zool A Ecol Genet Physiol. 2011;315A (9):544–552. doi: 10.1002/jez.703. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292 (1):H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- Houser DS, Champagne CD, Crocker DE. Lipolysis and glycerol gluconeogenesis in simultaneously fasting and lactating northern elephant seals. Am J Physiol Regul Integr Comp Physiol. 2007;293 (6):R2376–R2381. doi: 10.1152/ajpregu.00403.2007. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIF targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292 (5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36 (10):1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Johnson P, Elsner R, Zenteno-Savín T. Hypoxia-inducible factor in ringed seal (Phoca hispida) tissues. Free Radic Res. 2004;38 (8):847–854. doi: 10.1080/10715760410001725526. [DOI] [PubMed] [Google Scholar]
- Johnson P, Elsner R, Zenteno-Savín T. Hypoxia-inducible factor 1 proteomics and diving adaptations in ringed seal. Free Radic Biol Med. 2005;39 (2):205–212. doi: 10.1016/j.freeradbiomed.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Kanatous SB, Mammen PPA. Regulation of myoglobin expression. J Exp Biol. 2010;213 (16):2741–2747. doi: 10.1242/jeb.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu M, Kawauchi R, Tsunokawa M, Ueda K, Uchida E, Oikawa S, Higuchi H, Kawajiri T, Uchida S, Nagahata H. Comparison of serum lipid compositions, lipid peroxide,[alpha]-tocopherol and lipoproteins in captive marine mammals (bottlenose dolphins, spotted seals and West Indian manatees) and terrestrial mammals. Res Vet Sci. 2009;86 (2):216–222. doi: 10.1016/j.rvsc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Kelley EE, Khoo NKH, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48 (4):493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim I, Lee KY, Rhee S, Stadtman E. The isolation and purification of a specific “ protector” protein which inhibits enzyme inactivation by a thiol/Fe (III)/O2 mixed-function oxidation system. J Biol Chem. 1988;263 (10):4704. [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26 (1):221. doi: 10.1128/MCB.26.1.221–229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman G, Ponganis P. The physiological basis of diving to depth: birds and mammals. Annu Rev Physiol. 1998;60 (1):19–32. doi: 10.1146/annurev.physiol.60.1.19. doi:full/10.1146/annurev.physiol.60.1.19. [DOI] [PubMed] [Google Scholar]
- Kooyman GL. Diverse divers: physiology and behavior. Springer-Verlag; Berlin: 1989. [Google Scholar]
- Lawler JM, Hindle A. Living in a Box or Call of the Wild? Revisiting Lifetime Inactivity and Sarcopenia. Antioxid Redox Signal. 2011;15 (9):2529–2541. doi: 10.1089/ars.2011.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, OFarrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20 (14):2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Li RC, Morris MW, Lee SK, Pouranfar F, Wang Y, Gozal D. Neuroglobin protects PC12 cells against oxidative stress. Brain Res. 2008;1190:159–166. doi: 10.1016/j.brainres.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42 (1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Loshchagin O, Kovalenko R, Nozdrachev A, Yanvareva I, Krivoruchko B. Possible Role of Catalase in Adaptation to Diving of Semi-Aquatic Rodents Ondatra zibethica. J Evol Biochem Physiol. 2002;38 (1):90–95. doi: 10.1023/A:1015577607316. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7 (1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312 (3):159–163. doi: 10.1056/NEJM198501173120305. doi:full/10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide Dismutase. J Biol Chem. 1969;244 (22):6049–6055. [PubMed] [Google Scholar]
- Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am J Physiol Regul Integr Comp Physiol. 2009;297 (4):R927. doi: 10.1152/ajpregu.00247.2009. [DOI] [PubMed] [Google Scholar]
- Mitz SA, Reuss S, Folkow LP, Blix AS, Ramirez JM, Hankeln T, Burmester T. When the brain goes diving: glial oxidative metabolism may confer hypoxia tolerance to the seal brain. Neuroscience. 2009;163 (2):552–560. doi: 10.1016/j.neuroscience.2009.06.058. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5 (1):25. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Murphy BJ, Hochachka PW. Free amino acid profiles in blood during diving and recovery in the Antarctic Weddell seal. Can J Zool. 1981;59 (3):455–459. doi:pdfplus/10.1139/z81-066. [Google Scholar]
- Noren S, Iverson S, Boness D. Development of the blood and muscle oxygen stores in gray seals (Halichoerus grypus): implications for juvenile diving capacity and the necessity of a terrestrial postweaning fast. Physiol Biochem Zool. 2005;78 (4):482–490. doi: 10.1086/430228. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Crocker DE, Houser DS, Webb PM. Angiotensin II and aldosterone increase with fasting in breeding adult male northern elephant seals (Mirounga angustirostris) Physiol Biochem Zool. 2006;79 (6):1106–1112. doi: 10.1086/505996. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Houser DS, Wade CE, Leo Ortiz C. Hormonal changes associated with the transition between nursing and natural fasting in northern elephant seals (Mirounga angustirostris) Gen Comp Endocrinol. 2003a;130 (1):78–83. doi: 10.1016/s0016-6480(02)00572-5. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Noren DP, Ortiz CL, Talamantes F. GH and ghrelin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris) J Endocrinol. 2003b;178 (3):533–539. doi: 10.1677/joe.0.1780533. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Wade CE, Costa DP, Ortiz CL. Renal responses to plasma volume expansion and hyperosmolality in fasting seal pups. Am J Physiol Reg Integr Comp Physiol. 2002;282 (3):R805–R817. doi: 10.1152/ajpregu.00418.2001. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Wade CE, Ortiz CL. Prolonged fasting increases the response of the renin-angiotensin-aldosterone system, but not vasopressin levels, in postweaned northern elephant seal pups. Gen Comp Endocrinol. 2000;119 (2):217–223. doi: 10.1006/gcen.2000.7514. [DOI] [PubMed] [Google Scholar]
- Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol. 2001;280 (3):R790–795. doi: 10.1152/ajpregu.2001.280.3.R790. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70 (1):158. [PubMed] [Google Scholar]
- Parks D, Bulkley G, Granger D. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983;94 (3):415–422. [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266 (7):4244–4250. [PubMed] [Google Scholar]
- Ramirez JM, Folkow LP, Ludvigsen S, Ramirez PN, Blix AS. Slow intrinsic oscillations in thick neocortical slices of hypoxia tolerant deep diving seals. Neuroscience. 2011;177 (0):35–42. doi: 10.1016/j.neuroscience.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Ridgway S, Harrison R, Joyce P. Sleep and cardiac rhythm in the gray seal. Science. 1975;187 (4176):553–555. doi: 10.1126/science.163484. [DOI] [PubMed] [Google Scholar]
- Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34 (4):943–949. doi: 10.1161/01.HYP.34.4.943. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21 (1):55. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15 (1):551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000a;88 (4):1474. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000b;106 (7):809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273 (5271):59. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soñanez-Organis JG, Vázquez-Medina JP, Zenteno-Savín T, Aguilar A, Crocker DE, Ortiz RM. rolonged fasting increases purine recycling in postweaned northern elephant seals. J Exp Biol. doi: 10.1242/jeb.067173. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M, Sanz A, Gomez J, Pamplona R, Portero-Otin M, Gredilla R, Barja G. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res. 2006;40 (4):339–347. doi: 10.1080/10715760500250182. [DOI] [PubMed] [Google Scholar]
- Souza Rocha G, Fonseca AS, Rodrigues MP, Dantas FJS, Caldeira-de-Araujo A, Santos R. Comet assay to determine DNA damage induced by food deprivation in rats. Acta Biol Hung. 2008;59 (3):315–325. doi: 10.1556/ABiol.59.2008.3.5. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346 (25):1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- Stockard T, Levenson D, Berg L, Fransioli J, Baranov E, Ponganis P. Blood oxygen depletion during rest-associated apneas of northern elephant seals (Mirounga angustirostris) J Exp Biol. 2007;210 (15):2607. doi: 10.1242/jeb.008078. [DOI] [PubMed] [Google Scholar]
- Storey KB. Oxidative stress: animal adaptations in nature. Braz J Med Biol Res. 1996;29:1715–1733. [PubMed] [Google Scholar]
- Stowe DF, Camara AKS. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11 (6):1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98 (26):15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22 (1–2):269–285. doi: 10.1016/S0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Okulicz M, Bialik I, Szkudelska K. The influence of fasting on liver sulfhydryl groups, glutathione peroxidase and glutathione-S-transferase activities in the rat. J Physiol Biochem. 2004;60 (1):1–6. doi: 10.1007/BF03168215. [DOI] [PubMed] [Google Scholar]
- Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop Is Involved in Hydrogen Peroxide Formation by the NADPH Oxidase Nox4. J Biol Chem. 2011;286 (15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tift MS, Houser DS, Crocker DE. High-density lipoprotein remains elevated despite reductions in total cholesterol in fasting adult male elephant seals (Mirounga angustirostris) Comp Biochem Physiol B Biochem Mol Biol. 2011;159 (4):214–219. doi: 10.1016/j.cbpb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Umemura K, Itoh T, Hamada N, Fujita Y, Akao Y, Nozawa Y, Matsuura N, Iinuma M, Ito M. Preconditioning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem Biophys Res Commun. 2008;368 (4):948–954. doi: 10.1016/j.bbrc.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol. 2010;213 (14):2524–2530. doi: 10.1242/jeb.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Olguín-Monroy NO, Maldonado PD, Santamaría A, Königsberg M, Elsner R, Hammilll MO, Burns JM, Zenteno-Savín T. Maturarion increases superoxide radical production without increasing oxidative damage in the skeletal muscle of hooded seals (Cystophora cristata) Can J Zool. 2011a;89:206–212. doi: 10.1139/Z10-107. [DOI] [Google Scholar]
- Vázquez-Medina JP, Soñanez-Organis JG, Burns JM, Zenteno-Savín T, Ortiz RM. Antioxidant capacity develops with maturation in the deep diving hooded seal. J Exp Biol. 2011b;214:2903–2910. doi: 10.1242/jeb.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J Exp Biol. 2011c;214:1294–1299. doi: 10.1242/jeb.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Elsner R. Antioxidant enzymes in ringed seal tissues: potential protection against dive-associated ischemia/reperfusion. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142 (3–4):198–204. doi: 10.1016/j.cbpc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Elsner R. Glutathione protection against dive-associated ischemia/reperfusion in ringed seal tissues. J Exp Mar Biol Ecol. 2007;345 (2):110–118. doi: 10.1016/j.jembe.2007.02.003. [DOI] [Google Scholar]
- Vázquez-Medina JP, Zenteno-Savín T, Tift MS, Forman HJ, Crocker DE, Ortiz RM. Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. J Exp Biol. 2011d;214:4193–4200. doi: 10.1242/jeb.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra JA, Champagne CD, Crocker DE, Ortiz RM. 5′ AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J Endocrinol. 2011a;209 (3):317–325. doi: 10.1530/joe-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra JA, Vázquez-Medina JP, Crocker DE, Ortiz RM. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Reg Integr Comp Physiol. 2011b;300 (1):R150–R154. doi: 10.1152/ajpregu.00478.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm Filho D, Sell F, Ribeiro L, Ghislandi M, Carrasquedo F, Fraga CG, Wallauer JP, Simões-Lopes PC, Uhart MM. Comparison between the antioxidant status of terrestrial and diving mammals. Comp Biochem Physiol A Mol Integr Physiol. 2002;133 (3):885–892. doi: 10.1016/S1095-6433(02)00253-2. [DOI] [PubMed] [Google Scholar]
- Williams TM, Zavanelli M, Miller MA, Goldbeck RA, Morledge M, Casper D, Pabst DA, McLellan W, Cantin LP, Kliger DS. Running, swimming and diving modifies neuroprotecting globins in the mammalian brain. Proc Biol Sci. 2008;275 (1636):751–758. doi: 10.1098/rspb.2007.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HJ, Zhu XH, Luo Q, Wu YN, Kang Y, Jiao JJ, Gao WZ, Liu YX, Lou JS. Noninvasive delayed limb ischemic preconditioning in rats Increases antioxidant activities in cerebral tissue during severe ischemia-reperfusion Injury. J Surg Res. 2010 doi: 10.1016/j.jss.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Zenteno-Savín T, Clayton-Hernandez E, Elsner R. Diving seals: are they a model for coping with oxidative stress? Comp Biochem Physiol C Toxicol Pharmacol. 2002;133 (4):527–536. doi: 10.1016/S1532-0456(02)00075-3. [DOI] [PubMed] [Google Scholar]
- Zenteno-Savín T, St Leger J, Ponganis PJ. Hypoxemic and ischemic tolerance in emperor penguins. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152 (1):18–23. doi: 10.1016/j.cbpc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Yuan HJ, Wu YN, Kang Y, Jiao JJ, Gao WZ, Liu YX, Lou JS, Xia Z. Non-invasive limb ischemic pre-conditioning reduces oxidative stress and attenuates myocardium ischemia-reperfusion injury in diabetic rats. Free Radic Res. 2011;45 (2):201–210. doi: 10.3109/10715762.2010.522576. [DOI] [PubMed] [Google Scholar]