Abstract
Spontaneous respiration influences the body’s center-of-mass when standing. We contend that the healthy postural control system actively adapts to respiration, thereby minimizing its effect on postural sway. We therefore examined the interaction between respiration and postural sway, as measured by center-of-pressure (COP) oscillations, and quantified the extent to which this interaction resulted in “posturo-respiratory synchronization.” We hypothesized that synchronization would be stronger in elderly subjects and those with stroke, and when standing with eyes closed as compared to open, due to alterations in the physiologic mechanisms that normally regulate postural sway. Twenty-five subjects with chronic hemispheric infarction and 38 controls (50–80yrs) stood on a force platform for 3min with eyes-open and 3min with eyes-closed. Respiratory flow and COP dynamics were simultaneously recorded. The dominant oscillatory mode of respiration and the corresponding oscillatory modes of anterioposterior and mediolateral COP dynamics were extracted using ensemble empirical mode decomposition. The strength of posturo-respiratory synchronization was quantified from the regularity of instantaneous phase shifts between extracted respiratory and COP oscillations. Significant posturo-respiratory synchronization was only present in the anterioposterior direction. The strength of synchronization increased with age (p<0.01). Closing the eyes increased synchronization strength in both groups (p=0.01), but more so in stroke patients (p=0.01). These observations suggest that a control system actively regulates the effects of respiration on sagittal-plane postural sway, particularly during eyes-open standing. As evidenced by increased posturo-respiratory synchronization with advanced age and central lesion, this novel metric may be used as a clinical marker of altered postural control.
Keywords: Respiration, Postural Control, Balance, Coupling
INTRODUCTION
Standing requires a complex control system in which visual, vestibular and somatosensory elements, motor circuitry and cardio-respiratory activities function in concert to maintain the body’s center-of-mass within its base-of-support.1–5 Spontaneous breathing influences the body’s center-of-mass by altering trunk volume.6 When healthy adults stand with open eyes, respiration-induced trunk movements are accompanied by phasic muscle activation patterns and related angular movements of the hips and lower-extremity joints.7 It has therefore been postulated that the postural control system accounts for respiration, presumably to minimize its effect on postural sway.7–10 Understanding the dynamic interaction between respiration and postural sway may provide novel insight into fundamental control mechanisms associated with stance in health and disease.
As a first step to formally address the interaction of respiration and postural control, we have introduced a metric termed “posturo-respiratory synchronization.” This metric, which is derived from adaptive signal processing techniques that do not assume linearity or stationarity of the involved signals, estimates the degree to which respiration and postural sway, as measured by center of pressure (COP) excursions beneath the feet, are “synchronized” over time; i.e., stronger synchronization reflects greater influence of respiration on postural sway.
The neuro-anatomical changes to central control elements and/or sensory feedback circuitry that manifest with biological aging and/or disease often impair postural control.11–13 We hypothesized that damage to the postural control system related to aging and stroke impairs the ability to adjust one’s posture to the effects of respiration, leading to stronger posturo-respiratory synchronization. As vision is closely linked to postural control, we further hypothesized that the strength of posturo-respiratory synchronization would be greater when standing with eyes closed as compared to open. To test these hypotheses, we studied posturo-respiratory synchronization during eyes-open and eyes-closed standing in older adults aged 50 to 85 years with and without chronic hemispheric infarctions.
METHODS
Participants
Studies were conducted in the Syncope and Falls in the Elderly Laboratory and the Center for Advanced Magnetic Resonance Imaging at the Beth Israel Deaconess Medical Center. Communitydwelling older adults were consecutively recruited via medical records review and community advertisement. This study was approved by the local Committee on Clinical Investigations and written consent was obtained from all subjects. The entire cohort consisted of 25 participants with a history of stroke and 38 controls.
In the stroke group, we enrolled subjects at least six months after suffering their first large-vessel hemispheric infarct affecting less than one third of the MCA territory, as confirmed by examination of radiological MRI or CT images obtained during the acute phase and a Modified Rankin Scale < 4 (able to walk). Control subjects were free of major disease and musculoskeletal injury and were recruited to match the age, sex and hypertension frequency distributions of the stroke group.
Exclusion criteria were: 1) bilateral infarction; 2) intracranial or subarachnoid hemorrhage on MRI or CT; 3) unstable medical conditions; 4) vertebrobasilar or carotid disease (not associated with stroke), 5) diabetes mellitus; 6) Parkinson’s or other neurological disorder; 7) dementia; 8) valvular heart disease or clinically-significant arrhythmia; 9) morbid obesity (body mass index > 35); 10) severe hypertension (systolic BP>200 and/or diastolic BP>110, or subjects taking three or more antihypertensive medications) and 11) National Institutes of Health Stroke Scale (NIHSS) score > 20.
Protocol
Participants completed anthropometric and neurological assessments, autonomic, cognitive and balance testing as well as whole-brain magnetic resonance imaging. Balance was assessed using a sit-to-stand test. Subjects sat for five minutes with eyes open and legs elevated, and then stood for three minutes on a force platform (Kistler Instrument Corp., Amherst, NY). Subjects stood with eyes open, arms at their side and feet 15 cm apart, and were instructed to breathe normally and avoid extraneous movements. Subjects then repeated the test, except with their eyes closed. Respiratory flow was measured using an end-tidal volume gas monitor (Capnomac Ulitma, Ohmeda, Inc). COP displacement in anterioposterior (AP) and mediolateral (ML) directions were measured using the force platform. Signals were simultaneously recorded at 1000 Hz. The one minute of transition from sitting to standing was excluded from analysis.
Quantification of posturo-respiratory synchronization
All signals were down-sampled to 50 Hz using linear interpolation. We introduce a novel approach to quantify the strength of the phase synchronization between respiratory flow and COP fluctuations in each principle direction. The analysis calculates the degree of posturo-respiratory synchronization by a three-step procedure.
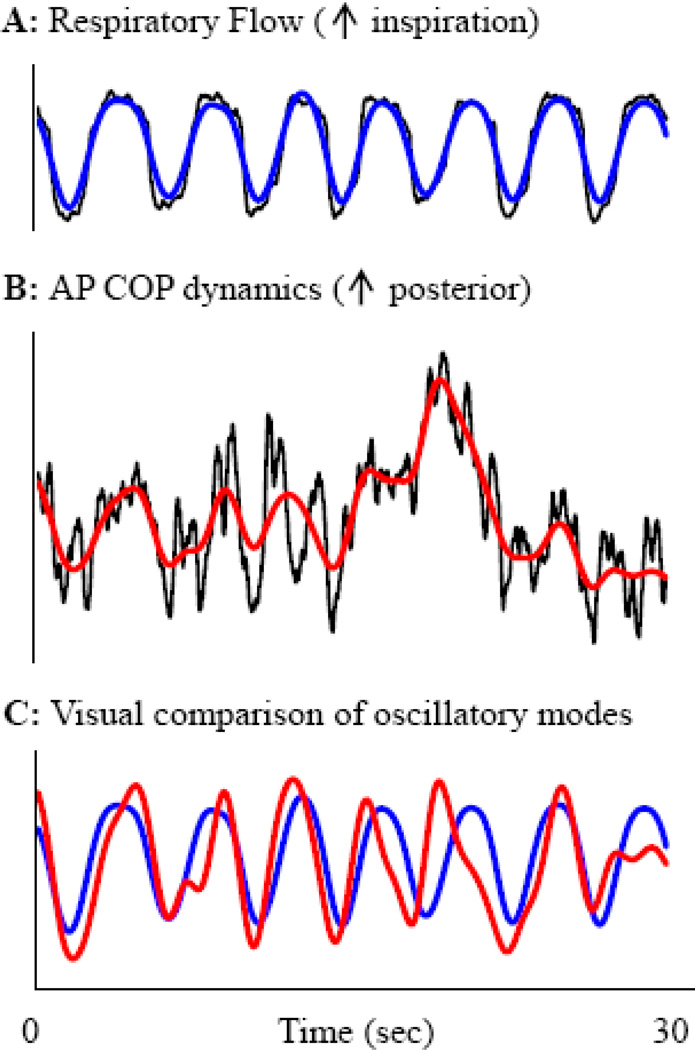
First, ensemble empirical mode decomposition (EEMD) was used to decompose each signal into multiple “intrinsic” or “oscillatory modes,” where each mode represents the frequency-amplitude modulation of the signal at a specific frequency range.14 The EEMD does not assume that signals are stationary or comprised of sinusoidal oscillations with constant amplitude and period. Thus, the EEMD-derived modes better represent nonstationary physiological oscillations than traditional decomposition methods, such as Fourier transform.15 The dominant oscillatory mode of respiration (frequency range: 0.16–0.27 Hz) was identified. This mode, along with the corresponding mode from both the AP and ML COP signals (Figure 1), was selected for further analysis.
Figure 1. Determination of oscillatory modes for posturo-respiratory synchronization analysis.
Respiratory flow (A) and anterioposterior center-of-pressure (B) dynamics were simultaneously recorded over time and compared (C). Empirical mode decomposition was used to decompose each signal into oscillatory modes, each representing the frequency-amplitude modulation of the signal at a specific frequency range (not pictured). The dominant mode of respiration (A, blue line), along with the corresponding mode of the AP COP signal (B, red line), were extracted. Here, for visual purposes, the blue and red curves in panels A and B reflect the dominant modes of each signal summed with all lowerfrequency modes of that signal. The same procedure was repeated for respiratory flow and mediolateral COP fluctuations.
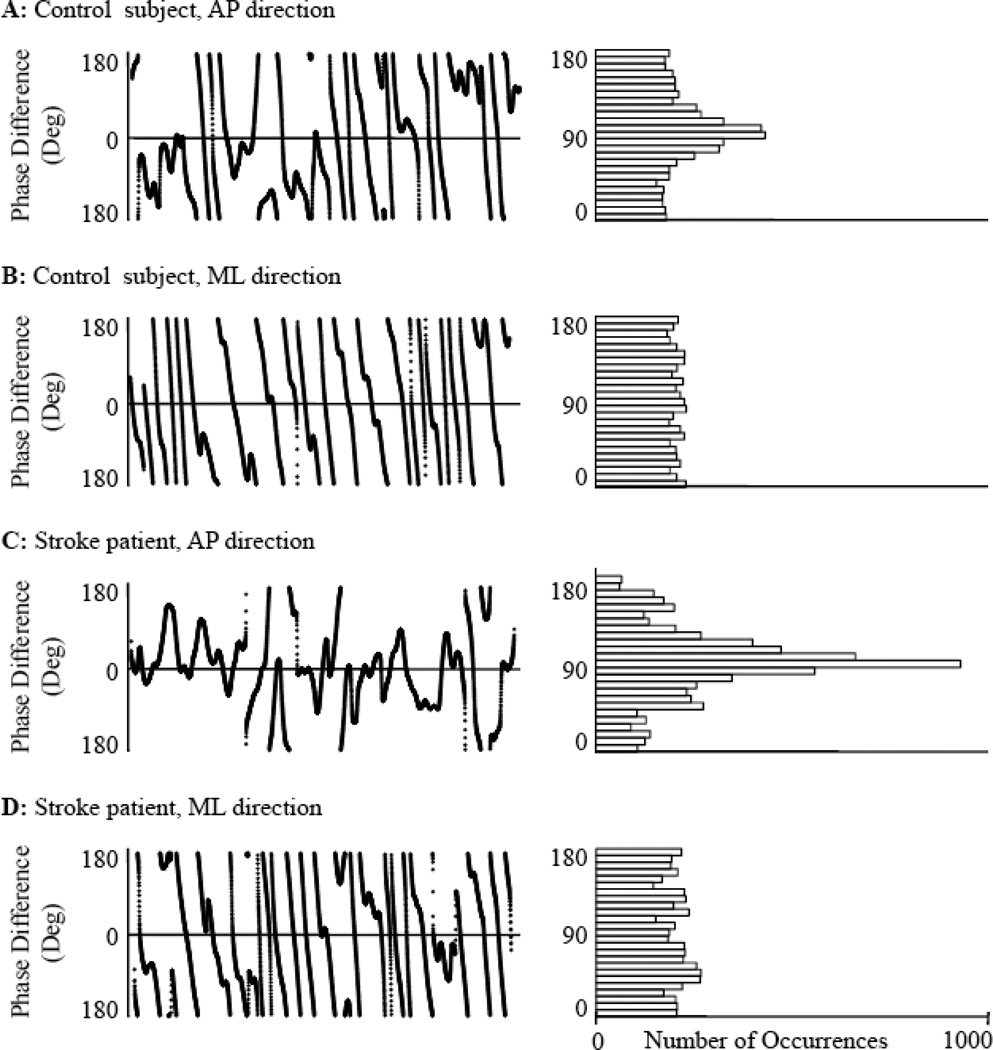
Second, the instantaneous phase difference at each time point along the extracted respiratory and COP oscillatory modes was calculated. Figure 2 illustrates examples of instantaneous phase differences (left panels) and their distribution (right panels) for a control (A-B) and a stroke subject (C-D) in AP and ML directions. As each of the original physiological signals contains inherent nonlinearities and nonstatitionarities (i.e., amplitude and period vary with time), instantaneous phase was calculated using the Hilbert transform.16
Figure 2. Instantaneous phase differences between respiratory flow and center-of-pressure dynamics during quiet standing with eyes open for a control and a stroke subject.
Phase differences (left panels) were calculated between the dominant oscillatory mode of respiratory flow and the corresponding modes of anterioposterior (AP) and mediolateral (ML) center-of-pressure fluctuations for a representative control (A-B) and stroke subject (C-D). Corresponding distributions of instantaneous phase differences are shown in the right panels. Compared to the ML direction for both subjects, the phase differences in the AP direction were more normally distributed from −180 to 180 degrees. Thus, Shannon entropy was smaller and the phase synchronization index was larger (i.e., stronger synchronization) in the AP direction.
Third, the degree of phase synchronization between the respiratory oscillation and the corresponding oscillatory component within the COP signal (AP or ML) was quantified with a synchronization index based on entropy analysis17 as follows: (1) The phase differences (range from −180o to 180o) between respiratory and the COP oscillations were calculated point by point. (2) The Shannon entropy, S, was calculated from the distribution of the phase differences by dividing the data points into non-overlapped bins that cover from −180o to 180o. Larger values of S are associated with more randomly distributed phase differences, thus indicating a weaker synchronization between the two oscillatory signals. Note that the number of bins was selected based on the total point number n (N = int{exp[0.626+0.4 ln(n−1.0)]}) to ensure optimal and reliable estimation of the distribution and the entropy.18 The synchronization index = 1-S/lnN ranges from 0 to 1 with larger values indicating stronger phase synchronization between oscillators (as characterized by more narrowly distributed phase differences, Figure 2).
We note that there are many different techniques to estimate physiological interaction, including mutual information19 and cross-recurrence analysis.20 Unlike our proposed EEMD-based analysis, these methods assume that either (1) each signal contains oscillations over a certain narrow frequency range (e.g., cross-recurrence analysis), or (2) the interaction between two physiological variables is independent of frequency or time scale (e.g., mutual information). These assumptions are often invalid because different physiological functions can affect physiological signals simultaneously at different time scales, leading to time-scale dependent interactions (e.g., blood pressure-flow relationship21). As postural sway contains fluctuations across numerous time-scales12 and respiration is not stationary (e.g., Figure 1), we utilized the EEMD method, which has been shown to be more reliable for extracting nonlinear oscillations from complex fluctuations.23 Moreover, we considered the phase interaction between the extracted COP and respiration oscillations because these phase dynamics are more sensitive to coupling strength than other amplitude-derived markers, especially for weakly coupled oscillators.24
Validation of posturo-respiratory synchronization
A surrogate data analysis technique was performed to determine if the strength of observed posturo-respiratory synchronization was greater than that expected due to chance. The original phase synchronization index was compared to indices derived from surrogate signals generated by shifting the respiration signal in time. We examined the surrogate respiration signals with time shifts from −9 sec to 9 sec (increment = 1sec). For posturo-respiratory synchronization to be non-random, it was expected that the original index value would be significantly larger than those indices generated from surrogate signals.
Quantification of traditional measures of respiration and postural control
Mean respiratory cycle duration (sec) and variability (i.e., standard deviation about the mean) were calculated from the extracted respiratory mode. Traditional measures of COP fluctuations were computed from original COP signals. Average ML and AP range (mm) and variability were computed by determining the scalar displacement mean and standard deviation, respectively, along each principal axis. Average COP speed (mm/s) was computed by dividing total path length by trial duration.
Statistical analysis
Analyses were performed using SAS 9.1 software (SAS Institute, Cary NC). To validate that the calculated strength of posturo-respiratory synchronization was not the result of randomly-generated phase synchronizations, standard least squares models were used to determine if shifting the respiratory time-series significantly altered the strength of posturo-respiratory synchronization. For each subject, separate analyses were completed for eyes-open and eyes-closed conditions, and for synchronization between respiratory flow and the COP time-series of each principle direction (AP and ML).
Descriptive statistics were used to summarize all variables. One-way ANOVAs or Wilcoxon rank-sum tests were used to examine demographics across groups. Repeated-measures ANCOVAs were used to determine the effects of visual condition, stroke, and age on the strength of posturo-respiratory synchronization. Model effects included visual condition as the repeated measure, group, age, and their interactions. Sex and BMI were included as covariates. As secondary analyses, similar models were used to determine the effects of visual condition, age, and stroke on traditional measures of respiration (i.e., respiratory rate and variability) and postural control (i.e., average COP speed, AP/ML range and variability). Significance was determined by p<0.05 for all models.
RESULTS
The stroke group (15 women, 10 men, age=69±4yrs, height=1.7±0.1, body mass=76±14kg) and control group (22 women, 13 men, age=67±5yrs, height=1.7±0.1, body mass= 71±11kg) were similar in gender distribution, age, height and body mass. Within the stroke group, time since stroke was 7.2±5.1yrs and infarct size was 1.3±0.05% of intracranial cavity volume. The degree of stroke-related impairment was mild-moderate, as indicated by NIHSS scores of 1.5±0.04 and modified Rankin Scores of 1.3±0.5.
Posturo-respiratory synchronization
Evidence of respiratory influence on COP fluctuations
It is of note that in Figure 2, for both the control and stroke subject, the distribution of instantaneous phase differences between extracted respiratory flow and COP oscillations appear more uniformly distributed in the AP direction (Panels A and C) as compared to the ML direction (Panels B and D). Surrogate data analysis indicated that in the AP direction, the strength of posturo-respiratory synchronization calculated from the original, non-shifted respiratory flow and COP oscillations was greater (p<0.01) than those derived from surrogate signals for all subjects except one (a 57 year-old control subject during eyes-open standing, p=0.07). Conversely, in the ML direction, synchronization strength derived from original signals did not differ from those derived from surrogate signals for any subject in either visual condition. Thus, posturo-respiratory synchronization in only the AP direction reflected a non-random influence of respiration on COP excursions for all subjects. Therefore, we present only those results of AP posturo-respiratory synchronization.
Effects of age, vision and stroke on posturo-respiratory synchronization
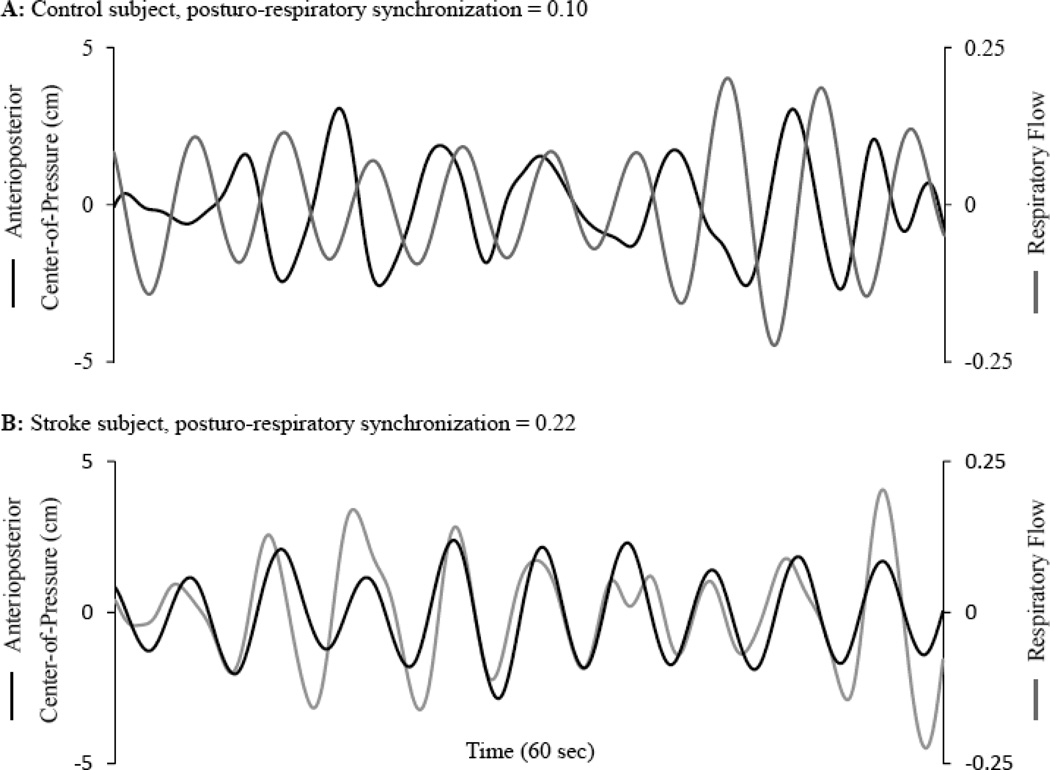
Figure 3 illustrates an example of the dominant oscillatory mode of respiratory flow and the corresponding mode of AP COP fluctuations during eyes-closed standing in a healthy control (A) and a participant with chronic infarction (B).
Figure 3. Relationship between respiratory flow and anterioposterior (AP) center-of-pressure (COP) dynamics during eyes-closed standing.
The dominant oscillatory mode of respiratory flow (black line) plotted against the corresponding oscillatory mode of AP COP fluctuations (gray line) for a healthy control (A) and subject with chronic stroke (B). A posturo-respiratory synchronization value of 0.22 for the subject with stroke, as compared to 0.10 for the healthy control, indicates stronger synchronization; i.e., greater regularity associated with the instantaneous phase-shift between signals over time.
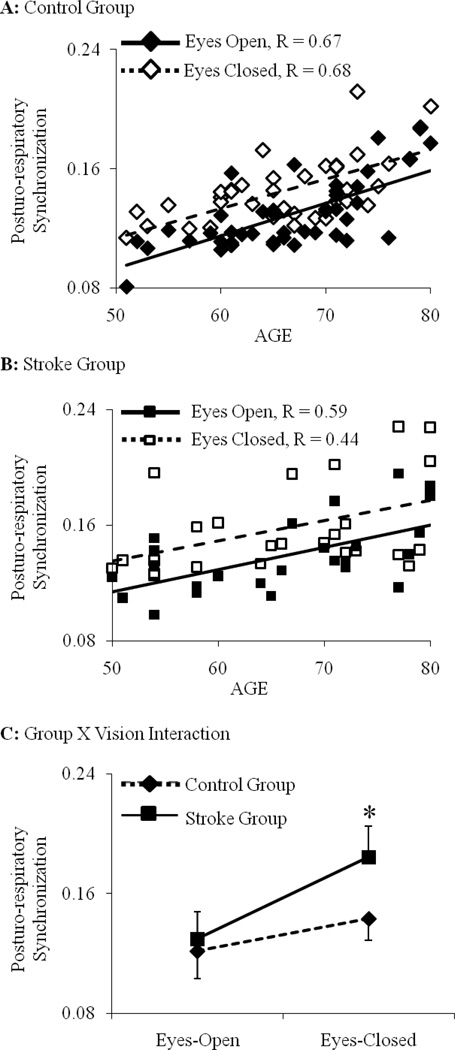
With all subjects combined, the strength of posturo-respiratory synchronization increased with advancing age (F1,63=5.1, p<0.001) (Figure 4). This relationship was independent of visual condition and history of stroke. The strength of posturo-respiratory coupling was similar between groups when standing with eyes open. Closing the eyes resulted in stronger posturo-respiratory synchronization in both groups (F1,63=5.2, p=0.01), yet this effect was more pronounced in the stroke group as compared to controls (F1,63=5.1, p=0.01) (Figure 4).
Figure 4. The effect of age, visual condition and stroke on posturo-respiratory synchronization.
The strength of posturo-respiratory synchronization during eyes-open and eyes-closed standing has been presented for each subject. The strength of synchronization increased as a function of age for (A) healthy older adults and (B) chronic stroke patients in both visual conditions (p < 0.01). This relationship was similar between groups and visual condition. With all subjects combined, the strength of posturo-respiratory synchronization was not different between groups during standing with eyes open (C). Closing the eyes increased the strength of synchronization in both groups, and this increase was significantly greater in subjects with chronic stroke as compared to controls. * indicates a significant interaction between the effects of group and visual condition (p = 0.01).
Traditional measures of respiration and postural control
Respiratory parameters (average rate and variability) were not different between groups or visual condition. Significant interactions between group and visual condition were observed for average COP speed (F1,63=6.7, p=0.009), ML range (F1,63=7.1, p=0.001) and ML variability (F1,63=4.1, p=0.01) (Table 1). For these variables, means were similar between groups during eyes-open standing. Closing the eyes did not affect control group means, but increased speed, ML range, and ML variability in the stroke group. No effects of stroke or visual condition were observed for AP range or variability.
Table 1.
The effect of stroke and visual feedback on traditional measures of respiration and postural control (Means ± SD)
| Control Group | Stroke Group | ||||
|---|---|---|---|---|---|
| Eyes-Open | Eyes-Closed | Eyes-Open | Eyes-Closed | p value* | |
| Respiratory Rate (bpm) | 12.4 ± 3.4 | 11.9 ± 4.4 | 12.1 ± 3.8 | 12.1 ± 3.7 | 0.29 |
| Postural Control (i.e., center-of-pressure) | |||||
| Speed (mm/s) | 27.3 ± 6.4A | 28.1 ± 7.3A | 29.3 ± 7.4A | 37.3 ± 9.9B | 0.009 |
| ML range (mm) | 28.7 ± 16.1A | 26.0 ± 18.0A | 29.2 ± 12.7A | 42.9 ± 19.9B | 0.001 |
| ML variability (mm) | 4.1 ± 0.8A | 3.8 ± 0.3A | 4.6 ± 0.9A | 5.9 ± 0.4B | 0.01 |
| AP range (mm) | 44.4 ± 18.6 | 42.8 ± 17.3 | 43.0 ± 16.3 | 46.2 ± 22.9 | 0.54 |
| AP variability (mm) | 7.6 ± 2.9 | 7.9 ± 3.5 | 8.8 ± 4.4 | 9.0 ± 4.0 | 0.18 |
bold p values represent significant interaction between group and visual condition.
Homogeneous groups within each row. Means with different superscripts are significantly different from one another.
DISCUSSION
We introduced a novel metric, termed posturo-respiratory synchronization, to quantify the influence of respiration on standing postural sway. Our analyses indicated that non-random synchronization exists in the AP direction, but not in the ML direction. The strength of posture-respiratory synchronization in the AP direction increased with advancing age. When standing with eyes open, synchronization strength was similar between the control and stroke groups. Removing visual feedback increased synchronization strength in both groups; however, this increase was significantly greater in those with chronic MCA infarctions.
The relationship between age and increased strength of posturo-respiratory synchronization in the sagittal plane suggests the degradation of an interactive control system that normally adjusts one’s postural movements to the effects of respiration. Although the physiological and/or mechanical mechanisms involved in this phenomenon are unknown, linear-based “time-locked” averaging techniques indicate that when healthy adults stand with eyes-open, respiratory-induced AP trunk movements are accompanied by phasic muscle activation patterns and associated angular movements of the hips and lower-extremities.7 Thus, we contend that respiratory-induced alterations in the body’s center of mass are actively accounted for by the postural control system, at least in part through neuromuscular mechanisms that are altered with advancing age.
Closing the eyes resulted in increased posturo-respiratory synchronization in both healthy older adults and those with history of stroke. In other words, the effects of respiration on postural sway were less when standing with eyes open as compared to eyes closed. One potential explanation for this phenomenon may be that when the eyes are open, the postural control system adjusts for the effects of respiration on postural sway in order to stabilize the head and/or visual system. This notion is supported by previous observations that when standing with eyes open, and particularly when performing visual-based perceptual tasks, upright posture is adjusted to facilitate visual fixation on an object within the environment.24,25 Our finding of age-related increase in the impact of respiration on postural sway during eyes-open standing may therefore partially explain reports of diminished visual-based perceptual performance when standing in older adults.26
It is of further note that in both groups, aging was additionally associated with increased strength of posturo-respiratory coupling when standing with eyes-closed (Figure 4). This observation indicates that the interaction between postural sway and respiration is also regulated in the absence of vision, and that aging affects the non-visual pathways involved in this regulatory process. As such, future research is warranted that utilizes the outlined signal processing techniques to study the dynamic interactions between postural sway, respiration and head/eye movements, together with their relationship to visual condition and performance in cognitive and perceptual tasks.
As compared to controls, stroke patients exhibited stronger posturo-respiratory synchronization during eyes-closed standing, yet maintained similar strength of synchronization during eyes-open standing. Stroke therefore further impairs the non-visual pathways involved in adapting postural control to respiration during eyes-closed standing. Future work should explore specific regions of the MCA territory for their role in regulating respiratory-induced movements of the trunk. At the same time, this observation suggests that stroke patients were able to compensate for stroke-induced neuromuscular impairments when standing with eyes open. Vision may therefore play an active role in regulating the respiratory effects on postural sway, and patients with stroke may be more dependent upon visual feedback. This notion is supported by previous studies.13,27 For example, we observed increased visual dependence for standing postural control in patients with chronic stroke.13 Patients with right hemisphere MCA infarcts demonstrated greater sway velocity, ML range and ML variability when standing with eyes closed as compared to open, whereas control subjects showed no significant changes in these measures across visual conditions. In terms of posturo-respiratory synchronization, our current analyses suggest that non-visual control pathways were unable to fully compensate for the absence of vision, as indicated by significant increases in synchronization strength when both controls and stroke patients closed their eyes.
The influence of respiration on postural sway in the AP direction, but not the ML direction, may reflect biomechanical differences in postural alignment. Previous reports indicate that respiration induces greater movement of the body’s trunk in the AP direction.1 Respiration may thus not induce large enough ML perturbations to significantly impact sway excursions in this direction. Additionally, the presence of posturo-respiratory synchronization only in the AP direction, together with the observation that chronic infarction increased average postural sway speed and magnitude during eyes-closed standing only in the ML direction, supports the notion that AP and ML fluctuations are regulated using fundamentally different control strategies.28 In this case, chronic brain damage in the MCA territory, which is known to degrade the ability to determine the body’s vertical position in the frontal plane in the absence of visual feedback,27 may disrupt the capacity to minimize postural sway excursions in the ML direction while simultaneously altering the interaction between respiration and postural control in the AP direction.
Changes in posturo-respiratory synchronization between conditions and across groups may have stemmed from alterations in respiratory patterns. This is unlikely, however, as no differences in the mean rate or variability of respiration were observed among subjects of different age, across visual conditions or between groups. As we utilized an end-tidal volume monitor to measure respiratory flow, research is needed that combines the current analytical techniques with kinematic and electromyographic instrumentation to determine the relationship between posturo-respiratory synchronization, postural muscle activation and movements of the trunk, hips and lower-extremities.
In conclusion, we have introduced a novel metric to quantify posturo-respiratory synchronization by determining the degree of phase synchronization between the dominant oscillations of respiration and corresponding oscillations within postural sway dynamics. Using this metric, we observed a delicatelymaintained interaction between respiration and standing posture in the AP direction. Increased strength of posturo-respiratory synchronization with advancing age and stroke may indicate a more simple control system that possesses less capacity to compensate for the stressors of everyday life.
Highlights.
We introduced a new metric, posturo-respiratory synchronization, to study the influence of respiration on postural sway.
Nonrandom posturo-respiratory synchronization was observed only in the anterioposterior direction.
Synchronization strength increased with advancing age, indicating greater influence of respiration on postural sway.
When standing with eyes open, synchronization strength was similar between healthy older adults and patients with stroke.
Removing visual feedback increased the strength of synchronization, particularly in those who had suffered a stroke.
Acknowledgements
This study was supported by the NIH (R01-NS045745, AG023480), the American Diabetes Association (1-06-CR-25), and the National Center for Research Resources Clinical Translational Science Award (UL 1RR025758) to Harvard University and the Beth Israel Deaconess Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Hunter IW, Kearney RE. Respiratory components of human postural sway. Neurosci Lett. 1981;25:155–159. doi: 10.1016/0304-3940(81)90324-4. [DOI] [PubMed] [Google Scholar]
- 2.Horak F, MacPherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepard JT, editors. Handbook of Physiology. New York: Oxford University Press; 1996. [Google Scholar]
- 3.Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12:635–658. [PubMed] [Google Scholar]
- 4.Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandevia SC, Butler JE, Hodges PW, Taylor JL. Balancing acts: respiratory sensations, motor control and human posture. Clin Exp Pharmacol Physiol. 2002;29:118–121. doi: 10.1046/j.1440-1681.2002.03611.x. [DOI] [PubMed] [Google Scholar]
- 6.Gurfinkel VS, Kots YM, Paltsev EI, Feldman AG. The compensation of respiratory disturbances of erect posture of man as an example of the organisation of interarticular interaction. In: Gelfand IM, Gurfinkel VS, Formin SV, Tsetlin ML, editors. Models of the structural functional organisation of certain biological systems. Cambridge: MIT Press; 1971. [Google Scholar]
- 7.Hodges PW, Gurfinkel VS, Brumagne S, Smith TC, Cordo PC. Coexistence of stability and mobility in postural control: evidence from postural compensation for respiration. Exp Brain Res. 2002;144:293–302. doi: 10.1007/s00221-002-1040-x. [DOI] [PubMed] [Google Scholar]
- 8.Sakellari V, Bronstein AM, Corna S, Hammon CA, Jones S, Wolsley CJ. The effects of hyperventilation on postural control mechanisms. Brain. 1997;120:1659–1673. doi: 10.1093/brain/120.9.1659. [DOI] [PubMed] [Google Scholar]
- 9.Schmid M, Conforto S, Bibbo D, D'Alessio T. Respiration and postural sway: detection of phase synchronizations and interactions. Hum Mov Sci. 2004;23:105–119. doi: 10.1016/j.humov.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Coppieters MW, Hodges PW. Effect of experimentally induced low back pain on postural sway with breathing. Exp Brain Res. 2005;166:109–117. doi: 10.1007/s00221-005-2352-4. [DOI] [PubMed] [Google Scholar]
- 11.Barra J, Marquer A, Joassin R, Reymond C, Metge L, Chauvineau V, Pérennou D. Humans use internal models to construct and update a sense of verticality. Brain. 2010;133:3552–3563. doi: 10.1093/brain/awq311. [DOI] [PubMed] [Google Scholar]
- 12.Manor B, Costa MD, Hu K, Newton E, Starobinets O, Kang HG, Peng CK, Novak V, Lipsitz LA. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol. 2010;109:1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manor B, Hu K, Zhao P, Selim M, Alsop D, Novak P, Lipsitz L, Novak V. Altered control of postural sway following cerebral infarction: a cross-sectional analysis. Neurology. 2010;74:458–464. doi: 10.1212/WNL.0b013e3181cef647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang NE, Wu ML, Long SR, Shen SS, Qu WD, Gloersen P, Fan KL. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc Roy Soc Long A. 2009;454:903–993. [Google Scholar]
- 15.Xu L, Chen Z, Hu K, Stanley HE, Ivanov PC. Spurious detection of phase synchronization in coupled nonlinear oscillators. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73 doi: 10.1103/PhysRevE.73.065201. 065201. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. New Jersey: Prentice Hall; 1999. [Google Scholar]
- 17.Tass P, Rosenblum MG, Weule J, Kurths J, Pikovsky A, Volkmann J, Schnitzler A, Freund HJ. Detection of n:m phase locking from noisy data: Application to magnetoencephalography. Phys Rev Lett. 1998;81:3291–3294. [Google Scholar]
- 18.Otnes R, Enochson L. Digital Time Series Analysis. New York: Wiley; 1972. [Google Scholar]
- 19.Palus M, Hoyer D. Detecting nonlinearity and phase synchronization with surrogate data. IEEE Eng Med Biol Mag. 1998;17:40–45. doi: 10.1109/51.731319. [DOI] [PubMed] [Google Scholar]
- 20.Shockley K, Butwill M, Zbilut JP, Webber CL. Cross recurrence quantification of coupled oscillators. Phys Let. 2002;305:59–69. [Google Scholar]
- 21.Novak V, Yang AC, Lepicovsky L, Goldberger AL, Lipsitz LA, Peng CK. Multimodal pressure-flow method to assess dynamics of cerebral autoregulation in stroke and hypertension. Biomed Eng Online. 2004;3:39. doi: 10.1186/1475-925X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Huang NE, Long SR, Peng CK. One the trend, detrending, and variability of nonlinear and nonstationary time series. Proc Natl Acad Sci U S A. 2007;104:14889–14894. doi: 10.1073/pnas.0701020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996;76:1804–1807. doi: 10.1103/PhysRevLett.76.1804. [DOI] [PubMed] [Google Scholar]
- 24.Bardy BG, Marin L, Stoffregen TA, Bootsma RJ. Postural coordination modes considered as emergent phenomena. J Exp Psychol Hum Percept Perform. 1999;25:1284–1301. doi: 10.1037//0096-1523.25.5.1284. [DOI] [PubMed] [Google Scholar]
- 25.Stoffregen TA, Hove P, Bardy BG, Riley MA, Bonnet CT. Postural stabilization of perceptual but not cognitive performance. J Mot Behav. 2007;39:126–138. doi: 10.3200/JMBR.39.2.126-138. [DOI] [PubMed] [Google Scholar]
- 26.Prado JM, Stoffregen TA, Duarte M. Postural sway during dual tasks in young and elderly adults. Gerontology. 2007;53:274–281. doi: 10.1159/000102938. [DOI] [PubMed] [Google Scholar]
- 27.Pérennou DA, Mazibrada G, Chauvineau V, Greenwood R, Rothwell J, Gresty MA, Bronstein AM. Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain. 2008;131:2401–2413. doi: 10.1093/brain/awn170. [DOI] [PubMed] [Google Scholar]
- 28.Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]