Abstract
Curcumin is a widely-used herbal medicine for various human diseases including inflammation and cancer. The demonstration and optimization of curcumin’s activities in the clinical setting, however, has been compromised by its poor bioavailability and the lack of analytic methods to monitor its absorption. In this paper, we report the first validated liquid chromatography-tandem mass spectrometric method for simultaneous quantification of curcumin and its major metabolite: curcumin-O-glucuronide (COG), in the linear range of 2.0–2000 ng/mL in human plasma. The intra-day and inter-day accuracies of curcumin and COG in human plasma were in the range of 91.3–111.5% and 82.7–109.2% and their co-efficiency of variations were in the range of 3.5–12.7% and 3.1–11.3%, respectively. This method was capable of detecting only COG in human plasma samples from two healthy volunteers after an oral ingestion of curcumin.
Keywords: curcumin, curcumin-O-glucuronide, liquid chromatography-tandem mass spectrometry, Pharmacokinetics
1.0. INTRODUCTION
Curcumin is a natural polyphenol extracted from the rhizome of turmeric (Curcuma longa) [1, 2]. Curcumin possesses a wide range of biological and pharmacological activities including anti-oxidant, anti-inflammatory and anti-tumor effects, most of which have been elucidated from preclinical studies [3]. Due to the lack of analytic methods to monitor its absorption, current application of curcumin in clinical setting has not been optimized [4]. Pharmacokinetic (PK) studies of curcumin in humans have consistently reported low plasma curcumin levels (<50 ng/mL) after oral ingestion of curcumin up to 12 g/day [5, 6]. Factors contributing to its low oral bioavailability include its poor solubility and absorption, and rapid metabolism [4]. Metabolic studies have demonstrated that orally ingested curcumin is extensively transformed to curcumin-O-glucuronide (COG) and curcumin-O-sulfate (COS) in both rodents [7, 8] and human [6]. As the major metabolite, COG may be explored as a potential marker for evaluating curcumin absorption and monitoring the compliance of curcumin consumption. Up to date, the plasma level of COG has been determined indirectly by quantifying curcumin generated from glucuronidase hydrolysis of plasma samples [6]. In this study, a sensitive LC-MS/MS method for direct quantification of COG and curcumin has been established and validated in human plasma.
2.0. MATERIALS AND METHODS
2.1. Reagents and chemicals
Curcumin (>98%) was purchased from Acros Organics (Acros Organics, Morris Plains, NJ) and used for developing a calibration curve without further purification. Curcumin O-glucuronide (COG) was custom-synthesized from Cell Mosaic (Worcester, MA). Curcumin C3 complex (CC3C) was purchased as a dietary supplement from Ageless Inc. (Ann Arbor, MI). The internal standard (I.S.), hesperetin, was obtained as a white powder from the National Cancer Institute (NCI) and used without further purification. Analytical HPLC grade acetonitrile, methanol, ethyl acetate and formic acid were obtained from Fisher Scientific (Waltham, MA). Heparin-treated human plasma was obtained from LAMPIRE Biological Laboratories, Inc. (Pipersville, PA). A Barnstead E-pure water purification system (Dubuque, IA.) was used to obtain HPLC grade water (>18 mΩ).
2.2. HPLC chromatographic and mass spectrometric conditions
Liquid chromatography was performed on a Shimadzu HPLC system (Shimadzu, Columbia, MD) consisting of a CBM-20A system controller, an LC-20 AD pump, a SIL-20AC auto-sampler, and a DGU-20A5 degasser. Curcumin, COG and I.S. (hesperetin) were separated on a BetaBasic C8 column (2.1mm×50mm, 5 µm, Thermo Hypersil-Keystone, Bellefonte, PA) coupled with a Beta C8 guard column (2.1mm×10mm, 2 µm, Thermo Hypersil-Keystone, Bellefonte, PA) under an isocratic elution at a flow rate of 0.2 mL/min. The mobile phase consisted of 50% acetonitrile in water containing 0.1% formic acid.
Curcumin, COG and the I.S. were monitored using a Finnigan TSQ Quantum EMR Triple Quadrupole mass spectrometer (Thermo Fisher Scientific Corp., San Jose, CA) equipped with an electro-spray ionization (ESI) source. Xcalibur software (Home Page Version 1.4 SR1) was used for system control and data processing. The mass spectrometer was operated in positive ESI mode with a collision gas (Argon) pressure of 1.5 mTorr, a typical electro-spray needle voltage of 4900 V, a sheath nitrogen gas flow of 49 (arbitrary unit) and a heated capillary temperature of 325°C. Curcumin, COG and the I.S. were analyzed by the multiple reaction monitor (MRM) mode using ion transitions at a proper collision energy as follows: curcumin m/z 369 >m/z 177 (E = 20%), COG m/z 545 >m/z 369 (E = 15%), and the I.S. m/z 303 >m/z 177 (E = 20%). The mass spectrometer was tuned to its optimal sensitivity by direct infusion of curcumin.
2.3. Preparation of calibration standards and quality controls for LC-MS/MS analysis
For construction of the calibration curve, a 10 µL aliquot of the appropriate standard working solutions of curcumin and COG (20 to 20, 000 ng/mL) and 10 µL hesperetin (10 µg/mL) were added to 100 µL human plasma to give plasma samples containing 2, 5, 10, 20, 50, 100, 200, 500, 1000 and 2000 ng/mL curcumin and COG, respectively. Quality controls (QCs) were prepared separately at 2, 5, 50 and 500 ng/mL concentrations. The solution was vortex-mixed and diluted with 900 µL 0.15 M phosphate saline buffer (PBS). The resulting solutions were extracted with ethyl acetate and the organic layer was collected and transferred to glass tubes. Ethyl acetate was evaporated under a stream of nitrogen. The residue was then reconstituted in 100 µL of mobile phase. The reconstituted solution was centrifuged at 12,000 g for 2 min and a 25 µL aliquot of the supernatant was subjected to LC-MS/MS analysis.
2.4. Stability and carry over evaluation of curcumin and COG in human plasma
The carry-over of curcumin and COG was evaluated at the highest test concentration of curcumin and COG (2.0 µg/mL) spiked in human plasma. To evaluate the stability of curcumin and COG in human plasma, an appropriate volume of curcumin or COG stock solution was added to human plasma (2 mL) to yield concentrations of 0.5 and 2 µg/mL. The mixtures were then incubated in an ice-water container (4 °C), room temperature (22–25°C), and 37°C water bath, respectively. A 200 µL aliquot of each sample was collected at 0 and 30 min, and 1, 2, 4 h (for all temperatures), 8 and 24 h (for 37°C only) and stored immediately at −80°C till analysis. To evaluate the two-week storage stability of curcumin and COG, human plasma samples were spiked with 50, 500, and 2000 ng/mL curcumin and COG, respectively, was prepared on day 1, 7 and 13, and then these samples were stored in an −80 °C freezer. One day 14, all these samples were analyzed using the LC-MS/MS method. To evaluate the freeze-thaw stability of curcumin and COG, the above prepared human plasma samples were thawed at 4 °C for approximately 30 min and were restored at −80 °C for the next freezing cycle at 6 h intervals. To evaluate the stability of curcumin and COG in the reconstituted solution of human plasma extract in the auto-sampler, these samples were prepared from the reconstituted solution of human plasma extract spiked with curcumin and COG (10, 50, 500 ng/mL), and then subjected to LC-MS/MS analysis every hour up to 6 h.
2.5. Recovery and matrix effects
A post-extraction spike experiment was performed to evaluate the recovery and matrix effects of hesperetin, curcumin and COG in human plasma as follows: Three separate groups of curcumin and COG samples at concentrations of 5, 50 and 500 ng/mL, and the I.S. at 1000 ng/mL, were prepared as follows: Group A, the I.S., curcumin and COG were prepared directly in mobile phase; Group B, blank human plasma was acidified with PBS and then extracted with ethyl acetate, the residue of the ethyl acetate layer was reconstituted in mobile phase, then, the I.S., curcumin and COG were spiked into the reconstituted solution, and Group C, blank human plasma spiked with the I.S., curcumin and COG was acidified with PBS and then extracted with ethyl acetate, the residue of the ethyl acetate layer was reconstituted in mobile phase. The recovery of the I.S., curcumin and COG were calculated by the ratio of peak areas of the I.S., curcumin and COG of the Group C samples to those of Group B samples. Their matrix effects were evaluated by the ratio of peak areas of Group B to that of Group A samples.
2.6. LC-MS/MS assay validation
The intra-day validation was determined in six replicates at concentrations of 2, 5, 50 and 500 ng/mL curcumin and COG and the inter-day validation was determined across these concentrations in triplicates on three different days. The mean concentrations and the coefficient of variation (CV) of intra-day were calculated as the relative standard deviation (%) from these replicates and the CV of inter-day was calculated as the relative standard deriviation (%) of the respective mean concentrations on each individual day for three days. The accuracy of the assay was determined by comparing the corresponding calculated mean concentrations with the nominal concentrations. The lower limit of quantification (LLOQ) was defined as the lowest concentration with a signal/noise ratio of the analyte peak >10.
2.7. Analysis of curcumin and COG in human plasma samples
Two healthy human subjects provided written informed consent for study participation in accordance with the protocol ((2010H0189) approved by the Institutional Review Board of the Ohio State University. A 100 µL aliquot of human plasma collected from healthy volunteers after an oral ingestion of curcumin C3 complex was mixed with 10 µL I.S. solution (10 µg/mL) and the mixture was extracted with ethyl acetate as described in Section 2.3.. An aliquot of 25 µL reconstituted solution was injected for LC-MS/MS analysis.
3.0. RESULTS and DISCUSSIONS
3.1. Mass spectrometric characterization of curcumin and COG
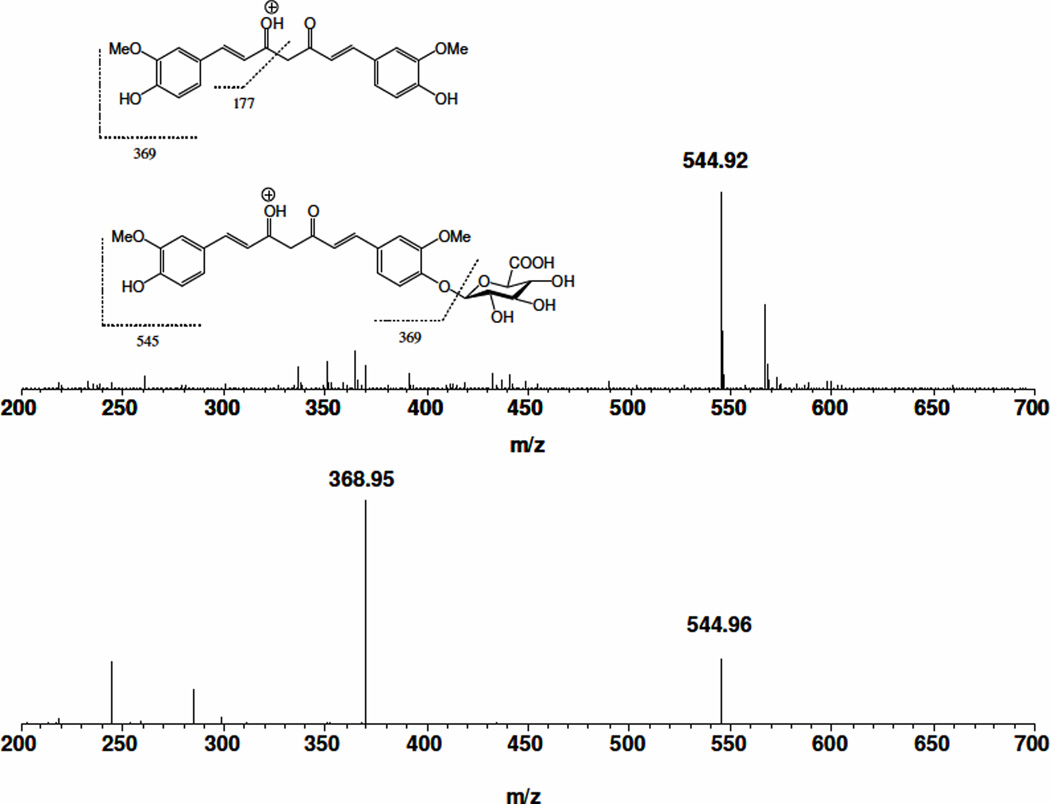
The chemical authenticity and mass and tandem mass spectra of curcumin have been reported [9]. Chemical authenticity of COG was confirmed on a TSQ quantum triple quadruple mass spectrometer. Under a positive ion mode, standard solutions of 10 µg/mL COG in 50% acetonitrile containing 0.1% formic acid were infused with an LC flow of 0.2 mL/min into a TSQ mass spectrometer electro-spray ion (ESI) source. The full scan mass spectrum (Figure 1A) of COG showed a predominant ion at m/z 545, corresponding to its [M+H]+ ion. The [M+H]+ ion of COG was subjected to collision induced dissociation at appropriate collision energy. The parent ions underwent significant fragmentation to yield the most abundant fragment ion at m/z 369 (Figure 1B), corresponding to the protonated molecular ion of curcumin. This fragment is formed by glycosidic cleavage of COG by removal of dehydrated glucuronic acid. Therefore, the following transitional channel: m/z 545→369 was selected for monitoring COG,
Figure 1.
Full scan mass spectrum (A, 200–700 Th) of COG and tandem mass spectrum (B) of the protonated molecular ion of COG at m/z 545. The putative fragmentation pathways of curcumin and COG are inserted as an inset in A.
3.2. Optimization of extraction conditions of curcumin and COG from human plasma
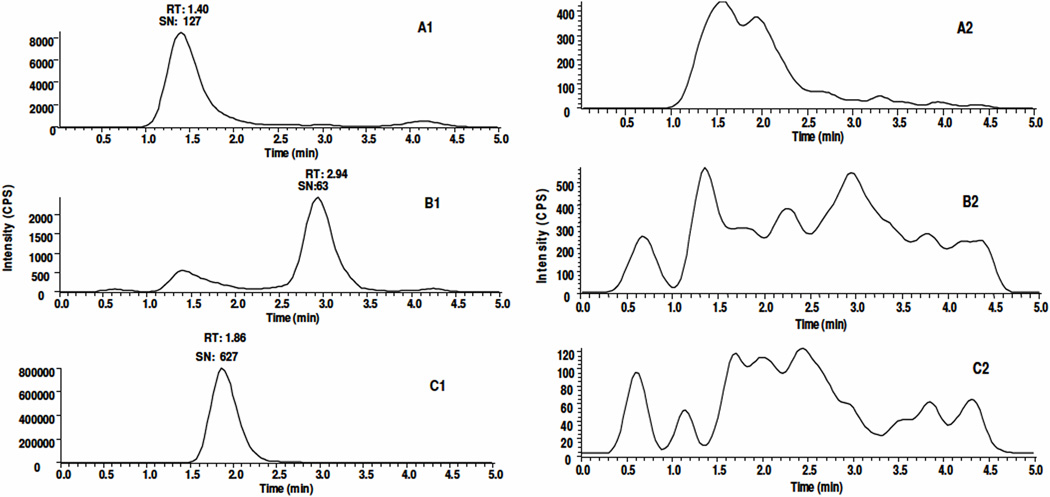
COG is relatively hydrophilic compared to curcumin due to its conjugation to glucuronic acid. We predicted therefore, that COG would elute ahead of curcumin on a reversed-phase column. As shown in Figure 2, there were peaks at 1.40, 2.94, 1.86 min in the extracted ion chromatograms representing COG (A1), curcumin (B1) and I.S. (C1), respectively. Although COG and I.S. eluted within a similar time frame, their mass chromatograms could be clearly distinguished since they have a different protonated molecular ion and corresponding fragment ion. There were no interference peaks at their corresponding retention times as shown in Figures 2A2, 2B2, and 2C2 in the reconstituted residue from blank human plasma, which demonstrates the specificity of this method for the detection and quantification of curcumin and COG in human plasma. To extract curcumin and COG from human plasma, an established extraction method for curcumin in mouse plasma was tested using ethyl acetate [9]. A consistent recovery rate of curcumin (~40%) from human plasma at neutral pH was observed. However, at neutral pH, the recovery of COG was extremely low (<10%) and fluctuated significantly. This could be due to its higher hydrophilicity when compared to curcumin. Additionally, COG is a weak acid and the pKa value of COG is much smaller than curcumin. Therefore, the major form of COG is deprotonated anion and exists as a metal ion adduct under neutral condition, which could limit its extraction from plasma. Hence, acidification of human plasma could decrease the ionization form of COG and facilitate its extraction from aqueous solution using organic solvents. Previous studies have shown that curcumin has greater stablility in a weakly acidic environment (pH 3.0–6.5) [1, 6]. After several attempts for pH optimization, we found that the recovery of both curcumin and COG reached an optimal yield at pH 3.2. Next, matrix effects and extraction recoveries were evaluated for curcumin and COG at concentrations of 5, 50 and 500 ng/mL and for I.S. at 1000 ng/mL in each matrix. The matrix effects and recovery of I.S. were determined to be approximately 86–93% and 73–75%, respectively. The matrix effects of curcumin and COG were determined to be about 99–116 and 80–105% and its recovery was about 49–57% and 35–48%, respectively (Table 1).
Figure 2.
Extracted ion chromatograms of COG (545>369, A1), curcumin (369>173 B1), and I.S. (303>177, C1) in human plasma spiked with 2 ng/mL curcumin and COG and 1000 ng/mL I.S. A2, B2 and C2 are the corresponding extracted ion chromatograms of the analytes in blank human plasma, respectively. The retention time of COG, curcumin and I.S. are 1.40, 2.94, 1.86 min, respectively. RT: Retention Time, SN: Signal to Noise Ratio
Table 1.
Assay validation parameters of curcumin (A) and COG (B) in human plasma
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Curcumin (ng/mL) |
Recovery (%) |
Matrix effects (%) |
Intra-day | Inter-day | ||||
| Mean | C.V. (%) |
Accuracy (%) |
Mean | C.V. (%) |
Accuracy (%) |
|||
| 2 | ND* | ND | 2.23 | 8.32 | 112 | 1.99 | 11.1 | 99.5 |
| 5 | 48.8 | 116 | 4.98 | 7.03 | 99.6 | 4.99 | 5.18 | 99.9 |
| 50 | 54.6 | 99.2 | 45.7 | 3.45 | 91.3 | 50.7 | 6.93 | 101 |
| 500 | 57.1 | 99.8 | 506 | 5.61 | 101 | 503 | 12.7 | 101 |
| B | ||||||||
|---|---|---|---|---|---|---|---|---|
| COG (ng/mL) |
Recovery (%) |
Matrix effects (%) |
Intra-day | Inter-day | ||||
| Mean | C.V. (%) |
Accuracy (%) |
Mean | C.V. (%) |
Accuracy (%) |
|||
| 2 | ND | ND | 1.65 | 9.73 | 82.7 | 2.17 | 11.3 | 109 |
| 5 | 47.9 | 105 | 4.63 | 4.14 | 92.6 | 5.26 | 6.08 | 105 |
| 50 | 37.5 | 80.2 | 48.9 | 6.44 | 97.9 | 53.8 | 6.26 | 107 |
| 500 | 35.3 | 82.0 | 511 | 3.12 | 102 | 546 | 8.21 | 109 |
ND: not determined
3.3. Method development and validation
Using the aforementioned extraction method and LC conditions, the linearity of the method was tested over the concentration range of 2.0–2000 ng/mL for both curcumin and COG. Linearity was found with a linear regression coefficient greater than 0.99. The method was validated for both curcumin and COG at concentrations of 2, 5, 50 and 500 ng/mL with six replicates per concentration. The intra-day and inter-day accuracies along with percentage relative standard derivation values for human plasma are presented in Table 1. As shown, the accuracy values of curcumin and COG were in the range of 91.3–111% and 82.7–109%, respectively. The CVs of curcumin and COG were in the range of 3.5–12.7% and 3.1–11.3%, respectively. All the intra-day and inter day precision and accuracy values are acceptable according to the FDA criterion of a GLP analytic method validation (Guidance for Industry Bioanalytical Method Validation, p.5, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf).
3.4. The carry-over and stability of curcumin and COG in human plasma
The carry-over and stability of curcumin and COG in human plasma was evaluated under several conditions as detailed in the Experimental Section 2.4. No carry-over of curcumin and COG was noticed at the highest test concentration of curcumin and COG.
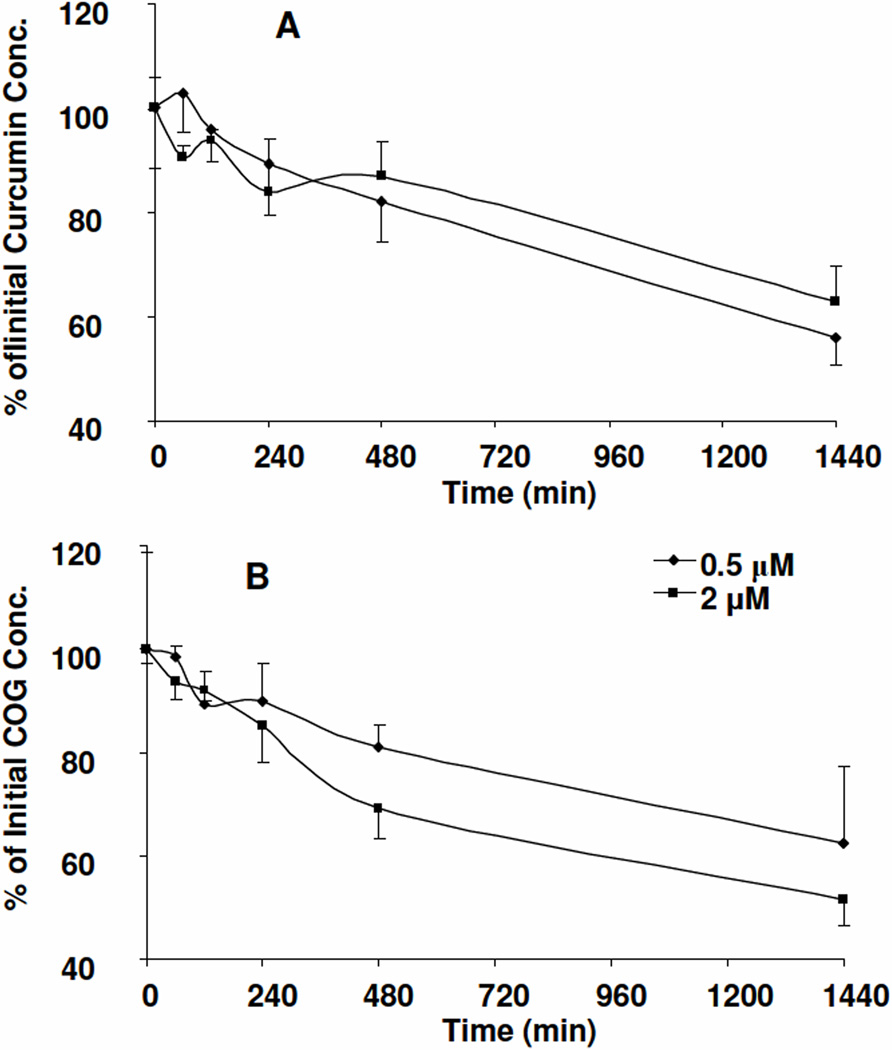
For their stability at 4 °C, room temperature, and 37 °C, it was found that >90% curcumin and COG (0.5 to 2.0 µg/mL) remains unchanged in human plasma in 2 h at 37 °C (Figure 3) and > 85% curcumin and COG remains unchanged in human plasma at 4 °C and room temperature. These data suggested that both curcumin and COG are quite stable in human plasma for the first 2 h at 37 °C, and 4 h at room temperature and 4 °C. Therefore, no special treatment and precautions are needed for clinical samples process for future clinical pharmacokinetic studies of curcumin and COG. Kinetics analysis of the concentration-time profile of COG and curcumin at 37 °C demonstrated that the half-lives of curcumin at 0.5 and 2.0 µg/mL in human plasma were 36.9±15.9 h and 23.1±2.8 h, respectively (Figure 3A), and the half-lives for COG at 0.5 and 2.0 µg /mL were 27.2±6.07 h and 34.6 ±6.87 h, respectively (Figure 3B). The half-lives of curcumin and COG at these two concentrations were not significantly different (p>0.05, paired test). Therefore, curcumin and COG had similar stability profiles in human plasma. Notably, the degradation half-life of curcumin in human plasma (23 to 36.9 h) was much longer than that in mouse plasma (3.1 h) [9].
Figure 3.
Stability profile of curcumin (A) and COG (B) in human plasma at 37 °C for 24 h (n=3).
For freeze-thaw stability, after three freeze-thaw cycles, 104%, 91%, and 111% of curcumin and 120%, 110%, and 108% of COG remained unchanged at concentrations of 50, 500, and 2000 ng/mL respectively. This result indicated that curcumin and COG are relatively stable in human plasma over three freeze-thaw cycles.
For the storage stability of curcumin and COG at concentrations of 10, 50 and 500 ng/mL in a −80 °C freezer was evaluated in human plasma for 8 and 15 days. It was found that 103.3, 101.9, 95.5 of curcumin, 105.5, 127.9% and 117.4% of COG were retained at concentrations of 10, 50, and 500 ng/mL respectively when compared to freshly prepared samples. This indicated that curcumin and COG are relatively stable in human plasma over two weeks. A long-term stability study up to 1 year is ongoing.
Last, we have evaluated sample stability on the reconstituted solution of human plasma extract in an auto-sampler (4 °C), it was found that 6 h storage in an autosampler 93% of curcumin and 91% of COG remained unchanged. This indicates that extracted curcumin and COG are quite stable in the auto-sampler over 6 h.
3.5. Quantification of curcumin and COG plasma levels in healthy volunteers after an oral administration of curcumin C3 complex
With the afore-described method for quantification of curcumin and COG in human plasma, the curcumin and COG levels in plasma samples collected from two healthy volunteers (S1 and S2) after an oral ingestion of 4 g curcumin as curcumin C3 complex were determined. No curcumin was detected in all plasma samples from these two subjects. COG was detected from 30 min (10.6 and 10.9 ng/mL) up to 24 h (2.1 and 8.7 ng/mL) with peak plasma levels of 35.3 and 167.5 ng/mL at 45 or 60 min in S1 and S2, respectively. This result is consistent with non-detectable level of curcumin at the low dose and low levels of curcumin at high dose of curcumin C3 complex (8–10 g) in human subjects [6, 10].
4.0. CONCLUSIONS
An LC-MS/MS method for direct quantification of COG and curcumin was developed in human plasma and this method is capable of detecting COG in human plasma up to 24 hrs after an oral administration of 4 g curcumin as curcumin C3 complex. This direct method is more efficient in COG quantification when compared with currently-used indirect methods through avoiding repeat sampling and analysis, and the pretreatment of the biological matrices with β-glucuronidase. Therefore, this method provided a convenient translational tool for the future clinical pharmacological study of curcumin.
Highlights.
First direct method to measure curcumin-O-glucuronide (COG) in human plasma
The LLOQ is 2 ng/mL with CVs (<15%) and Accuracy (85% to 115%)
Capable of characterizing the pharmacokinetics of COG up to 24 hr in human
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health (NIH) grants [R21 CA135478], the National Center for Research Resources [UL1RR025755] and Biomedical Mass Spectrometric Laboratory at The Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G. J Colloid Interface Sci. 2011;354:116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Mukerjee A, Vishwanatha JK. Anticancer Res. 2009;29:3867–3875. [PubMed] [Google Scholar]
- 3.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 6.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai A, Miyazawa T. Life sciences. 2000;67:2785–2793. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 8.Pan MH, Huang TM, Lin JK. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 9.Vijaya Saradhi UV, Ling Y, Wang J, Chiu M, Schwartz EB, Fuchs JR, Chan KK, Liu Z. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3045–3051. doi: 10.1016/j.jchromb.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. BMC complementary and alternative medicine. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]