Abstract
Objective
Type II collagen (CII) is a cartilage-specific protein to which a loss of immune tolerance may trigger autoimmune reactions and cause arthritis. The major T cell epitope on CII, aa259-273, can be presented by several HLA-DRB1*04 alleles in its native or posttranslational-glycosylated form. Here, we aimed to functionally explore and compare CII-autoreactive T cells from blood and synovial fluid of patients with rheumatoid arthritis (RA).
Methods
Peripheral blood were obtained from HLA-DRB1*04 RA and control subjects (n=10 each), then stimulated in vitro with several variants of the CII259-273 epitope: (a) unmodified, or glycosylated on (b) lysine-264, (c) lysine-270, or (d) both lysine-264 and 270. Upregulation of CD154 was used to identify responding T cells. These cells were further characterized by intracellular staining for IL-17, IFNγ and IL-2 by flow cytometry. For RA patients, synovial T cells were investigated in parallel.
Results
Multifunctional T cell responses towards all examined variants of the CII259-273 peptide could be detected in RA patients and to a lesser extent also in healthy HLA-matched individuals (p<0.001). In RA, a comparison between blood and joint-derived T cell function revealed a significant increase of the proinflammatory cytokine IFNγ (p=0.027) in synovial T cells. Studies of longitudinal samples show that T cell responses were sustained over the course of disease, and even included epitope spreading.
Conclusions
The identification of inflammatory T cell responses to both glycosylated and non-glycosylated variants of the major CII epitope in RA patients suggests that CII autoreactivity may be more common than previously appreciated.
Introduction
CD4 T cells are believed to have a central role in the autoimmune pathogenic mechanisms occurring in rheumatoid arthritis (RA). High number of activated CD4 T cells in the inflamed joint and a strong genetic association with several HLA-DRB1 alleles support this notion (1).
Type II collagen (CII), the main constituent of articular cartilage, is a possible autoantigen in RA. Indeed, an RA-like disease, collagen-induced arthritis (CIA), can be induced in rodents and monkeys following immunization with CII in adjuvant (2), and in humans autoantibodies towards both native and citrullinated CII are present in the serum and synovial fluid (SF) of RA patients (3, 4).
Susceptibility to CIA is associated with the murine MHC class II molecule Aq (5), and also with transgenic expression of the human RA-associated MHC class II molecule HLA-DRB1*0401 (DR4) (5, 6). Importantly, these MHC class II molecules (from mouse and humans) select and present CII259-273 as the immunodominant T cell epitope in a similar manner. Likewise, epitopes recognized by CII-specific antibodies are shared between mice affected by CIA and RA patients (4). Hence, MHC presentation as well as T and B cell recognition of CII show striking similarities in mouse and man.
Interestingly, CII259-273 represents a T cell epitope with several variants. It contains two lysines at positions 264 and 270 that can be hydroxylated and further glycosylated with mono- or disaccharides. These modifications are recognized by T cells and play an important role in the development of CIA and also influence T cell tolerance to self-CII in both Aq- and DR4 expressing mice (7–9).
There are only a few reports that address T cell responses to glycosylated and non-glycosylated CII in humans (7, 10). In the present work, we have studied the frequencies and characteristics of T cell responses to different variants of the dominant HLA-DR*04 restricted T cell epitope on CII in RA patients. The approach we chose consists of: (1) parallel studies of several variants of the epitope, (2) detection of multiple effector functions, i.e. IL-2, IL-17 and IFNγ production, (3) comparison of these in T cells purified from paired blood and synovial fluid, and (4) longitudinal studies to assess changes during the course of disease.
Material and methods
Patients
Nine HLA-DRB1*0401 and one HLA-DRB1*0404 RA patients seropositive for anti-CII antibodies and with established disease (according to the 1987 American College of Rheumatology criteria (11)) and ten HLA-DRB1*0401 healthy controls were recruited for this study. Disease activity measurements (DAS28) were included when available within a 2-week time period of sampling. Mononuclear cells were obtained from heparinized peripheral blood (PB) and synovial fluid (SF) from one (n=5) or up to three (n=5) time points and passed over Ficoll-Hypaque gradients. Isolated PBMCs and SFMCs were cryopreserved in liquid nitrogen in 10% DMSO and 90% heat-inactivated FBS and thawed before use. Informed consent was obtained from all subjects under protocols approved by the Karolinska Hospital Ethical Review Board or the IRB at Benaroya Research Institute.
Peptides
The following native and galactosylated variants of CII259-273 were synthesized as previously described (12). (1) CII-K, an unmodified CII259-273 with lysine residues at position 264 and 270); (2) CII-Gal264, galactosylated with a β-D-galactopyranose residue on l-hydroxylysine at position 264; (3) CII-Gal270, galactosylated exclusively at position 270; and (4) CII-Gal264/270, galactosylated at position 264 and 270.
Functional cellular assay
PBMCs and SFMCs were thawed and rested overnight in RPMI-1640 supplemented with 10% pooled human AB serum, 1.5×106 cell/well. Cells were stimulated with the respective peptide variants at a concentration of 50 μg/ml for 7 hours together with anti-CD28 at 1 μg/ml (BioLegend). Brefeldin A (Sigma-Aldrich), 10 μg/ml, was added for the last 5 hours. For positive control and determination of CD154 assay sensitivity, staphylococcal enterotoxin B (SEB) was added to a separate culture at 1 μg/ml (Sigma-Aldrich). For sensitivity assay SEB-stimulated cells were diluted in 13 consequent two-fold dilutions, down to 1/8192. Following stimulation, cells were treated with LIVE/DEAD Fixable Green Dead Cell Stain (Invitrogen) and then stained for surface expression of CD3, CD4, for positive gating and for CD19 and CD14 for exclusion of monocytes and B cells. Cells were then fixed, permeabilized (BD Biosciences) and stained for IL-17A, INFγ, IL-2 (BioLegend) and CD154 (BD Bioscience). Background levels were determined by unstimulated cells (treated with anti-CD28 and Brefeldin A) and further subtracted from the CII-stimulated cultures. Samples were run on a CyAn ADP Analyzer (Beckman Coulter) and the data was analyzed by FlowJo software version 8.6.36 (Tree star, Ashland, OR, USA).
Statistics
One-way Anova (Kruskal-Wallis test) followed by Dunn’s multiple comparison tests were used to analyze differences between groups, and paired t-test (Wilcoxon singed rank test) was used for comparison between levels of cytokine production by T cells from synovial fluid and peripheral blood. Spearman’s rank correlation was use for correlation analysis between disease activity (DAS28) and T cell responses. P<0.05 was considered statistically significant.
Results
RA patients display T cell reactivity to both native and glycosylated CII259-273
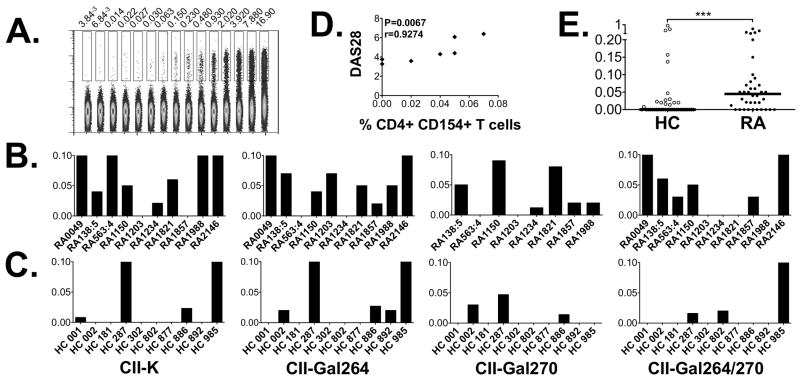
Recently activated antigen-specific CD4 T cells can be detected by an upregulation of CD154 (13). Here we used CD154 upregulation to study autoreactive CD4 T cell responses against CII in RA. We first defined the lowest level of detection of CD154 upregulation (i.e. the sensitivity of this assay). To this aim cells were stimulated with the superantigen SEB and further diluted in a two-fold serial dilution, demonstrating a linear reduction and a clear positive CD154 signal from CD4 T cells at frequencies below 0.01% (figure 1A). Encouraged by this we stimulated PBMCs from ten RA patients and ten healthy controls (HC) carrying HLA-DRB1*04 alleles with the different variants of the CII259-273 epitope and looked for positive responses. As depicted in figure 1B and 1C, both RA patients and HC responded to CII, with CII-Gal264 evoking the strongest response followed by CII-K, CII-Gal270 and the CII-Gal264/270 (figure 1B and 1C). We had disease activity measurements for 7/10 samples and when performing correlation analysis between T cell response and DAS28 we found a significant correlation (r=0.92, p<0.007) with the degree of CD154 upregulation following stimulation with the CII-264 peptide (figure 1D). Also, when taken together the responses against all four CII259-273 variants, the patient samples displayed a stronger response than healthy controls (p<0.001, figure 1E).
Figure 1. CD154 upregulation in response to the different variants of CII259-273.
(A). Expression of CD154 in CD4+ T cells stimulated with the superantigen SEB and further diluted in a two-fold serial dilution from 1/1 down to 1/8192. The threshold of detection was set to <0.01%. (B). T cell responses towards the four variants of CII259-273; CII-K, CII-Gal264, CII-Gal270 and CII-Gal264/270 was monitored by multicolor flow cytometry in 10 RA patients following short term in vitro stimulation of PBMC. The expression levels (%) of CD154 after subtracting background levels are displayed. (C). CD154 upregulation after stimulation with the CII variants in 10 HLA-DR*0401 matched healthy control samples. The bars for B and C have been cut at 0.1% to emphasize the lower responses. (D). The magnitude of CD154 upregulation following CII-Gal264 stimulation demonstrated a strong association with disease activity (DAS28). (E). Comparison of the sum of CD154 upregulation in healthy controls versus RA subjects following stimulation with all four CII259-273 variants. *** P < 0.001
RA patients responded to CII with a diverse cytokine response
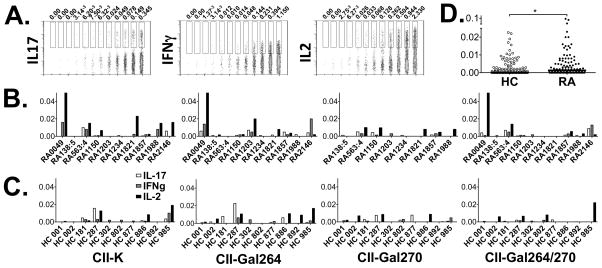
Having established that RA patients carry CII-reactive T cells we next wanted to determine the effector functions these T cells display. Using a multiparameter flow cytometry approach, we examined cytokine production together with CD154 upregulation. The combination of the two read outs reduces background and was postulated to increase sensitivity. Again, we first determined the detection levels for the assay, i.e. cytokines IL-17, IFNγ and IL-2. Following SEB stimulation we concluded that also here the detection limits were lower than 0.01% (figure 2A). We then investigated the production of IL-17, IFNγ and IL-2 in response to the different variants of the CII259-273 epitope. As can be seen in figure 2B and C, CD4 T cells from both patients and healthy donors could secrete cytokines in response to all variants of CII259-273. Again, CII-K and CII-Gal264 induced stronger responses in comparison with the two remaining CII259-273 variants. With regard to magnitude, a trend towards higher production of IFNγ and IL-2 from the patients samples could be monitored, yet did not reach statistical significance. However, the overall production of cytokines (i.e. plotting of IL-17, IFNγ and IL2 together) was significantly higher from RA samples as compared to healthy subjects, p<0.05 (figure 2D).
Figure 2. Cytokine production in response to CII259-273 variants.
(A). Expression of intracellular IL-17, IFNγ and IL-2 in CD4+ CD154+ T cells stimulated with the superantigen SEB and further diluted 10 times in a two-fold serial dilution. The threshold of detection was set to <0.01% (B). T cell responses towards the four variants of CII259-273; CII-K, CII-Gal264, CII-Gal270 and CII-Gal264/270 was monitored by multicolor flow cytometry in 10 RA patients following short term in vitro stimulation of PBMC. The expression levels (%) of IL-17, IFNγ and IL-2 after subtracting background levels are displayed. (C). IL-17, IFNγ and IL-2 expression following stimulation with the CII259-273 variants in 10 HLA-DR*0401 matched healthy control samples are displayed. (D). Combined analysis of the sum of cytokines produced in response to the CII peptide variants. *P < 0.05
RA synovial T cells are prominent IFNγ producers
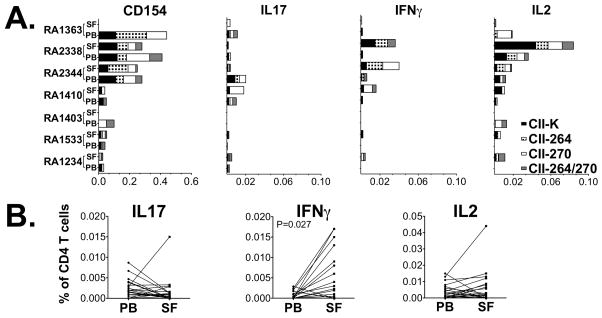
We further wished to examine T cell reactivity to the CII259-273 variants at the site of inflammation and further compare responses in the circulation with that in affected joints. Hereby, T cell responses to the four variants of CII259-273 were examined in seven paired samples from peripheral blood and synovial fluid (collected from five different patients). The level of CD154 upregulation varied between patients (Figure 3A), but a robust proportion of the CD154+ T cells produced cytokines in response to peptide stimulation. Upon direct comparison, the level of IFNγ production was significantly higher in SF as compared to PB, p=0.027 (figure 3B).
Figure 3. Comparative analysis of T cell reactivity to CII in PB and SF.
(A). Paired peripheral blood and synovial fluid-derived mononuclear cells from 5 different patients were stimulated with CII-K (black), CII-Gal264 (black and white checkered), CII-270 (white) and CII-Gal264/270 (grey) and the frequency of T cell response to these peptides were measured by flow cytometry. To ensure correct detection of T cell reactivity, patients with low CII responses were re-analyzed. Thus RA 1403 and RA 1410 are consecutive cell samples from one RA patient, and RA 1234 and RA 1533 from another. (B) Pairwise comparison of the frequency of positive cells from blood and synovial fluid for the respective cytokines, where IFNγ gave a significant difference between the compartments (p=0.027).
CII reactive T cells persist over time
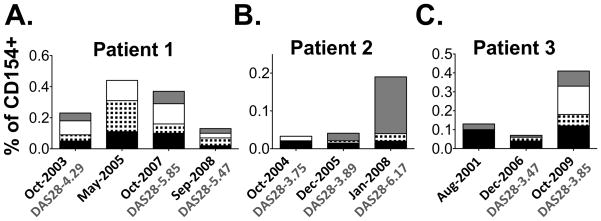
Next, we decided to dissect variations in T cell reactivity towards CII during the course of RA. Hence, we utilized longitudinal cell samples from three HLA-DRB1*0401 RA patients and examined their T cell responses in comparison with disease activity. From patient 1 we had access to four consecutive samples spanning over a 5-year period, for patient 2 the corresponding time was 4 years and 3 samples and for patient 3 three samples over a period of 8 years.
As shown in figure 4, the T cell responses varied both in magnitude and fine specificity. Patient 1 displayed multireactivity through out the study period, while patient 2 had a dramatic increase in Gal264/270-reactivity that coincided with high disease activity. Patient 3 developed new sub-specificities over the study period even though this individual already had a disease duration of 40 years at the start of this study.
Figure 4. Longitudinal studies of T cell responses to CII.
T cell responses to the four different variants of CII259-273, i.e. CII-K (black), CII-Gal264 (black and white checkered), CII-270 (white) and CII-Gal264/270 (grey), were monitored by upregulation of CD154 on CD4 T cells in 3 RA patients over time. The disease activity measurement DAS28 is displayed where such information was available.
Discussion
Our understanding of the role of T cells in RA has increased due to both improved methods for detecting rare antigen-specific T cells (13) and the emergence of new candidate autoantigens dependent on post-translationally modified epitopes (14, 15). Hence, detailed T cell studies on well characterized patient material has a good potential to enhance our current understanding of autoimmune responses in different subsets of RA patients, and their contribution to disease development and/or chronicity. Here we studied T cell responses to native and glycosylated variants of the immunodominant T cell epitope from type II collagen, CII259-273, in HLA-DRB1*0401 and HLA-DRB1*0404 RA patients. We show that CII-specific T cells recognizing different variants of CII259-273 are frequent in HLA-DRB*04 RA and are also found in HLA matched control subjects. Importantly, the magnitude of the response was significantly higher in samples derived from RA patients. In fact, most samples displayed CII reactivity, and for one of the peptides (CII-264) we could correlate the observed response to disease activity (DAS28) values in this small cohort.
When moving into the rheumatic joint and comparing the magnitude of response between blood and synovial fluid, IFNγ production in response to CII stimuli was significantly higher in synovial fluid as compared to peripheral blood in our patient samples. Also here we attempted to correlate T cell effector function with disease activity. We had disease activity measurements for 4/7 samples, but the magnitude of response could not be correlated to DAS28 values in this small cohort.
Our data show that many RA patients display T cell responses to both native and glycosylated CII, with only few patients exclusively responding to either native or glycosylated CII. This type of response pattern mirrors what has previously been reported in Aq mice (8), while DR4 transgenic mice display a biased T cell response against the non-glycosylated self-CII peptide (9). We still lack knowledge of whether T cell responses to the different CII epitopes contribute to early RA (i.e. disease development), or whether they are a consequence of joint destruction. Notably, our present study demonstrates that DR4-restricted CII recognition towards all variants of the CII epitope occurs in the human setting (and is thus different than the transgenic DR4 mouse setting) and that the functional outcome of CII recognition was cytokine production, most prominently IFNγ, but also IL-17 and IL-2.
Our study was not powered to dissect the influence of disease activity to any high degree, but notably, the sample with the highest T cell responses was taken at a time point of high disease activity (DAS28: 6.63), suggesting that the level of anti-CII reactivity may directly influence, or indirectly be influenced by, disease activity in RA. Hence, T cells residing at the site of inflammation might have higher pathogenic capacity than those in periphery. Neither could we addressed the effect of treatment, all our patients have chronic RA and the majority of them was on methotrexate in combination with TNF blocking therapy. In fact, this underscores the difference between healthy controls and subjects, that the observed differences could potentially have been even greater if the patients had not been immune-compromised by treatment.
Our results from longitudinal sampling obtained from the same patients at different time points of disease also demonstrate that CII reactivity may vary over time but clearly the responses were persistent and even included epitope spreading late in disease. Patient 3 displayed T cell autoreactivity to new versions of the CII epitope after 40 years of disease duration.
RA is a heterogeneous disease in which different antigens, genes and environmental factors are likely to be involved in various subsets of the disease. In this work we focused on the cartilage-restricted candidate autoantigen type II collagen, and studied the function of PB- and SF-derived T cells in HLA-DRB1*04 RA patients. Our study demonstrates the feasibility of renewed studies of T cell reactivities to candidate autoantigens in RA using the present refined methods. In addition to glycosylation, citrullination is a critical post translational modification in RA, and future T cell studies obviously need to be focused also on citrullinated antigens. Thus, by studying T and B cell reactivities to different potential arthritogenic autoantigens, including those that have undergone various post-translational modifications, in different subsets of RA and in different genetic contexts, we may be able to better understand which T and B cell reactivities that may contribute to disease development and/or chronicity in different subsets of RA.
Acknowledgments
FUNDING
This work was supported by Margaretha af Ugglas Foundation, the Swedish Research Council, the EU FP7 project Masterswitch (HEALTH-F2-2008-223404), the IMI JU funded project BTCure 115142-2, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01-AR-051394, the Swedish Combine program, the Swedish Association against Rheumatism, the King Gustaf the V:s 80 year Foundation, and the Swedish Foundation for Strategic Research.
The authors would also like to thank all personal and patients at the rheumatology clinic at the Karolinska University Hospital, as well as Eva Jemseby and Gull-Britt Almgren for organizing sampling, storage and administration of biomaterial and the Benaroya Research Institute Translational Research Program and Clinical Core for recruitment of healthy subjects, sample processing and handling.
Footnotes
COMPETING INTERESTS
No competing interests
References
- 1.Klareskog L, Forsum U, Scheynius A, Kabelitz D, Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982;79(11):3632–6. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 3.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62(1):44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 4.Uysal H, Nandakumar KS, Kessel C, Haag S, Carlsen S, Burkhardt H, et al. Antibodies to citrullinated proteins: molecular interactions and arthritogenicity. Immunol Rev. 2010;233(1):9–33. doi: 10.1111/j.0105-2896.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 5.Brunsberg U, Gustafsson K, Jansson L, Michaelsson E, Ahrlund-Richter L, Pettersson S, et al. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994;24(7):1698–702. doi: 10.1002/eji.1830240736. [DOI] [PubMed] [Google Scholar]
- 6.Holmdahl R, Andersson EC, Andersen CB, Svejgaard A, Fugger L. Transgenic mouse models of rheumatoid arthritis. Immunol Rev. 1999;169:161–73. doi: 10.1111/j.1600-065x.1999.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 7.Backlund J, Carlsen S, Hoger T, Holm B, Fugger L, Kihlberg J, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263–270) in humanized transgenic mice and in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2002;99(15):9960–5. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backlund J, Nandakumar KS, Bockermann R, Mori L, Holmdahl R. Genetic control of tolerance to type II collagen and development of arthritis in an autologous collagen-induced arthritis model. J Immunol. 2003;171(7):3493–9. doi: 10.4049/jimmunol.171.7.3493. [DOI] [PubMed] [Google Scholar]
- 9.Batsalova T, Dzhambazov B, Merky P, Backlund A, Backlund J. Breaking T cell tolerance against self type II collagen in HLA-DR4-transgenic mice and development of autoimmune arthritis. Arthritis Rheum. 2010;62(7):1911–20. doi: 10.1002/art.27460. [DOI] [PubMed] [Google Scholar]
- 10.Londei M, Savill CM, Verhoef A, Brennan F, Leech ZA, Duance V, et al. Persistence of collagen type II-specific T-cell clones in the synovial membrane of a patient with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989;86(2):636–40. doi: 10.1073/pnas.86.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Holm b, Broddefalk J, Flodell S, Wellner E, Kihlberg J. An Improved Synthesis of a Galactosylated Hydroxylysine Building Block and its use in Solid-Phase Glycopeptide Synthesis. Tetrahedron. 2000;56(11):1579–86. [Google Scholar]
- 13.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11(10):1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 14.James EA, Moustakas AK, Bui J, Papadopoulos GK, Bondinas G, Buckner JH, et al. HLA-DR1001 presents “altered-self” peptides derived from joint-associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. 2010;62(10):2909–18. doi: 10.1002/art.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, et al. Identification and functional characterization of T cells reactive to citrullinated-vimentin in HLA-DRB1*0401 humanized mice and RA patients. Arthritis and rheumatism. 2011;63(10):2873–83. doi: 10.1002/art.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]