Abstract
Background
High midlife body mass index (BMI) has been linked to a greater risk of dementia in late life, but few have studied the effect of BMI across midlife on cognitive abilities and cognitive change in a dementia free sample.
Methods
We investigated the association between body mass index (BMI), measured twice across midlife (mean age 40 and 61 years, respectively), and cognitive change in four domains across two decades in the Swedish Adoption/Twin Study of Aging (SATSA).
Results
Latent growth curve models fitted to data from 657 non-demented participants showed that persons who were overweight/obese in early midlife had significantly lower cognitive performance across domains in late life and significantly steeper decline in perceptual speed, adjusting for cardio-metabolic factors. Both underweight and overweight/obesity in late midlife were associated with lower cognitive abilities in late life. However, the association between underweight and low cognitive abilities did not remain significant when weight decline between early and late midlife was controlled for.
Conclusions
There is a negative effect on cognitive abilities later in life related to being overweight/obese across midlife. Moreover, weight decline across midlife rather than low weight in late midlife per se was associated with low cognitive abilities. Weight patterns across midlife may be prodromal markers of late life cognitive health.
Keywords: Aging, Body Mass Index, Cognition, Cognitive aging, Cognitive decline, Overweight, Obesity, Trajectories, Underweight, Weight changes
Introduction
Overweight/obesity is health problem that has reached epidemic proportions. A growing body of evidence links high midlife body mass index (BMI) to an increased risk of dementia, the main threat against maintained cognitive function in late life. However, even persons that not are diagnosed with dementia experience cognitive decline, which is a source of irritation and worries among the elderly. Little is known about the association between BMI and cognitive ability among those without dementia. Given the high prevalence of overweight/obesity even a small adverse effect of overweight/obesity on cognitive abilities might have a serious effect on public health.
Two prospective studies, The Whitehall II Study and the Framingham Offspring Study (FOS), have shown that overweight/obesity in midlife (and to some extent underweight) is associated with lower scores on tests of memory,1 executive abilities,1, 2 and spatial abilities,2 while overweight/obesity was not associated with lower cognitive test performance on measures of semantic memory in FOS.2 In the longitudinal study Origins of Variance in the Old-Old (OCTO-twin), which includes a wider range of cognitive tests, higher BMI values in late midlife were associated with lower test performance on episodic memory, semantic memory, perceptual speed, spatial ability, and verbal ability thirty years later, when participants were aged 80 years and above; however, BMI was not associated with steeper decline in any cognitive ability.3 In the same sample as the present study, we found that high BMI in early midlife was related both to lower general cognitive ability and to steeper decline,4 but domain specific trajectories have not previously been reported. Although previous studies rather consistently reports a negative effect of being overweight/obese in midlife across cognitive domains there is a need for studies including wider assessments of cognitive functions, and especially studies with longitudinal assessments to be able to evaluate if overweight/obese persons not only have a lower cognitive performance but also a steeper decline.
In late life, the association between BMI and cognitive abilities is less consistent. Weight decline,5-7 weight increase,5 low BMI,6 and high BMI8-10 have each been associated with lower cognitive abilities. Short follow-up times and inclusion of persons diagnosed with dementia, might blur the association between weight and cognition, as weight loss in late life might be a prodromal marker of dementia.5-7 Additionally, most research studying weight and cognition in late life has focused on current weight in relation to cognition. A life course perspective on overweight/obesity with several assessments might be important, as the negative effect of being overweight/obese is proposed to be delayed,11 being overweight/obese over a longer period of time is associated with more severe negative health outcomes,12 and both weight increase and decreases might be signs of pathological processes, which in turn might affect cognitive functioning.
Hence, to extend our knowledge about the association between weight and cognition we studied the effect of being underweight and overweight/obese at different time points, being underweight and overweight/obese over long periods of time, and changes in BMI on the longitudinal change in four cognitive domains in a dementia-free sample.
Methods
Design/participants
The study sample was drawn from the Swedish Adoption/Twin Study of Aging (SATSA) which in turn came from the Swedish Twin Registry (STR).13 The STR includes two cohorts of same-sex twin pairs born either 1896-1926 or 1926-1958. These participants were mailed questionnaires in 1963 or 1973, constituting the baseline assessment. As detailed elsewhere,14 questionnaires (Q) were sent to SATSA beginning in 1984 and in-person testing (IPT) started in 1986, including 645 twins 50 years of age and older. Since then, these twins and all twins who turned 50 years of age since the last IPT have been assessed on a battery of cognitive tests every three years by trained research nurses and responded to questionnaires. For the present analyses cognitive assessments from five IPTs were available spanning over eighteen years. The first IPT in which the individual participated is called IPTentry, and the first Q is called Qentry.
In total, 859 persons participated in at least one IPT. Among these, 813 individuals had an early midlife BMI score. Persons diagnosed with dementia during the course of the study (n = 78) were excluded. Dementia was screened for during each IPT and diagnosed during a consensus conference.15, 16 Persons older than 50 at baseline (1963/1973) (n =77) were excluded. One person had missing data on educational level. After these exclusions, 657 persons remained for analyses.
SATSA has been approved by an ethics committee at the Karolinska Institutet, Stockholm, Sweden. Written informed consent was obtained from all participants.
Measurements
Body mass index
BMI was calculated from self-reported height and weight at baseline (1963/1973) and from assessed height and weight at IPTentry (kg/m2). BMI was analyzed as a categorical variable: underweight (BMI<20 kg/m2), normal weight (20≤BMI<25 kg/m2), overweight (25≤BMI<30 kg/m2), and obese (≥30 kg/m2). For underweight persons a higher cut-off point than the usual 18.5 kg/m2 was used (i.e., 20 kg/m2), as only 15 persons (2.3 % of the sample) at baseline and 6 persons (0.9 %) at IPTentry had a BMI below 18.5. We considered BMI change defined as a gain or loss of five percent between baseline and IPTentry, with remaining persons defined as stable.
Cognitive testing
Four cognitive domains are represented in the SATSA cognitive battery: verbal, spatial/fluid, memory and perceptual speed.17 Verbal abilities are indexed by: (1) the Swedish WAIS Information subtest of general knowledge,18 (2) Synonyms, where participants evaluate which of five words is a synonym to a target word;19 and (3) Analogies where participants evaluate a word pair and complete a second word pair representing a similar relationship.20 Spatial/fluid abilities are assessed by: (1) the Figure Logic test of inductive reasoning where participants identify which figure among five is not constructed according to a principle shared by four others,19 (2) the Block Design test where participants copy a given pattern with a set of colored blocks,21 (3) and Card Rotations,where participants identify one picture that is identical to a target item but is rotated.22 Memory tests include: (1) the Thurstone Picture Memory test where participants identify which of 28 line drawings were previously shown to them using a forced recognition format,23 (2) the Digit Span test where participants repeat a sequence of digits as originally presented (Digit Span forward), or in reversed order (Digit Span backward),18 and (3) Names and Faces (immediate and delayed) where the participants recall the names of 16 persons whose pictures they viewed.24 Perceptual speed is comprised of: (1) the Symbol Digit task where participants verbally respond with the appropriate digit that matches a printed symbol,25 (2) and the Figure Identification task where the participants detect which item among five alternatives that is identical to a target item.19 Principal components analysis was used to create component scores for each domain.26
Covariates
Covariates included age, sex, education dichotomized as low (≤9 years) or high (>9 years), cohort dichotomized as older (born between 1900-1926) or younger (1927-1948), alcohol based on self-report at baseline and IPTentry and dichotomized as abstainers (never reported drinking alcohol) and drinkers, smoking based on self-report at baseline and IPTentry and dichotomized as never-smokers and ever-smokers, and exercise based on the twins’ self-reported estimated amount of exercise in midlife assessed at baseline and dichotomized as no “exercise/light” and “moderate/ heavy”. Self-reported data on cardiovascular diseases from Qentry were coded as absence or presence of stroke, myocardial infarction, heart failure, and angina pectoris. Prevalence of hypertension was defined as resting diastolic blood pressure above 140 and/or systolic blood pressure above 90 at IPTentry, and/or self-reported use of antihypertensive medication at Qentry. Diabetes was based on self-reports of diabetes and/or diabetic agents at Qentry. Responses to later IPTs and Qs were used to create late-midlife cardio-metabolic variables. Physical health was based on answers to 51 health items,27 reduced to a single scale which reflects general physical disease status.28
Statistical analysis
All analyses were performed in SAS 9.1.29 Differences in baseline characteristics between underweight, normal weight, overweight, and obese persons and differences between persons with stable, declining and increasing BMI were assessed by one-way analyses of variance controlling for unequal variance employing the Welch statement in PROC GLM or χ2-tests when appropriate.
To measure change in cognitive performance over time and to explore the potential effect of midlife BMI on cognitive performance over time we employed latent growth curve modeling. A full maximum-likelihood estimate (MLE) technique was used,30 employing PROC MIXED. Models were adjusted to account for the correlation within twin pairs. Growth curves were fit to establish linear and nonlinear age trajectories using mean-centered age (linear) and its square (nonlinear, or quadratic). The centering age of 65 years was chosen for all cognitive domains except for verbal abilities, where we chose age 70 based on previous SATSA results 31. Additional models were fit considering BMI change status.
A stepwise procedure was adopted to evaluate longitudinal trajectories for each cognitive domain. All models included linear and quadratic age, sex, educational level, cohort, alcohol use, and smoking and interaction terms between BMI and both linear and quadratic age. In further analyses we also adjusted for cardio-metabolic factors. Interaction terms with sex were also tested for, but since no significant interactions were found we did not proceed further. To take into account preclinical cognitive function we also conducted analyses where verbal ability was included as a covariate in the models. As the cognitive component scores are scaled as t-scores (SD = 10), eight-, five-, and 2-point differences would translate to large (0.8), medium (0.5), and small (0.2) effect sizes, respectively.
While baseline models indicated non-zero variances of the growth trajectory features, after entry of predictors it was necessary to set the between-pair variance for the age2 term to zero for spatial and memory abilities to achieve convergence. However, when the other models were run excluding the nonlinear age term as a random effect, the fixed effects were not altered in any substantial way.
Results
Participant characteristics as a function of BMI categories are presented in Table 1. Persons who were underweight at baseline were younger, female, belonged to the younger cohort, and had a lower prevalence of hypertension and diabetes across the study period. Overweight and obese persons were older and had higher prevalence of hypertension and diabetes. The mean follow-up time from baseline to IPTentry was 21.4 years (range 12-37 years).
Table 1.
Sample Characteristics, Total Sample and by Body Mass Index (BMI) groups.
| Total | Underweight | Normal weight | Overweight | Obese | P- value | |
|---|---|---|---|---|---|---|
|
|
||||||
| n=657 | n = 73 | n = 407 | n = 151 | n = 26 | ||
| Age, mean (SD) | 39.9 (5.9) | 36.7 (6.6) | 39.7 (5.8) | 41.6 (5.1) | 41.5 (5.1) | <.0001 |
| BMI at baseline, mean (SD) | 23.5 (3.1) | 19.1 (0.7) | 22.6 (1.4) | 26.7 (1.3) | 32.2 (2.2) | <.0001 |
| BMI at IPTentry, mean (SD) | 25.8 (3.9) | 22.0 (2.1) | 24.9 (2.8) | 28.6 (3.8) | 33.8 (2.9) | .0002 |
| Men, n (%) | 280 (42.6) | 7 (9.6) | 180 (44.2) | 83 (55.0) | 10 (38.5) | <.0001 |
| Education ≤9 years, n (%) | 411 (62.6) | 38 (52.0) | 254 (62.4) | 100 (66.2) | 19 (73.1) | 0.136 |
| Older cohort, n (%) | 293 (44.6) | 15 (20.5) | 190 (46.7) | 77 (51.0) | 11 (42.3) | 0.0001 |
| Alcohol abstainers, n (%) | 96 (14.6) | 10 (13.7) | 55 (13.5) | 23 (15.2) | 8 (30.8) | 0.115 |
| Never smokers, n (%) | 306 (46.6) | 36 (49.3) | 184 (45.2) | 69 (45.7) | 17 (65.4) | 0.234 |
| No/light exercise, n (%) | 203 (31.5) | 27 (37.0) | 122 (30.3) | 45 (31.5) | 9 (34.6) | 0.710 |
| Stroke, n (%) | 9 (1.4) | 2 (2.7) | 4 (1.0) | 3 (0.02) | 0 (0.0) | 0.521 |
| Myocardial infarction, n (%) | 19 (2.9) | 1 (1.4) | 12 (2.9) | 3.3 (5) | 1 (3.8) | 0.853 |
| Heart failure, n (%) | 2 (2.7) | 11 (2.7) | 11 (7.3) | 2 (7.7) | 26 (4.0) | 0.062 |
| Angina pectoris, n (%) | 42 (6.4) | 2 (2.7) | 23 (5.6) | 15 (9.9) | 2 (7.7) | 0.155 |
| Hypertension, n (%) | 475 (72.6) | 37 (51.4) | 286 (70.3) | 127 (84.1) | 25 (96.1) | .0001 |
| Diabetes, n (%) | 30 (4.6) | 0 (0.0) | 10 (2.5) | 17 (11.3) | 3 (11.5) | <.0001 |
Early midlife BMI and late life cognitive abilities
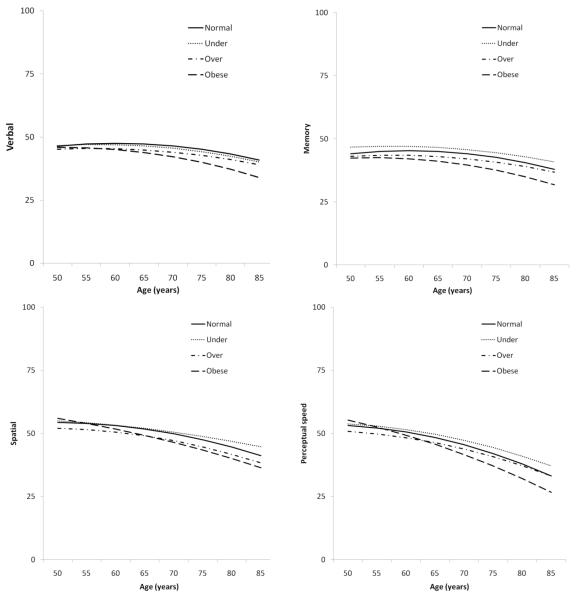
Being overweight or obese was associated with lower cognitive test performance in all cognitive domains (Table 2, Figure 1). The effect size differences (d) in expected cognitive performance at age 65 (intercept) varied from −.20 to −.38 for overweight and obese versus normal weight, thus representing small effects. The interaction effects between BMI and age indicate that persons who were obese in early midlife had a steeper cognitive decline in perceptual speed. The effect size difference (d) in linear rate of change at age 65 between normal versus obese was medium at −.53 when considering 20 years of follow-up. A similar but non-significant pattern was observed for verbal and spatial abilities, with near moderate effect sizes observed (d = −.37 and −.41) for verbal and spatial abilities, respectively). No interaction between BMI and quadratic age was seen on the slope; therefore, those results are not shown in the table.
Table 2.
Associations between Early Midlife BMI (Age 25-50) and Late Midlife BMI (Age 51-75), and Cognitive Mean Level Test Performance and Longitudinal Trajectories of Change in Late Midlife. a
| Verbal | Spatial | Memory | Perceptual Speed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Effect | BMI Group | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value |
| Early Midlife | |||||||||||||
| Intercept65 | Normal (Ref) | 47.28 | 1.13 | <.0001 | 51.75 | 1.23 | <.0001 | 44.97 | 1.26 | <.0001 | 48.37 | 1.15 | <.0001 |
| Underweightb | −0.77 | 1.04 | 0.4625 | 0.23 | 1.07 | 0.8277 | 1.61 | 1.17 | 0.1701 | 1.31 | 1.05 | 0.2156 | |
| Overweightb | −2.39 | 0.74 | 0.0016 | −2.69 | 0.79 | 0.001 | −1.99 | 0.85 | 0.0208 | −2.06 | 0.76 | 0.0081 | |
| Obeseb | −3.37 | 1.58 | 0.0348 | −2.55 | 1.68 | 0.1326 | −3.84 | 1.80 | 0.0351 | −2.69 | 1.62 | 0.1011 | |
| Linear Age65 | Normal (Ref) | −0.10 | 0.02 | <.0001 | −0.32 | 0.02 | <.0001 | −0.12 | 0.03 | 0.003 | −0.51 | 0.03 | <.0001 |
| Underweightc | −0.03 | 0.07 | 0.6213 | 0.06 | 0.07 | 0.4054 | −0.01 | 0.07 | 0.8897 | 0.08 | 0.07 | 0.2178 | |
| Overweightc | −0.03 | 0.04 | 0.4593 | −0.02 | 0.05 | 0.7099 | −0.02 | 0.06 | 0.7596 | 0.05 | 0.05 | 0.287 | |
| Obesec | −0.18 | 0.11 | 0.105 | −0.21 | 0.11 | 0.0546 | −0.13 | 0.13 | 0.311 | −0.26 | 0.11 | 0.0156 | |
| Quadratic Age | ---- | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 |
| Late Midlife | |||||||||||||
| Intercept65 | Normal (Ref) | 46.88 | 1.05 | <.0001 | 51.30 | 1.16 | <.0001 | 44.94 | 1.17 | <.0001 | 48.40 | 1.08 | <.0001 |
| Underweightb | −3.40 | 1.34 | 0.0123 | −1.79 | 1.51 | 0.2377 | −3.95 | 1.62 | 0.0156 | −1.84 | 1.45 | 0.2066 | |
| Overweightb | −1.85 | 0.62 | 0.0035 | −0.78 | 0.68 | 0.2497 | −0.76 | 0.72 | 0.2935 | −1.55 | 0.65 | 0.0177 | |
| Obeseb | −1.64 | 0.93 | 0.0791 | −3.47 | 1.02 | 0.0009 | −3.11 | 1.09 | 0.0051 | −3.66 | 0.97 | 0.0002 | |
| Linear Age65 | ---- | −0.09 | 0.02 | 0.0002 | −0.33 | 0.03 | <.0001 | −0.11 | 0.03 | 0.0019 | −0.51 | 0.03 | <.0001 |
| Quadratic Age | ---- | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 |
Analyses also controlled for sex, educational level, cohort, alcohol use, and smoking.
Intercept65, performance level difference at age 65 due to BMI category.
Linear Age65, linear trend difference at age 65 due to BMI category.
Figure 1.
Longitudinal Association between Early Midlife Body Mass Index and Cognitive Abilities in Late Life
Late midlife BMI, BMI across midlife and late life cognitive abilities
Participant characteristics as a function of BMI categories in late midlife are presented in Table 3. High BMI in late midlife - i.e., overweight and/or obese - was associated with lower mean level test performance in all cognitive domains (Table 2). Effect size differences (d) ranged from −.08 to −.35. Associations between overweight or obesity in late midlife and lower mean cognitive test performance remained significant when change in BMI was controlled for (d = −.10 to −.43). Overweight and obesity in late midlife were also associated with steeper linear decline in verbal abilities, −.05 (SE 0.03), p = .089 [d = −.39], and −.10 (SE .05), p = .045 [d =−.25], respectively, but not with steeper decline in any other cognitive domain (not shown).
Table 3.
Age, Body Mass Index (BMI) in Late Midlife (Age 50-75).
| BMI Category in Late Midlife |
||||||
|---|---|---|---|---|---|---|
| All | Underweight | Normal weight | Overweight | Obese | P- value | |
|
|
||||||
| n=657 | n = 28 | n = 286 | n = 261 | n = 82 | ||
| Age, mean (SD) | 61.1 (6.6) | 59.4 (6.7) | 61.2 (6.7) | 61.6 (6.6) | 59.5 (6.0) | 0.030 |
| BMI, mean (SD) | 25.8 (3.9) | 19.1 (0.7) | 23.1 (1.2) | 27.1 (1.3) | 33.4 (3.1) | <.0001 |
Persons who were underweight in late life had lower mean level test performance on verbal abilities and memory in late life compared to persons who were normal weight. However, the association between underweight and lower cognitive test performance did not remain significant when change in BMI was controlled for (Table 4), instead decrease in BMI was associated with lower cognitive test performance across domains. Persons whose BMI was lower in later than in earlier midlife (n=43, 6.5 % of the sample) had lower mean cognitive test performance across all cognitive domains. These persons had the highest BMI in early midlife (25.3 kg/m2) compared to the BMI gainers (22.9 kg/m2) and the stable BMI group (24.3 kg/m2), F (655, 2) =18.37, p = <.0001. Persons whose BMI was lower in later than in earlier midlife was also older (mean agebaseline 47.9 years), than persons who remained stable (43.7 years) or whose BMI increased (40.2 years), F (655, 2) = 33.5, p = <.0001. Additionally, they had higher prevalence of general physical disease (mean 2.3, range 0-8) than persons who remained stable (mean 1.4) or whose BMI became higher (1.7), F (626, 2) =3.96, p = .02.
Table 4.
Associations between Late Midlife Body Mass Index (BMI) (Age 50-70) and Cognitive Performance when Change in BMI is Controlled for and the Association between Change in BMI and Cognitive Performance.a
| Verbal | Spatial | Memory | Perceptual Speed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Effect | BMI Group | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value |
| Intercept65 | Normal (Ref) | 47.69 | 1.14 | <.0001 | 52.58 | 1.22 | <.0001 | 46.16 | 1.26 | <.0001 | 49.36 | 1.15 | <.0001 |
| Underweightb | −1.68 | 1.50 | 0.2659 | 0.28 | 1.61 | 0.8629 | −2.76 | 1.73 | 0.1128 | −0.13 | 1.54 | 0.9323 | |
| Overweightb | −2.11 | 0.67 | 0.0020 | −1.14 | 0.71 | 0.1122 | −1.16 | 0.76 | 0.1307 | −2.16 | 0.68 | 0.0019 | |
| Obeseb | −1.90 | 0.99 | 0.0575 | −4.29 | 1.06 | <.0001 | −3.87 | 1.14 | 0.0009 | −4.26 | 1.01 | <.0001 | |
| Linear Age65 | ---- | <0.01 | 0.03 | 0.7543 | −0.33 | 0.03 | <.0001 | −0.11 | 0.03 | 0.0012 | −0.51 | 0.03 | 0.0012 |
| Quadratic Age | --- | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 | −0.01 | <0.01 | <.0001 |
| BMI Decline | ---- | −3.47 | 1.10 | 0.0020 | −4.02 | 1.18 | 0.0009 | −4.11 | 1.15 | 0.0005 | −2.70 | 1.12 | 0.0168 |
| BMI Gain | --- | 0.71 | 0.63 | 0.2602 | −0.12 | 0.65 | 0.8559 | −0.03 | 0.67 | 0.9618 | −0.04 | 0.61 | 0.9527 |
Analyses also controlled for sex, educational level, cohort, alcohol use, and smoking.
Intercept65, performance level difference at age 65 due to BMI category.
Persons who were underweight at both time points (n=19) and those who were overweight and/or obese at both time points (n=159) were compared to the remainder of the sample, who were either normal weight at both times or normal weight at one point and underweight, overweight or obese at the other point (479). The consistently underweight group performed significantly worse on mean verbal abilities and memory, −5.8 (SE 1.9), p = 0.003, and −6.6 (SE 2.1), p = 0.002, respectively. The consistently overweight/obese group scored significantly worse across domains, with the estimates rather similar for all four cognitive domains (range −2.2 to −1.7). No effect on cognitive decline was found.
Controlling for potential causal mechanisms
Adding cardio-metabolic factors from either a midlife or life time perspective did not substantially change any association reported above (not shown). Neither did the addition of a measure of general physical health. Adding verbal abilities did not significantly change the association between BMI and cognitive abilities, although the mean level estimates became slightly weaker for spatial abilities −.29 (SE 0.10), p = 0.006 and memory −0.9 (SE 0.11), p = 0.007.
Discussion
Overweight/obesity and mean level cognitive performance
In the present study we show that being overweight/obese had a negative effect on mean cognitive test performance across domains for those who were obese/overweight in early midlife, late midlife, or across midlife. Consistent with our prior findings from OCTO-twin3 we show a negative effect of being overweight or obese in midlife on the mean level performance across cognitive domains (verbal and spatial abilities, memory, and perceptual speed). Similarly, a negative effect of midlife overweight/obesity on tests of memory and executive abilities has been reported from the Whitehall II Study,1 and on spatial abilities in FOS.2 Furthermore, consistent with the Whitehall II Study1 we also found that persons who were overweight/obese across midlife had a higher risk of lower cognitive test performance in late life, suggesting a potential cumulative effect. This negative effect of high midlife BMI on the mean level cognitive performance is important as persons with lower cognitive abilities are at a higher risk of cognitive impairments when an additional cognitive decline occurs.
Overweight/obesity and change in cognitive functioning
To our knowledge, we are the first to report a negative effect of high BMI across midlife on change in cognitive functioning. In particular, early midlife overweight/obesity was associated with a steeper decline in perceptual speed and a similar although non-significant pattern of decline in verbal and spatial abilities. Given that overweight/obese persons already have a lower initial level of cognitive function, an additional effect on the slope is detrimental. There was no significant effect of midlife BMI on memory trajectories. This lack of association between higher midlife BMI and late life memory abilities is puzzling. However, there are studies of cardiovascular risk factors and memory that also report a lack of association.32-34
In late midlife, there were no negative effects of being overweight/obese on the trajectories of cognitive change, except for verbal abilities (which will be discussed later), consistent with our prior findings from OCTO-Twin.3 There are several possible explanations; (1) late midlife overweight/obesity might have less impact on cognitive decline than early midlife overweight/obesity, (2) the association between overweight/obesity and cognition might be blurred by preclinical states causing changes in both cognition and weight, (3) the negative effect of being overweight/obese might be delayed, or (4) cumulative (as shown).
A negative effect of overweight/obesity on verbal abilities measured at a single time point has also been reported from the Lothian Birth Cohort.10 The effect of overweight/obesity seen on decline in verbal abilities is interesting, since verbal abilities represent the cognitive domain that is considered to be most resistant to aging.35 As there is less change in verbal abilities due to aging, it might be easier to pinpoint factors such as adiposity causing change in this domain.
The association between higher BMI and lower cognitive functioning an artifact of early life cognitive ability?
It has been hypothesized that the association between a higher BMI and lower cognitive test performance in old age might be an artifact of confounding by early life cognitive ability.10 As cognitive abilities were not assessed in early midlife, we used the best available proxies for early midlife cognitive functioning: educational level and verbal abilities at IPTentry. The rationale is that verbal abilities remain rather stable over the adult life span and are considered to be least affected by aging. Furthermore, the decline in verbal abilities is considered to start later than the decline in other cognitive domains, around the age of 70 years,31 i.e. nine years after our first assessment of verbal abilities. While including verbal abilities from IPTentry in the present study, as in the OCTO-twin analyses,3 did to some extent attenuate the association between being overweight/obese in midlife and poorer mean level cognitive performance at the age of 65, the association remained significant. It is likely that the point estimates declined because of shared variance between cognitive abilities. Given these findings and the fact that there is also an effect of early midlife overweight/obesity on the slope, we think it is likely that the BMI effect reflects exposure and is not an artifact, although we believe that the association probably is bidirectional.
Causal pathways
The association between high midlife BMI and later cognitive abilities was not attenuated in the present study by considering cardio-metabolic factors, including diabetes and exercise, in midlife or in both midlife and late life, in OCTO-twin,3 or in the Whitehall II study.1 There are several potential methodological limitations such as undiagnosed type II diabetes and hypertension (although this should be less common among the SATSA participants as they receive feedback on their health after the IPT visit), the effects of the preclinical phase, and treatment and treatment adherence (not captured by SATSA). There may be other causal pathways that we did not assess. For example, the adipose tissue is the body’s largest endocrine organ and secretes hormones, cytokines, and growth factors that can cross the blood-brain barrier36 and affect brain health. Likewise, these factors may interact directly with blood vessels37, 38 and contribute to disrupting homeostasis. Leptin and adiponectin have been suggested as possible pathways between overweight and brain health.39 Another proposed causal pathway is through inflammatory processes. Overweight persons have higher levels of inflammation than normal-weight persons.40 In longitudinal studies, serum C-reactive protein and pro-inflammatory cytokines like IL-6 have been associated with cognitive decline.41, 42
Decline in BMI and cognitive functioning
It has been proposed that low BMI in late life is a risk factor for various health outcomes, including cognitive decline5, 7 and dementia.43, 44 However, we found that low BMI in late midlife was not a risk factor for low cognitive abilities when change in BMI was controlled for. Weight decline might be a sign of various diseases. In particular, declining weight is associated with the preclinical phase and the progression of dementia.5-7, 45 This explanation is less likely in SATSA, considering the extensive work-up and strict exclusion criteria for persons diagnosed with dementia in the present analyses. Other plausible pathways are through diseases associated with both weight loss and lower cognitive abilities. Indeed, persons with declining BMI had lower general physical health and were older, however, controlling for general physical health and age did not substantially change the association between declining BMI and cognitive test performance. It is worth mentioning that the persons who lost five percentage points or more of their BMI from early to late midlife had the highest BMI in early midlife (still not very high). It might seem a little bit contradictory given that high weight often is associated with a higher risk of weight gain.46 As mentioned, the decline in BMI and cognition might stem from some consequences of being overweight/obese in early midlife. It should also be remembered that this group of persons who had a declining BMI was rather small (6.5 % of the sample). Finally, it should be highlighted that we don’t know if their weight loss was intentional or not. Future studies need to address these questions.
Underweight and cognitive functioning
There were a small number of people (2.9 % of the sample) who were underweight at both times of measurement. These persons had a higher risk of low cognitive test performance, especially on tests measuring verbal abilities and memory, even when general physical health was controlled for. Similar associations between BMI and executive abilities have previously been reported1 as well as between underweight and dementia,47, 48 suggesting a need to explore potential pathways in further studies with sufficient numbers of persons being underweight.
It should be noticed that we used a higher cut-off point (20 kg/m2) for underweight as there were few persons with a BMI below 18.5 kg/m2. As weight increases over the life span, underweight is an uncommon phenomena in old age with its strict definitions.44, 49 Furthermore, the optimal cut-off scores for BMI for the elderly are under discussion, and it is suggested that the cut-offs should be moved upward.50, 51 Given this, and to be able to evaluate a potential U-shaped association, as reported in other studies,1, 6 and to increase the statistical power it seemed reasonable to use a higher cut-off point. Using the less restrictive cut-off point of underweight and still finding a moderate negative effect on cognitive abilities in late life contributes to the discussion about optimal cut-off points.
Strengths and limitations
The strengths of our study include its population-based design and the long follow-up period with repeated measures of cognitive function using a battery of cognitive tests. Nevertheless, there are limitations that need to be discussed. Although BMI is correlated with fat mass,52, 53 it does not assess body fat distribution. The number of participants in this study who were overweight or obese in early midlife was relatively low, which might be due to the fact that height and weight were self-reported. However, a previous study of SATSA have shown high accuracy of self-reported height and weight.54 Moreover, the proportions of overweight and obese persons from this study were also representative of the estimated proportions in the Swedish population in 1960 and 1970.
Conclusions
In summary, there is a negative effect of being overweight/obese across midlife on cognitive abilities, especially on the mean level performance. Furthermore, weight loss between early and late midlife is associated with lower cognitive performance in late life. These findings indicate that BMI patterns across midlife may be important risk markers of late life cognitive health. This study also shows the importance of considering change in BMI when the association between BMI and cognitive health is studied.
Funding/Acknowledgements
This study is supported by National Institute of Aging (AG04563, AG10175, AG08724), The MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Working Life and Social Research (FAS) (97:0147:1B, 2009-0795, postdoc grant 2010-0704, FLARE postdoc grant 2010-1852), Swedish Research Council (825-2007-7460, 825-2009-6141), and The Bank of Sweden Tercentenary Foundation.
Footnotes
Conflict of Interest
All of the authors (Anna K. Dahl, Linda B. Hassing, Eleonor I. Fransson, Margaret Gatz, Chandra A. Reynolds & Nancy L. Pedersen) report no disclosure.
References
- 1.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009;89(2):601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadra S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alz Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 3.Hassing LB, Dahl AK, Pedersen NL, Johansson B. Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement Geriatr Cogn Disord. 2010;29(6):543–552. doi: 10.1159/000314874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, et al. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci. 2010;65(1):57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brubacher D, Monsch A, Stähelin H. Weight change and cognitive performance. Int J Obes. 2004;28:1163–1167. doi: 10.1038/sj.ijo.0802721. [DOI] [PubMed] [Google Scholar]
- 6.Buchman A, Wilson R, Bienias J, Shah R, Evans D, Bennett D. Change in body mass index and risk of incident Alzheimers disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 7.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res. 2004;12(9):1519–1526. doi: 10.1038/oby.2004.189. [DOI] [PubMed] [Google Scholar]
- 8.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 9.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26(1, Supplement 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Corley J, Gow AJ, Starr JM, Deary IJ. Is body mass index in old age related to cognitive abilities? the Lothian birth cohort 1936 study. Psychol Aging. 2010;25:867–875. doi: 10.1037/a0020301. [DOI] [PubMed] [Google Scholar]
- 11.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 12.Jeffreys M, Lawlor DA, Galobardes B, McCarron P, Kinra S, Ebrahim S, et al. Lifecourse weight patterns and adult-onset diabetes: the Glasgow Alumni and British Women’s Heart and Health studies. Int J Obes. 2005;30(3):507–512. doi: 10.1038/sj.ijo.0803161. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein P, De faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 14.Finkel D, Pedersen N. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish adoption/twin study of aging. Aging Neurpsych Cogn. 2004;11(2-3):325–345. [Google Scholar]
- 15.Gatz M, Pedersen N, Berg S, Johansson B, Johansson K, Mortimer J, et al. Heritability for Alzheimer’s disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52A(2):M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 16.Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26(4):439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychol Sci. 1992;3(6):346–352. [Google Scholar]
- 18.Jonsson CO, Molander L. Manual till CVD-skalan. Psykologi Förlaget; Stockholm: 1964. [Google Scholar]
- 19.Dureman I, Kebbob L, Osterberg E. Manual till DS-batteriet. Psykologi Förlaget; Stockholm: 1971. [Google Scholar]
- 20.Westrin PA. WIT III manual. Skandinaviska Test Förlaget; Stockholm: 1969. [Google Scholar]
- 21.Arthur G. A point scale of performance tests, revised form II. The Psychological Corporation; New York: 1947. [Google Scholar]
- 22.Ekström R, French J, Harman H. Manual kit of factor referenced cognitive tests. Educational Testing Service; Princeton: 1976. [Google Scholar]
- 23.Thurstone L. Primary mental abilities. University of Chicago Press; Chicago: 1938. [Google Scholar]
- 24.DeFries JC, Plomin R, Vandenberg SG, Kuse AR. Parent-offspring resemblance for cognitive abilities in the Colorado Adoption Project - Biological, adoptive, and control parents and one-year-old children. Intelligence. 1981;5(3):245–277. [Google Scholar]
- 25.Smith A. Symbol Digit Modalities Test (SDMT) manual. Western Psychological Services; Los Angeles: 1982. rev. ed. [Google Scholar]
- 26.Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22(3):558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- 27.Duke University Center for the Study of Aging and Human Development . Multidimensional functional assessment: the OARS methodology. Duke University Medical Center; Durham, NC: 1978. [Google Scholar]
- 28.Harris JR, Pedersen NL, McClearn G, Plomin R, Nesselroade JR. Age differences in genetic and enviromentaö influences for health from Swedish Adoption/Twin Study of Aging. J Gerontol B Psych Sci Soc Sci. 1992;47(3):P213–220. doi: 10.1093/geronj/47.3.p213. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc . SAS system for Microsoft Windows. 9.1 ed SAS Institute Inc.; 2002-2003. [Google Scholar]
- 30.McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. In: Schinka J, Velicer W, editors. Comprehensive handbook of psychology. Vol. 2. Wiley; New York: 2003. pp. 447–480. [Google Scholar]
- 31.Finkel D, Reynolds C, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39(3):535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- 32.Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67(1):24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- 33.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 34.Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. J Clin Epidemiol. 2005;58(12):1308–1315. doi: 10.1016/j.jclinepi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Schaie KW. Developmental influences on adult intelligence - the Seattle Longitudinal Study. OUp USA; New York: 2005. [Google Scholar]
- 36.Lathe R. Hormones and the hippocampus. J Endocrinol. 2001;169(2):205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- 37.De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33(12):2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 38.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26(6):754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 39.Barrett-Connor E. An introduction to obesity and dementia. Curr Alz Res. 2007;4:97–101. doi: 10.2174/156720507780362074. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Buring JE, Cook NR, Rifai N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events: An 8-Year Follow-Up of 14 719 Initially Healthy American Women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 42.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 43.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen project. J Am Geriatr Soc. 2008;56(1):111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 44.Dahl A, Lopponen M, Isoaho R, Berg S, Kivela SL. Overweight and obesity in old age are not associated with greater dementia risk. J Am Geriatr Soc. 2008;56(12):2261–2266. doi: 10.1111/j.1532-5415.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 45.Beydoun MA, Lhotsky A, Wang Y, Forno GD, An Y, Metter EJ, et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am J Epidemiol. 2008;168(10):1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry C, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br Med J. 2005;330:1360–1364. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in the elderly: a 25-year longitudinal study of a population aged 70 to 95 years. Eur J Nutr. 1999;53:905–914. doi: 10.1038/sj.ejcn.1600852. [DOI] [PubMed] [Google Scholar]
- 50.Ferrucci L, Studenski SA, Alley DE, Barbagallo M, Harris TB. Obesity in aging and art. J of Gerontol A Biol Sci Med Sci. 2010;65(1):53–56. doi: 10.1093/gerona/glp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161(9):1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 53.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 54.Dahl AK, Hassing LB, Fransson EI, Pedersen NL. Agreement between self-reported and measured height, weight and body mass index in old age--a longitudinal study with 20 years of follow-up. Age Ageing. 2010;39(4):445–451. doi: 10.1093/ageing/afq038. [DOI] [PMC free article] [PubMed] [Google Scholar]