Highlights
► Allergen-specific immunotherapy is based on therapeutic vaccination with allergens. ► The genes encoding allergen molecules and their structures have been identified. ► Detailed knowledge of allergen structures and epitopes allows engineering new vaccines. ► These vaccines target different mechanisms of the allergic immune response. ► New allergy vaccines will increase safety, efficacy and convenience of immunotherapy and are currently tested in immunotherapy trials.
Abstract
Vaccines aim to establish or strengthen immune responses but are also effective for the treatment of allergy. The latter is surprising because allergy represents a hyper-immune response based on immunoglobulin E production against harmless environmental antigens, i.e., allergens. Nevertheless, vaccination with allergens, termed allergen-specific immunotherapy is the only disease-modifying therapy of allergy with long-lasting effects. New forms of allergy diagnosis and allergy vaccines based on recombinant allergen-derivatives, peptides and allergen genes have emerged through molecular allergen characterization. The molecular allergy vaccines allow sophisticated targeting of the immune system and may eliminate side effects which so far have limited the use of traditional allergen extract-based vaccines. Successful clinical trials performed with the new vaccines indicate that broad allergy vaccination is on the horizon and may help to control the allergy pandemic.
Introduction
Allergy is an immunoglobulin E (IgE)-mediated hypersensitivity disease affecting more than 25% of the population [1]. Allergic patients suffer from a broad variety of symptoms (e.g., rhinoconjunctivitis, asthma, dermatitis, gastrointestinal symptoms, life-threatening systemic anaphylaxis) upon allergen contact. Allergen-specific immunotherapy (SIT), the therapeutic vaccination with the disease-causing allergens is a clinically effective treatment for allergy which unlike symptomatic pharmacotherapy, modifies the underlying pathological immune response and seems to have long-lasting effects [2]. In 1911 L. Noon published the first successful immunotherapy trial which he had performed in grass pollen-allergic patients using pollen extracts administered by subcutaneous immunization [3]. In his classical publication (Figure 1) he referred to the work by W.P. Dunbar, who not only had identified pollen grains as allergen source but also had succeeded in producing antisera by immunizing animals with pollen extracts [4]. Moreover, Dunbar showed that these antisera prevented allergic symptoms when patients were exposed to allergens in provocation tests. The fact that vaccination with allergens can be used to treat a hypersensitivity disease is surprising. The dominant explanation for this effect is that SIT induces counter IgG responses towards the disease-causing allergens which interfere with allergen-specific IgE responses. The latter has been demonstrated in a classical study already in 1935 [5]. Since then, many studies support the concept that the induction of allergen-specific blocking IgG antibodies is a major mechanism of SIT but also cellular mechanisms are discussed and investigated [2].
Figure 1.
Classical publication of the first immunotherapy trial conducted by L. Noon from 1911, highlighting the earlier experiments performed by WP Dunbar supporting that SIT is a vaccine approach. Reprinted from [3] with permission.
Although SIT is a clinically effective treatment, it has been recognized that the use of natural allergen extracts for the preparation of the vaccines is a major bottleneck for its further development towards a broadly used treatment. In this context, several studies have demonstrated the poor quality of allergen extracts which may lack important allergens and show unpredictable compositions [6–8,9•]. Another major problem of SIT is that the administration of allergens can cause severe side effects [9•]. Therefore, SIT is limited to specially trained physicians, it requires the multiple application of increasing allergen doses to reach the therapeutically effective maintenance doses and thus only relatively few allergic patients can benefit from SIT.
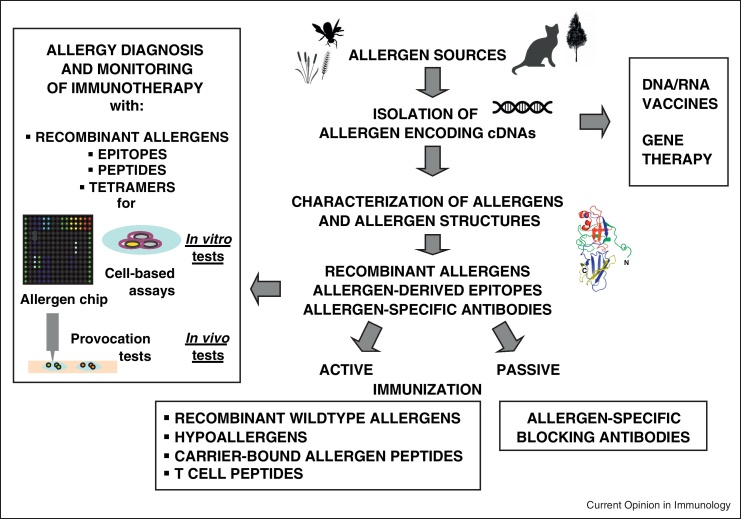
However, during the last 25 years, molecular cloning techniques have enabled the isolation of allergen-encoding cDNAs from various allergen sources and the subsequent production of allergens in recombinant form [10•]. Recombinant allergens have facilitated the investigation of the pathomechanisms of allergy using clinically relevant allergens in patients. In vitro and in vivo diagnostic tests based on recombinant allergens, epitopes and peptides have been developed which allow for a more precise diagnosis of allergy and monitoring of immune responses during treatment (Figure 2) (reviewed in [10•]). Most importantly, the availability of allergen sequences and structures has allowed the development of new sophisticated allergy vaccines which have entered successful clinical evaluation and hold promise that more effective, safe and convenient allergy vaccines become soon available and it even seems that prophylactic vaccination is on the horizon [10•].
Figure 2.
Demonstration of the impact of the knowledge of allergen genes and allergen sequences/structures for the development of new forms of allergy diagnosis, therapy monitoring and treatment.
Allergen-based diagnosis and monitoring of immune responses: Towards better prescription and understanding of SIT mechanisms.
Allergen-SIT is a form of therapeutic vaccination and hence requires that the disease-causing allergens are identified for prescription of the correct vaccine. Diagnostic tests based on recombinant allergen molecules allow the precise determination of IgE reactivities to the individual allergen molecules leading to better understanding of cross-reactivities [11]. Furthermore, component testing identifies the allergen sources primarily responsible for allergic sensitization in different populations and thus forms a basis for better prescription of SIT. A recent study demonstrated that the detailed knowledge of the molecular IgE reactivity profiles profoundly changed the prescription of allergy vaccines among allergologists [12•]. Using diagnostic tests based on recombinant bee and wasp allergens devoid of cross-reactive carbohydrate epitopes [13] it was shown that it is possible to discriminate genuinely bee and wasp-sensitized patients from double-sensitized patients, allowing more precise prescription of venom SIT. In fact, clinically non-relevant cross-reactive carbohydrate epitopes were also shown to impair the diagnosis of cat allergy [14] which can be achieved with non-glycosylated recombinant cat allergens [15].
Several studies conducted in food allergic patients indicate that component-resolved allergy diagnosis allows predicting whether individuals are prone to develop mild or more severe manifestations of food allergy [16–18]. Such tests are useful for preventive measures such as diet and may pave the road towards vaccination against food allergy. In this context, clinical studies have shown that oral immunotherapy, may be effective for the treatment of milk, egg and peanut allergy [19–21]. Several studies highlight the usefulness of diagnostic allergy tests based on micro-arrayed allergens which allow the simultaneous detection of IgE reactivities against a large number of allergens with small blood samples [16,22,23].
Recombinant allergen components, epitopes and peptides have been used to study the effects of SIT in patients (reviewed in [2,9•,10•]). Studies using recombinant allergens for monitoring of SIT re-emphasized the importance of blocking IgG antibodies for the prevention of allergen-induced effector cell degranulation [24,25]. On the basis of the availability of allergen sequences it has become possible to produce MHCII tetramers for monitoring allergen-specific T cell responses in the course of disease and during SIT [26,27].
Mechanisms of SIT
Besides classical injection immunotherapy (SCIT) also other application routes for SIT have been investigated. Many clinical studies based on sublingual administration of allergens (SLIT) have been conducted and it seems that this treatment is clinically effective, albeit less than injection immunotherapy. Also the immunological mechanisms underlying SLIT are less well understood than those of SCIT. A recent study proposed that IgE-mediated allergen uptake by mucosal APCs may lead to an increased production of immunoregulatory cytokines like IL-10 and TGF-β [28•]. Yet SLIT induces only low levels of allergen-specific IgG but rather boosts allergen-specific IgE [29]. By contrast, it has been demonstrated in immunotherapy trials using purified recombinant wildtype allergens for subcutaneous vaccination that the induction of allergen-specific IgG antibodies was associated with the reduction of symptoms, medication intake and sensitivity to the allergen [30]. Vaccination-induced allergen-specific IgG thus occupies the binding sites for IgE on the allergens and blocks allergen-induced effector cell activation [25] as well as IgE-facilitated antigen presentation of allergens to T cells [31]. The latter mechanism may explain the reduced allergen-specific T cell activation in SIT-treated patients as an antibody-mediated effect. Interestingly, it was suggested that besides epitope-specificity, affinity may play an important role for the effects of blocking antibodies [32•]. Already in earlier SCIT trials with recombinant hypoallergenic allergen derivatives and CpG-modified allergens it has been shown that the induction of allergen-specific IgG is associated with a reduction of the boosts of allergen-specific IgE production occurring after seasonal allergen exposure and represents another important mechanism for the effects of SCIT [33,34]. The mechanisms underlying SCIT with hypoallergenic allergen-derivatives have been summarized in a recent review [35].
In addition to established subcutaneous injection of allergens and allergen-derivatives intralymphatic administration of allergy vaccines has been suggested for reducing side effects and obtaining increased allergen-specific IgG but the latter has not yet been convincingly demonstrated [36]. Furthermore, epicutaneous allergen administration has been proposed as an alternative route to subcutaneous injection. Studies performed in experimental animal models indicate that epicutaneous allergen application may have effects on systemic allergen-specific immune responses [37,38] but effects on systemic immunity have not been studied in allergic patients [39].
Studies performed with T cell epitope-containing peptides which do not induce allergen-specific IgG responses indicate that SIT may also selectively address allergen-specific T cell responses. The administration of peptides from the major cat allergen Fel d 1 induced a reduction of delayed type, presumably T cell-mediated symptoms [40]. The induction of IL-10 producing T regulatory cells and the existence of linked immunological suppression have been suggested as possible underlying mechanisms [41]. Indeed, it has been demonstrated in a TCR-transgenic model that Th1 cells as well as Fox p 3+ regulatory T cells are able to suppress the effector functions of Th2 cells [42,43]. However, the effects of T cell peptide therapy on allergen-induced mast cell degranulation and acute allergic inflammation observed in clinical trials are modest and deserve further investigation [44].
New molecules and strategies for SIT: sophisticated targeting of the immune system
When the sequences of the first major allergens became available and their T cell epitopes could be mapped using peptide technology, the concept of inducing T cell tolerance with non-IgE reactive but T cell epitope-containing peptides emerged as the first targeted molecular SIT approach [45,46]. Currently several immunotherapy studies with allergen-derived T cell peptides are ongoing of which a recent study performed for cat allergy showed beneficial effects on late phase allergic symptoms [40]. Although there is evidence for linked immunological suppression [41], it is very likely that the T cell peptide approach will require that several different allergen-derived peptides are included in the vaccine in order to cover the diverse spectrum of T cell epitopes recognized by allergic patients.
Several research groups have therefore developed recombinant hypoallergenic allergen derivatives which are characterized by reduced IgE reactivity but preserved T cell epitopes to reduce IgE-mediated side effects during SIT (reviewed in [35,47]). These molecules are made by reducing IgE reactivity and maintaining the spectrum of the T cell epitopes of the original wildtype allergens by various recombinant DNA technologies such as mutation, reassembly, or fragmentation. The first immunotherapy trial with recombinant allergens was actually performed with recombinant hypoallergenic derivatives of the major birch pollen allergen Bet v 1 eleven years ago. Meanwhile, successful phase III SIT trials with recombinant Bet v 1 hypoallergens have been performed [48]. The analyses of SCIT trials which have been performed with recombinant hypoallergenic allergen derivatives revealed the induction of allergen-specific blocking IgG antibodies as a major underlying mechanism. Several recombinant hypoallergens for the treatment of allergy to house dust mites, birch pollen and cat have been constructed and characterized in in vitro and experimental animal models recently [49–53,54•]. Different strategies for their construction have been used such as reassembly of sequence elements [49,50,55], oligomerization to increase IgG responses [51], epitope grafting inducing altered fold [54•], coupling to Vitamin D3 for immunomodulation [52], random PCR-based sequence evolution [56] and mutations [53].
Successful clinical SCIT trials have been performed with recombinant allergens made exactly as their natural counterparts for birch and grass pollen allergy [30,57]. These studies also demonstrated that clinical efficacy and reduction of sensitivity are associated with the development of allergen-specific IgG antibodies [48].
In the course of clinical studies performed with non-IgE-reactive T cell peptides and recombinant hypoallergenic allergen derivatives it has been recognized that allergen-derived T cell epitopes can induce late phase, non-IgE-mediated side effects. In an attempt to eliminate IgE as well as T cell-mediated side effects a new type of allergy vaccines has therefore been developed [10•]. This approach is based on the identification of allergen peptides which are part of the major IgE binding sites on allergens but per se lack IgE and allergenic activity [58]. These peptides which are typically between 20 and 35 amino acids in length can either be coupled chemically to a carrier protein [59–61] or produced as recombinant fusion proteins with a carrier protein [62,63•]. Immunization with carrier-bound allergen peptides induces and focuses IgG antibodies towards the IgE epitopes on allergens with the T cell help of the carrier protein and thus do not activate allergen-specific T cells. Since the presence of allergen-specific T cell epitopes is reduced in these vaccines they should bypass T cell-mediated side effects [9•]. Viral carrier proteins have been suggested in order to achieve immunomodulation and to induce a beneficial antiviral immunity in addition to protection against allergens [64]. A similar approach aiming at the induction of allergen-specific IgG antibodies is based on the use of allergen mimotopes [65].
Since allergen-specific IgG antibodies seem to play a major role in antagonizing allergic immune responses, passive immunization with allergen-specific IgG antibodies has emerged as another possibility for allergy vaccination [66]. In fact, human allergen-specific IgG antibodies capable of blocking even polyclonal IgE recognition of allergens have been developed [67,68]. Moreover, it has been demonstrated in experimental animal models that maternal immunization generates allergen-specific IgG which is transmitted to the offspring and can prevent allergic sensitization [69,70,71•].
On the basis of the finding that transfer of hematopoetic stem cells expressing major allergens can induce robust allergen-specific tolerance in a preventive model of allergy [72,73], membrane-anchored and secreted allergen-expressing stem cells have been recently engineered to delineate the mechanisms of tolerance [74]. Gene therapy is an interesting preventive approach for allergy but will require the simultaneous assembly of major allergen epitopes recognized in certain populations and it will be necessary to develop safe treatment protocols [74] (Figure 2).
Another interesting approach for immunomodulation is vaccination with allergen-encoding RNA which in experimental animal models has been shown to prevent allergic sensitization for several allergens [75].
Outlook: broad therapeutic vaccination and prophylactic vaccines?
Table 1 provides an overview of different strategies for SIT based on their putative immunological mechanisms. Certain strategies such as SCIT with recombinant allergen, hypoallergens, T cell peptides and carrier-bound allergen peptides are already in clinical evaluation and hold promise that allergen molecule-based vaccines will be soon available for broad, safe and convenient therapeutic vaccination. However, more controlled immunotherapy studies with defined allergen, allergen derivatives and epitopes are needed to further delineate the mechanisms of SIT and optimize the vaccines. Studies performed in experimental animal models and non-allergic individuals with engineered allergen derivatives and epitopes will be necessary to develop prophylactic vaccination and tolerance induction strategies for the prevention of allergies which may ultimately halt the allergy pandemic.
Table 1.
Strategies for SIT
| Human | Animal | |
|---|---|---|
| Active induction of a counter immune response | 24, 25, 30, 33, 34, 36, 57 | 49–55, 59–65 |
| Peripheral tolerance | 19–21, 40, 44, 45, | 37, 38, 41 |
| Central tolerance | 72, 73 | |
| Immune modulation | 75 | |
| Passive immunization | 69–71 |
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
Acknowledgements
This study was supported by grants F4605 and P23350-B11 from the Austrian Science Fund, by the Framework Program 7 (FP7) of the European Commission (FAST Project), the Christian Doppler Research Association, and a research grant from Biomay, Vienna, Austria.
References
- 1.Flöistrup H., Swartz J., Bergström A., Alm J.S., Scheynius A., van Hage M., Waser M., Braun-Fahrländer C., Schram-Bijkerk D., Huber M. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Larché M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 3.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 4.Dunbar W.P. Zur Ursache und spezifischen Heilung des Heufiebers. Dtsch Med Wochenschr. 1903;9:24–28. [Google Scholar]
- 5.Cooke R.A., Barnard J.H., Hebald S., Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy. J Exp Med. 1935;62:733–750. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetto B., Tinghino R., Braschi M.C., Antonicelli L., Pini C., Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–190. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 7.Curin M., Reininger R., Swoboda I., Focke M., Valenta R., Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–263. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 8.Blank S., Seismann H., Michel Y., McIntyre M., Cifuentes L., Braren I., Grunwald T., Darsow U., Ring J., Bredehorst R. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66:1322–1329. doi: 10.1111/j.1398-9995.2011.02667.x. [DOI] [PubMed] [Google Scholar]
- 9•.Focke M., Swoboda I., Marth K., Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]; This review provides a comprehensive description of problems associated with traditional allergen extract-based SIT and a recently developed immunotherapy concept based on allergen-derived peptides which addresses these bottlenecks.
- 10•.Valenta R., Ferreira F., Focke-Tejkl M., Linhart B., Niederberger V., Swoboda I., Vrtala S. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]; This article reviews the fine structure of allergens and discusses the impact of such knowledge on the development of new forms of allergy diagnosis and allergy vaccines.
- 11.Hiller R., Laffer S., Harwanegg C., Huber M., Schmidt W.M., Twardosz A., Barletta B., Becker W.M., Blaser K., Breiteneder H. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–416. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 12•.Sastre J., Landivar M.E., Ruiz-García M., Andregnette-Rosigno M.V., Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–711. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]; This study demonstrates how the knowledge of molecular IgE reactivity profiles gained by component-resolved allergy diagnosis affects the prescription of SIT in clinical practice.
- 13.Mittermann I., Zidarn M., Silar M., Markovic-Housley Z., Aberer W., Korosec P., Kosnik M., Valenta R. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–1307. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Arkestål K., Sibanda E., Thors C., Troye-Blomberg M., Mduluza T., Valenta R., Grönlund H., van Hage M. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α1,3-galactose. J Allergy Clin Immunol. 2011;127:1024–1028. doi: 10.1016/j.jaci.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Smith W., O’Neil S.E., Hales B.J., Chai T.L., Hazell L.A., Tanyaratsrisakul S., Piboonpocanum S., Thomas W.R. Two newly identified cat allergens: the von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int Arch Allergy Immunol. 2011;156:159–170. doi: 10.1159/000322879. [DOI] [PubMed] [Google Scholar]
- 16.Hochwallner H., Schulmeister U., Swoboda I., Balic N., Geller B., Nystrand M., Härlin A., Thalhamer J., Scheiblhofer S., Niggemann B. Microarray and allergenic activity assessment of milk allergens. Clin Exp Allergy. 2010;40:1809–1818. doi: 10.1111/j.1365-2222.2010.03602.x. [DOI] [PubMed] [Google Scholar]
- 17.Pastorello E.A., Farioli L., Pravettoni V., Scibilia J., Mascheri A., Borgonovo L., Piantanida M., Primavesi L., Stafylaraki C., Pasqualetti S. Pru p 3-sensitised Italian peach-allergic patients are less likely to develop severe symptoms when also presenting IgE antibodies to Pru p 1 and Pru p 4. Int Arch Allergy Immunol. 2011;156:362–372. doi: 10.1159/000324440. [DOI] [PubMed] [Google Scholar]
- 18.Alessandri C., Zennaro D., Scala E., Ferrara R., Bernardi M.L., Santoro M., Palazzo P., Mari A. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen's egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012;42:441–450. doi: 10.1111/j.1365-2222.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 19.Martorell A., De la Hoz B., Ibáñez M.D., Bone J., Terrados M.S., Michavila A., Plaza A.M., Alonso E., Garde J., Nevot S. Oral desensitization as a useful treatment in 2-year-old children with cow's milk allergy. Clin Exp Allergy. 2011;41:1297–1304. doi: 10.1111/j.1365-2222.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 20.Vickery B.P., Pons L., Kulis M., Steele P., Jones S.M., Burks A.W. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105:444–450. doi: 10.1016/j.anai.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varshney P., Jones S.M., Scurlock A.M., Perry T.T., Kemper A., Steele P., Hiegel A., Kamilaris J., Carlisle S., Yue X. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constantin C., Quirce S., Poorafshar M., Touraev A., Niggemann B., Mari A., Ebner C., Akerström H., Heberle-Bors E., Nystrand M. Micro-arrayed wheat seed and grass pollen allergens for component-resolved diagnosis. Allergy. 2009;64:1030–1037. doi: 10.1111/j.1398-9995.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- 23.Bublin M., Dennstedt S., Buchegger M., Antonietta Ciardiello M., Bernardi M.L., Tuppo L., Harwanegg C., Hafner C., Ebner C. The performance of a component-based allergen microarray for the diagnosis of kiwifruit allergy. Clin Exp Allergy. 2011;41:129–136. doi: 10.1111/j.1365-2222.2010.03619.x. [DOI] [PubMed] [Google Scholar]
- 24.Gadermaier E., Staikuniene J., Scheiblhofer S., Thalhamer J., Kundi M., Westritschnig K., Swoboda I., Flicker S., Valenta R. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–1182. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 25.Gadermaier E., Flicker S., Aberer W., Egger C., Reider N., Focke M., Vrtala S., Kundi M., Valenta R. Analysis of the antibody responses induced by subcutaneous injection immunotherapy with birch and Fagales pollen extracts adsorbed onto aluminum hydroxide. Int Arch Allergy Immunol. 2010;151:17–27. doi: 10.1159/000232567. [DOI] [PubMed] [Google Scholar]
- 26.Wambre E., Bonvalet M., Bodo V.B., Maillère B., Leclert G., Moussu H., Von Hofe E., Louise A., Balazuc A.M., Ebo D. Distinct characteristics of seasonal (Bet v 1) vs. perennial (Der p 1/Der p 2) allergen-specific CD4(+) T cell responses. Clin Exp Allergy. 2011;41:192–203. doi: 10.1111/j.1365-2222.2010.03641.x. [DOI] [PubMed] [Google Scholar]
- 27.Wambre E., Delong J.H., James E.A., Lafond R.E., Robinson D., Kwok W.W. Differentiation stage determines pathologic and protective allergen-specific CD4(+) T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–551. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Allam J.P., Würtzen P.A., Reinartz M., Winter J., Vrtala S., Chen K.W., Valenta R., Wenghoefer M., Appel T., Gros E. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–645. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]; This study investigates with defined allergen molecules in an in vitro system the specific interaction of allergens and oral Langerhans cells, resulting in immunoregulatory functions of these cells.
- 29.Dahl R., Kapp A., Colombo G., de Monchy J.G., Rak S., Emminger W., Riis B., Grønager P.M., Durham S.R. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121:512–518. doi: 10.1016/j.jaci.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Pauli G., Larsen T.H., Rak S., Horak F., Pastorello E., Valenta R., Purohit A., Arvidsson M., Kavina A., Schroeder J.W. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Pree I., Shamji M.H., Kimber I., Valenta R., Durham S.R., Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–1352. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 32•.James L.K., Shamji M.H., Walker S.M., Wilson D.R., Wachholz P.A., Francis J.N., Jacobson M.R., Kimber I., Till S.J., Durham S.R. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]; This study suggests that only SIT-induced allergen-specific IgG with high affinity to the allergen persists in treated patients.
- 33.Niederberger V., Horak F., Vrtala S., Spitzauer S., Krauth M.T., Valent P., Reisinger J., Pelzmann M., Hayek B., Kronqvist M. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(Suppl. 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creticos P.S., Schroeder J.T., Hamilton R.G., Balcer-Whaley S.L., Khattignavong A.P., Lindblad R., Li H., Coffman R., Seyfert V., Eiden J.J., Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 35.Linhart B, Valenta R: Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine 2011 Nov 17, PMID: 22100888. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 36.Senti G., Johansen P., Kündig T.M. Intralymphatic immunotherapy: from the rationale to human applications. Curr Top Microbiol Immunol. 2011;352:71–84. doi: 10.1007/82_2011_133. [DOI] [PubMed] [Google Scholar]
- 37.Dioszeghy V., Mondoulet L., Dhelft V., Ligouis M., Puteaux E., Benhamou P.H., Dupont C. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186:5629–5637. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 38.Mondoulet L., Dioszeghy V., Vanoirbeek J.A., Nemery B., Dupont C., Benhamou P.H. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011;154:299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 39.Senti G., von Moos S., Tay F., Graf N., Sonderegger T., Johansen P., Kündig T.M. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 40.Worm M., Lee H.H., Kleine-Tebbe J., Hafner R.P., Laidler P., Healey D., Buhot C., Verhoef A., Maillère B., Kay A.B. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Campbell J.D., Buckland K.F., McMillan S.J., Kearley J., Oldfield W.L., Stern L.J., Grönlund H., van Hage M., Reynolds C.J., Boyton R.J. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neunkirchner A., Leb-Reichl V.M., Schmetterer K.G., Mutschlechner S., Kueng H.J., Haiderer D., Schuch K., Wallner M., Jahn-Schmid B., Bohle B. Human TCR transgenic Bet v 1-specific Th1 cells suppress the effector function of Bet v 1-specific Th2 cells. J Immunol. 2011;187:4077–4087. doi: 10.4049/jimmunol.1003220. [DOI] [PubMed] [Google Scholar]
- 43.Schmetterer K.G., Haiderer D., Leb-Reichl V.M., Neunkirchner A., Jahn-Schmid B., Küng H.J., Schuch K., Steinberger P., Bohle B., Pickl W.F. Bet v 1-specific T-cell receptor/forkhead box protein 3 transgenic T cells suppress Bet v 1-specific T-cell effector function in an activation-dependent manner. J Allergy Clin Immunol. 2011;127:238–245. doi: 10.1016/j.jaci.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Oldfield W.L., Larché M., Kay A.B. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 45.Norman P.S., Ohman J.L., Jr., Long A.A., Creticos P.S., Gefter M.A. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–1628. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 46.Moldaver D., Larché M. Immunotherapy with peptides. Allergy. 2011;66:784–791. doi: 10.1111/j.1398-9995.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 47.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 48.Valenta R., Linhart B., Swoboda I., Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen K.W., Fuchs G., Sonneck K., Gieras A., Swoboda I., Douladiris N., Linhart B., Jankovic M., Pavkov T., Keller W. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–2498. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Campana R., Vrtala S., Maderegger B., Jertschin P., Stegfellner G., Swoboda I., Focke-Tejkl M., Blatt K., Gieras A., Zafred D. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J Allergy Clin Immunol. 2010;126:1024–1031. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Vrtala S., Fohr M., Campana R., Baumgartner C., Valent P., Valenta R. Genetic engineering of trimers of hypoallergenic fragments of the major birch pollen allergen Bet v 1, for allergy vaccination. Vaccine. 2011;29:2140–2148. doi: 10.1016/j.vaccine.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grundström J., Neimert-Andersson T., Kemi C., Nilsson O.B., Saarne T., Andersson M., van Hage M., Gafvelin G. Covalent coupling of vitamin D3 to the major cat allergen Fel d 1 improves the effects of allergen-specific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157:136–146. doi: 10.1159/000327546. [DOI] [PubMed] [Google Scholar]
- 53.Saarne T., Neimert-Andersson T., Grönlund H., Jutel M., Gafvelin G., van Hage M. Treatment with a Fel d 1 hypoallergen reduces allergic responses in a mouse model for cat allergy. Allergy. 2011;66:255–263. doi: 10.1111/j.1398-9995.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 54•.Wallner M., Hauser M., Himly M., Zaborsky N., Mutschlechner S., Harrer A., Asam C., Pichler U., van Ree R., Briza P. Reshaping the Bet v 1 fold modulates T(H) polarization. J Allergy Clin Immunol. 2011;127:1571–1578. doi: 10.1016/j.jaci.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of a hypoallergenic molecule for vaccination against birch pollen and related allergies.
- 55.Chen KW, Blatt K, Thomas WR, Swoboda I, Valent P, Valenta R, Vrtala S: Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J Allergy Clin Immunol 2012. [DOI] [PMC free article] [PubMed]
- 56.Nilsson O.B., Adedoyin J., Rhyner C., Neimert-Andersson T., Grundström J., Berndt K.D., Crameri R., Grönlund H. In vitro evolution of allergy vaccine candidates, with maintained structure, but reduced B cell and T cell activation capacity. PLoS One. 2011;6:e24558. doi: 10.1371/journal.pone.0024558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jutel M., Jaeger L., Suck R., Meyer H., Fiebig H., Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Gieras A., Cejka P., Blatt K., Focke-Tejkl M., Linhart B., Flicker S., Stoecklinger A., Marth K., Drescher A., Thalhamer J. Mapping of conformational IgE epitopes with peptide-specific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J Immunol. 2011;186:5333–5344. doi: 10.4049/jimmunol.1000804. [DOI] [PubMed] [Google Scholar]
- 59.Twaroch T.E., Focke M., Civaj V., Weber M., Balic N., Mari A., Ferrara R., Quirce S., Spitzauer S., Swoboda I. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Twaroch TE, Focke M, Fleischmann K, Balic N, Meyerweck S, Lupinek C, Blatt K, Mari A, Ebner C, Valent P, et al.: Carrier-bound Alt a 1 peptides without allergenic activity for vaccination againstAlternaria alternataallergy. Clin Exp Allergy 2012. [DOI] [PMC free article] [PubMed]
- 61.Chen K.W., Focke-Tejkl M., Blatt K., Kneidinger M., Gieras A., Dall’antonia F., Faé I., Fischer G., Keller W., Valent P. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients. Exp Allergy Immunol. 2012;67:609–621. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edlmayr J., Niespodziana K., Linhart B., Focke-Tejkl M., Westritschnig K., Scheiblhofer S., Stoecklinger A., Kneidinger M., Valent P., Campana R. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–6306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 63•.Niespodziana K., Focke-Tejkl M., Linhart B., Civaj V., Blatt K., Valent P., van Hage M., Grönlund H., Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–1570. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Development of a vaccine for the treatment of cat allergy based on fusing allergen peptides to the hepatitis PreS protein.
- 64.Edlmayr J., Niespodziana K., Focke-Tejkl M., Linhart B., Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–140. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- 65.Wallmann J., Epstein M.M., Singh P., Brunner R., Szalai K., El-Housseiny L., Pali-Schöll I., Jensen-Jarolim E. Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin Exp Allergy. 2010;40:650–658. doi: 10.1111/j.1365-2222.2009.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flicker S., Gadermaier E., Madritsch C., Valenta R. Passive immunization with allergen-specific antibodies. Curr Top Microbiol Immunol. 2011;352:141–159. doi: 10.1007/82_2011_143. [DOI] [PubMed] [Google Scholar]
- 67.Padavattan S., Flicker S., Schirmer T., Madritsch C., Randow S., Reese G., Vieths S., Lupinek C., Ebner C., Valenta R. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J Immunol. 2009;182:2141–2151. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- 68.Hecker J., Diethers A., Etzold S., Seismann H., Michel Y., Plum M., Bredehorst R., Blank S., Braren I., Spillner E. Generation and epitope analysis of human monoclonal antibody isotypes with specificity for the Timothy grass major allergen Phl p 5a. Mol Immunol. 2011;48:1236–1244. doi: 10.1016/j.molimm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Ellertsen L.K., Nygaard U.C., Melkild I., Løvik M. Maternal allergen immunisation to prevent sensitisation in offspring: Th2-polarising adjuvants are more efficient than a Th1-polarising adjuvant in mice. BMC Immunol. 2010;11:8. doi: 10.1186/1471-2172-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakata K., Kobayashi K., Ishikawa Y., Yamamoto M., Funada Y., Kotani Y., Blumberg R.S., Karasuyama H., Yoshida M., Nishimura Y. The transfer of maternal antigen-specific IgG regulates the development of allergic airway inflammation early in life in an FcRn-dependent manner. Biochem Biophys Res Commun. 2010;395:238–243. doi: 10.1016/j.bbrc.2010.03.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Victor J.R., Muniz B.P., Fusaro A.E., de Brito C.A., Taniguchi E.F., Duarte A.J., Sato M.N. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol. 2010;11:11. doi: 10.1186/1471-2172-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; These studies describe maternal allergy vaccination as a potential strategy to prevent allergic sensitization of the offspring.
- 72.Baranyi U., Linhart B., Pilat N., Gattringer M., Bagley J., Muehlbacher F., Iacomini J., Valenta R., Wekerle T. Tolerization of a type I allergic immune response through transplantation of genetically modified hematopoietic stem cells. J Immunol. 2008;180:8168–8175. doi: 10.4049/jimmunol.180.12.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baranyi U., Gattringer M., Boehm A., Marth K., Focke-Tejkl M., Bohle B., Blatt K., Valent P., Valenta R., Wekerle T. Expression of a major plant allergen as membrane-anchored and secreted protein in human cells with preserved T cell and B cell epitopes. Int Arch Allergy Immunol. 2011;156:259–266. doi: 10.1159/000323733. [DOI] [PubMed] [Google Scholar]
- 74.Baranyi U., Gattringer M., Valenta R., Wekerle T. Cell-based therapy in allergy. Curr Top Microbiol Immunol. 2011;352:161–179. doi: 10.1007/82_2011_127. [DOI] [PubMed] [Google Scholar]
- 75.Roesler E., Weiss R., Weinberger E.E., Fruehwirth A., Stoecklinger A., Mostböck S., Ferreira F., Thalhamer J., Scheiblhofer S. Immunize and disappear-safety-optimized mRNA vaccination with a panel of 29 allergens. J Allergy Clin Immunol. 2009;124:1070–1077. doi: 10.1016/j.jaci.2009.06.036. [DOI] [PubMed] [Google Scholar]