Graphical abstract
Keywords: UDP-glucose dehydrogenase, Thiohemiacetal and thioester intermediates, Catalytic mechanism, Deuterium incorporation, UDP-gluco-hexodialdose
Abstract
Human UDP-glucose 6-dehydrogenase (hUGDH) catalyzes the biosynthetic oxidation of UDP-glucose into UDP-glucuronic acid. The catalytic reaction proceeds in two NAD+-dependent steps via covalent thiohemiacetal and thioester enzyme intermediates. Formation of the thiohemiacetal adduct occurs through attack of Cys276 on C-6 of the UDP-gluco-hexodialdose produced in the first oxidation step. Because previous studies of the related enzyme from bovine liver had suggested loss of the C-5 hydrogen from UDP-gluco-hexodialdose due to keto-enol tautomerism, we examined incorporation of solvent deuterium into product(s) of UDP-glucose oxidation by hUGDH. We used wild-type enzyme and a slow-reacting Glu161→Gln mutant that accumulates the thioester adduct at steady state. In situ proton NMR measurements showed that UDP-glucuronic acid was the sole detectable product of both enzymatic transformations. The product contained no deuterium at C-5 within the detection limit (⩽2%). The results are consistent with the proposed mechanistic idea for hUGDH that incipient UDP-gluco-hexodialdose is immediately trapped by thiohemiacetal adduct formation.
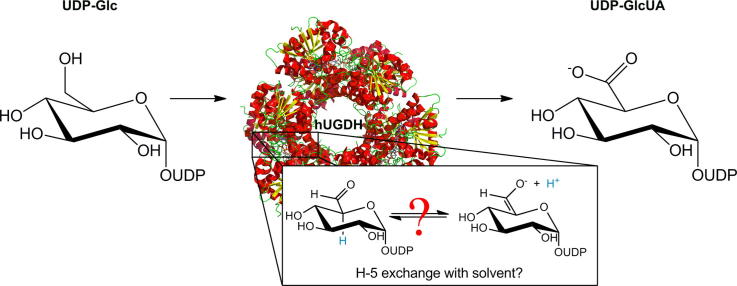
UDP-glucuronic acid (UDP-GlcUA) is an important metabolite of cellular physiology. It also serves a role in tissue development, because biosynthesis of extracellular matrix glycosaminoglycans in vertebrates requires UDP-GlcUA as precursor.1 UDP-GlcUA is derived from UDP-glucose (UDP-Glc) by two-step NAD+-dependent oxidation of the C-6 alcohol, catalyzed by UDP-glucose 6-dehydrogenase (UGDH).2 Genes encoding UGDH are present throughout all domains of life. Analysis of UGDH amino acid sequences reveals that enzymes from different lineages clearly share common ancestry.2 Basic elements of structure and function are therefore conserved among the different UGDHs.3,4 All known UGDHs fold into oligomers.2 The homodimeric quaternary structure adopted by bacterial enzymes appears to be the basic unit of UGDH enzyme function.3,5,6 Enzymes from eukaryotic origin form more complex ring-shaped homohexameric assemblies.4,7–10 The role of the particular structural architecture of UGDH from higher organisms is not entirely clear, but it could confer certain features of allosteric regulation.7,8 Residues of the active site are highly conserved among the different UGDHs, with only few exceptions.2 The catalytic mechanism of UGDH therefore seems to be highly conserved. Enzymatic oxidation of UDP-Glc occurs in three catalytic steps via covalent thiohemiacetal and thioester intermediates, as shown in Scheme 1 and Fig. 1.3,4,10–16 The proposed basic mechanism of UGDH is well supported by evidence from crystal structures and biochemical data for the wild-type enzyme and relevant mutants thereof.
Scheme 1.
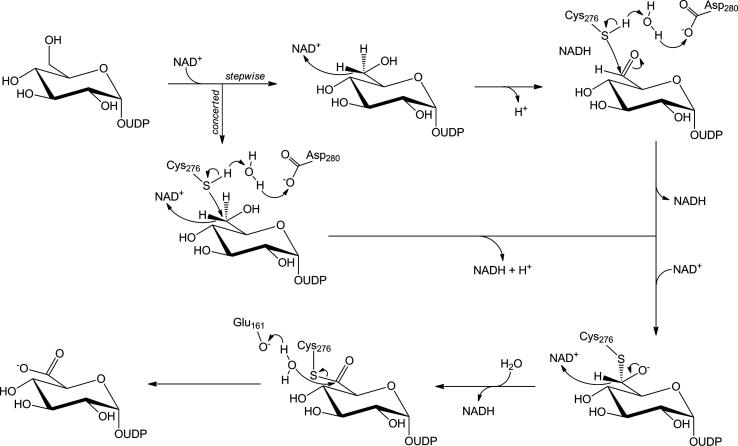
Proposed course of the catalytic reaction of UGDH. For clarity reasons, only the herein relevant amino acids of the active site of hUGDH are shown. The concerted mechanism would be inconsistent with exchange of the proton at glucosyl C-5 with solvent. The stepwise mechanism might allow proton exchange due to accumulation of enzyme-bound UDP-gluco-hexodialdose. Unless UDP-gluco-hexodialdose was released from the active site (which probably does not happen as shown later), exchange reaction would have to take place at the active site of the enzyme.
Figure 1.
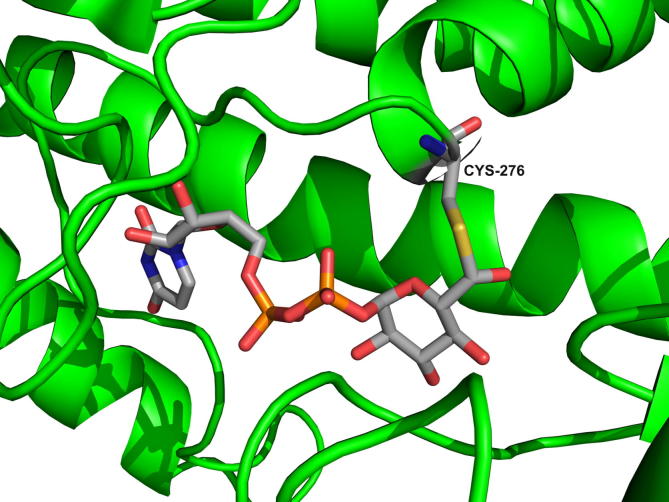
Close-up representation of the active site of E161Q in a trapped thiohemiacetal enzyme intermediate. Cys276 is the site of covalent modification. The PDB code of the structure is 3KHU.
Mechanistic proposals for UGDH differ in an important detail that is the kinetic significance of the enzyme-bound product of the first oxidation step, namely UDP-gluco-hexodialdose.12,13 Studies of UGDH from different sources concur that not even traces of UDP-gluco-hexodialdose are released from the enzyme during the reaction. One possibility considered was that the incipient UDP-gluco-hexodialdose is immediately trapped at the active site as a covalent thiohemiacetal adduct (Scheme 1).4 Hydride transfer oxidation by NAD+ and nucleophilic addition of the thiolate side chain of cysteine to the reactive C-6 of substrate would therefore take place in a kinetically coupled fashion, effectively reducing the lifetime of any UDP-gluco-hexodialdose formed in the reaction. We refer to this mechanism as concerted throughout the paper. The mechanistic idea is supported by kinetic data for human UGDH (hUGDH) from this laboratory, demonstrating that enzyme deprotonation, presumably at the active-site cysteine (Cys276), occurs kinetically in parallel with the initial substrate oxidation.4 Moreover, total disruption of covalent catalysis by Cys276 in a Cys→Ala mutant resulted in the massive impairment of already the first oxidation step.4,10 If the cysteine was required only in the second oxidation step, one would expect that a Cys→Ala mutant accumulates a detectable amount of NADH in a pre-steady burst, which results due to the relatively fast conversion of UDP-Glc to UDP-gluco-hexodialdose. Even though alcohol oxidation by NAD+ proceeds thermodynamically uphill, the non-favorable position of the reaction equilibrium would not prevent the formation of the aldehyde product as long as NAD+ and UDP-Glc are present in excess. Note that aldehyde reductases readily oxidize primary alcohols by NAD+ or NADP+.17 It is also relevant that trapping of the UDP-gluco-hexodialdose by chemical reduction with NaBH4 proved fruitless unless the active-site cysteine (of bovine liver UGDH) had been inactivated by chemical modification with cyanide.14
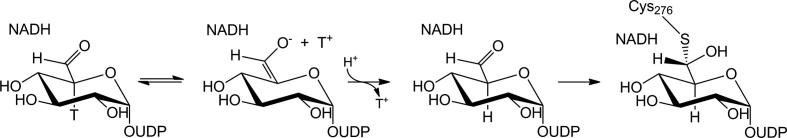
The alternative possibility is that substrate aldehyde and covalent thiohemiacetal adduct are formed in two sequential kinetic steps (stepwise mechanism). Release of UDP-gluco-hexodialdose from the enzyme complex with NADH may not be possible due to the (closed) protein conformation adopted at this point of the reaction.3,4 In support of this mechanistic view, chemically synthesized UDP-gluco-hexodialdose was a fairly good substrate for oxidation (with NAD+) or reduction (with NADH) by UGDH.12,13,15,18 Working with UGDH from the bacterium Streptococcus pyogenes, Tanner and co-workers showed that the wild-type enzyme, but also a mutated variant that had the key cysteine (Cys260) replaced by Ala, catalyzed oxidation and reduction of UDP-gluco-hexodialdose.12 These authors therefore concluded that the cysteine had no catalytic role in the first oxidation step.12 They also proposed that NADH was released from the enzyme complex with UDP-gluco-hexodialdose only after formation of the covalent thiohemiacetal adduct (Scheme 1). In their mechanistic studies of bovine liver UGDH, Feingold and colleagues observed that tritium label from C-5 of UDP-Glc was partly ‘washed out’ to solvent during the enzymatic reaction.19 Loss of label (30% of the original tritium) was explained by an enolization of the C-6 aldehyde in UDP-gluco-hexodialdose prior to covalent adduct formation (Scheme 2). No exchange of tritium was thought to occur in the thiohemiacetal and thioester intermediates. The proposed keto-enol tautomerism at the UGDH active site requires the C-6 aldehyde to become a rather long-lived intermediate of the enzymatic reaction and therefore, it would be clearly inconsistent with the idea of a strong kinetic coupling between substrate oxidation and thiohemiacetal formation. Utilization of UDP-gluco-hexodialdose as substrate for oxidation and reduction, by contrast, could be reconciled with the concerted mechanism, because the observed activities may not reflect exactly the steps that occur in the normal reaction pathway. Using UDP-galacto-hexodialdose (prepared from UDP-galactose by oxidation with galactose oxidase) as surrogate of UDP-gluco-hexodialdose, the authors demonstrated that the C-5 tritium readily exchanges with the solvent proton in a spontaneous (non-enzymatic) reaction.19 In contrast, chemically synthesized UDP-gluco-hexodialdose forms a hydrate upon contact with water, as shown by Campbell and Tanner.18 No enolization or C-5 proton exchange was observed in their study.
Scheme 2.
Enolization and proton exchange at C-5 of UDP-gluco-hexodialdose. If the aldehyde enolized prior to covalent intermediate formation, tritium label at C-5 could be replaced by hydrogen through label ‘wash-out’ to solvent. Similarly, deuterium could be incorporated (‘washed in’) if the reaction was performed in D2O.
Considering the mechanistic significance of the problem, and the partially contradictive results published so far, we decided to re-examine in this work the reported hydrogen exchange at C-5 during enzymatic oxidation of UDP-Glc. We took an approach distinct from that of Feingold and co-workers19 in that we measured incorporation of solvent deuterium by the UDP-GlcUA product. In situ proton NMR spectroscopy was employed to observe the enzymatic transformations in real time. We performed experiments with two forms of hUGDH, the wild-type enzyme and a point mutant that had Glu161 replaced by Gln (E161Q). Reason to compare the two enzymes was that each represented a distinct rate-determining step.4,10 Glu161 is the general catalytic base for hydrolysis of the thioester intermediate in the proposed mechanism of hUGDH (Scheme 1). The E161Q mutant is particularly impaired in the hydrolysis step and therefore accumulates the thioester adduct at steady state. The mutant is about 600 times less reactive in terms of turnover number (kcat) than wild-type hUGDH.10 In the wild-type enzyme at non-saturating concentration of NAD+ (0.5 mM), the thiohemiacetal intermediate accumulates at a steady state because the overall exchange of NADH by NAD+ becomes rate limiting.4 At saturating concentration of NAD+, none of the different catalytic steps in Scheme 1 appears to be distinctly rate determining in wild-type hUGDH. We considered that if hydrogen/deuterium exchange at C-5 of enzyme-bound UDP-gluco-hexodialdose took place at all in hUGDH, we should be able to accumulate sufficient amounts of the relevant intermediate in one of the two enzymes to make the exchange reaction eventually observable in the experiment. We performed in situ NMR experiments at pD 7.5 (wild type) and 8.3 (E161Q), because hUGDH is active and stable under these conditions. The previous work with bovine liver UGDH was done at a higher pH of 8.7.19 We therefore analyzed deuterium incorporation into the UDP-GlcUA product at different pD values in the range 5.9–8.8, using NMR measurement at a single time point of the reaction.
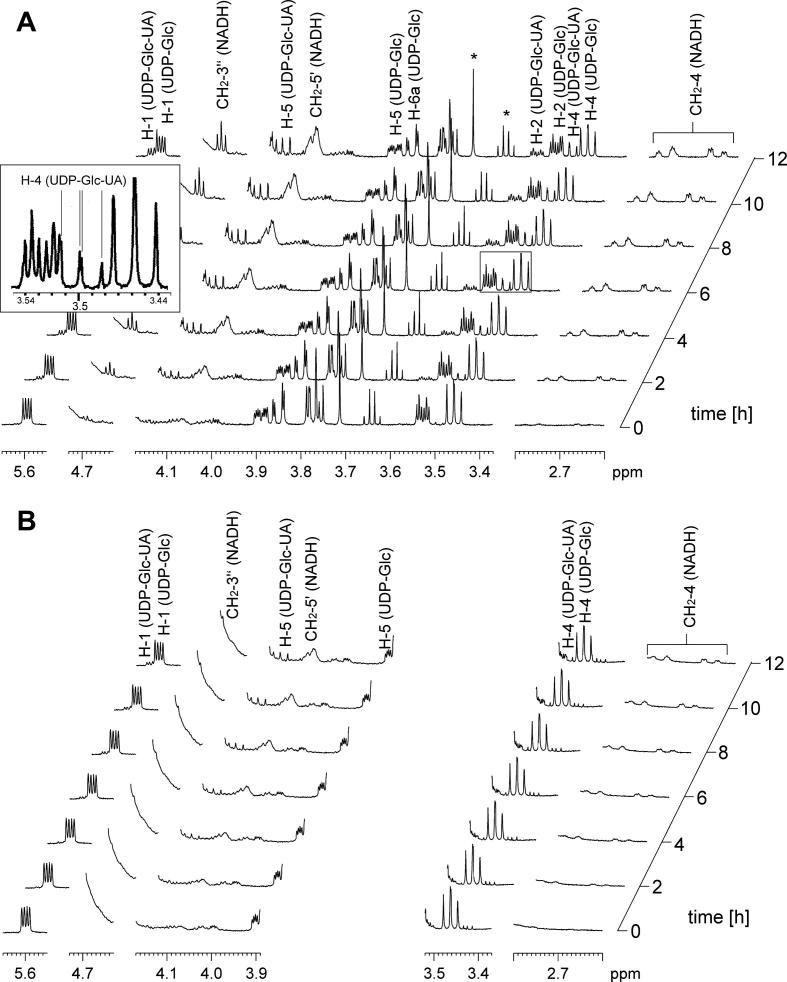
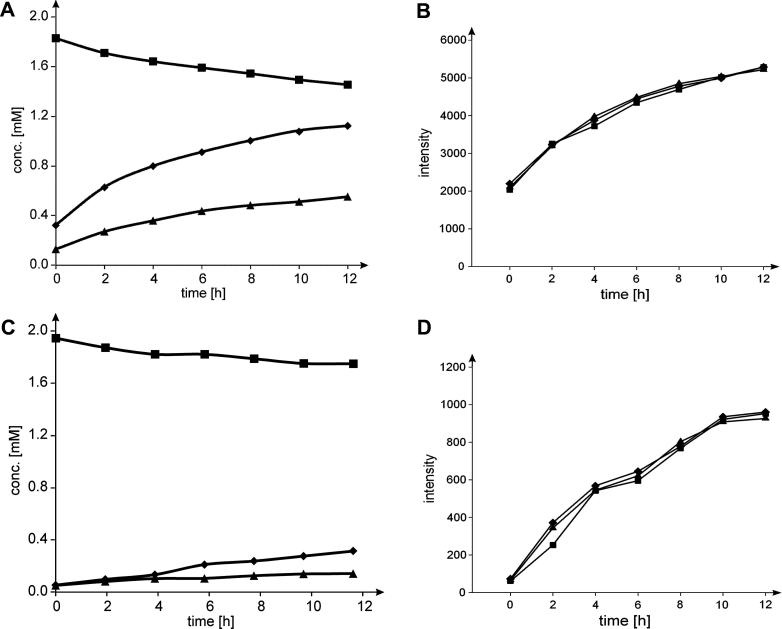
Figure 2 shows time-resolved proton NMR data recorded for oxidation of UDP-Glc by wild-type hUGDH (panel A) and E161Q (panel B). Results are presented in stack plots of seven selected spectra acquired over a reaction time of 12 h. Some selected signals from the UDP-Glc, UDP-GlcUA and NADH were assigned according to the literature and allow unambiguous identification of the reaction products. No intermediate UDP-gluco-hexodialdose was observed within detection limit, as expected. The presumed stoichiometry of the enzymatic transformation that two NADH and one UDP-GlcUA are produced for each UDP-Glc oxidized was confirmed in a quantitative analysis of the NMR data, as shown in Figure 3 (panels A and C). The rather low conversion of UDP-Glc observed in our experiments can be explained by a large solvent isotope effect on kcat for the respective enzyme used, resulting in a fourfold decreased reaction rate in D2O as compared to the corresponding reaction rate in water under the conditions applied. This large solvent isotope effect could deserve further attention in future mechanistic studies of hUGDH. The intrinsical low activity of E161Q combines with the isotope effect on kcat to give a very low overall activity of the mutant in the experiment. Substrate conversion did not exceed 20% after extended incubation times. However, it was sufficient to characterize the stoichiometry of the reaction and to determine the relevant proton signals in the UDP-GlcUA product. No UDP-gluco-hexodialdose was detectable in the reaction of E161Q.
Figure 2.
In situ NMR spectroscopic measurement of hUGDH catalyzed oxidation of UDP-Glc to UDP-GlcUA. The data are presented as stack plot of seven selected spectra in regular intervals of 2 h. Indicative signals of NADH, UDP-Glc, and UDP-Glc-UA are marked and assigned according to literature.24 Signals of impurities (TRIS from enzyme preparations, ethanol from commercial NAD+) are indicated with asterisks. No signals indicating presence of UDP-gluco-hexodialdose could be detected. Panels A and B show the reaction of wild-type hUGDH and E161Q, respectively. The spectral region from 3.89 ppm to 3.52 ppm is not shown in panel B due to presence of larger signals from impurities (glycerol). In panel A, the area of the spectrum showing the 1H NMR signal of proton H-4 in UDP-GlcUA is also presented enlarged in a box.
Figure 3.
Time courses of enzymatic oxidations of UDP-Glc, and determination of the associated change of relative H-5 signal intensity in UDP-GlcUA product. Time courses are shown in panels A (wild-type) and C (E161Q), the symbols used are square (UDP-Glc), triangle (UDP-GlcUA), and diamond (NADH). The low conversion can be explained by a large solvent isotope effect on kcat (see text). Two equivalents of NADH are formed per equivalent UDP-Glc utilized, as expected. Proton signal intensities in UDP-GlcUA are shown in panels B and D (square: H-1; triangle H-4; diamond: H-5) for reactions with wild-type enzyme and E161Q mutant, respectively. In case of deuterium incorporation at C-5, one would see a decrease of the respective proton signal compared to H-1 and H-4.
The 1H NMR signal of proton H-4 in resulting UDP-GlcUA appears at 3.50 ppm. Although it is slightly overlapped (Fig. 2A), its doublet of doublet structure is well detectable and the 3JH-H coupling constants can be determined to be 10.2 Hz and 9.2 Hz, respectively. Such quite large couplings indicate H-4 as well as both neighbored protons H-3 and H-5 to be in axial positions. The H-5 is hence not exchanged by deuterium. Furthermore, no additional signal of H-4 with one 3JH-H to H-3 and a 3JH-D to H-5 can be detected. The concomitant slight isotope shift would cause such signal to be in the spectral region of ±0.015 ppm around that of H-4 in the fully protonated UDP-GlcUA. This region is not overlapped by signals of further protons and hence allows a detection even of small amounts of byproducts. The absence of such signal can hence be used to exclude possible H/D exchange in an extent of more than 1%, which is the detection limit.
We analyzed the development of signal intensity of selected protons from UDP-GlcUA (H-1, H-4, H-5) in dependence of the incubation time. The results are summarized in Figure 3 for the reaction of wild-type UGDH (panel B) and E161Q (panel D). There was no loss of H-5 signal within error limit of 2%, which also indicates that exchange with the solvent had not occurred. Additionally, we tested the effect of lowering the concentration of NAD+ from 15 mM, which is completely saturating, to just 0.5 mM, which is limiting at the steady state, on H-5 signal evolution in product. It was shown in recent work that at 0.5 mM NAD+ the overall oxidation of thiohemiacetal intermediate becomes rate determining.4 We considered that under the conditions of limiting NAD+ the proton exchange reaction might therefore be favored. The reaction was performed for that reason directly in the NMR tube and NAD+ was supplemented in regular intervals to promote the conversion. NMR data showed that H-5 signal intensity was not affected as compared to reference proton signals.
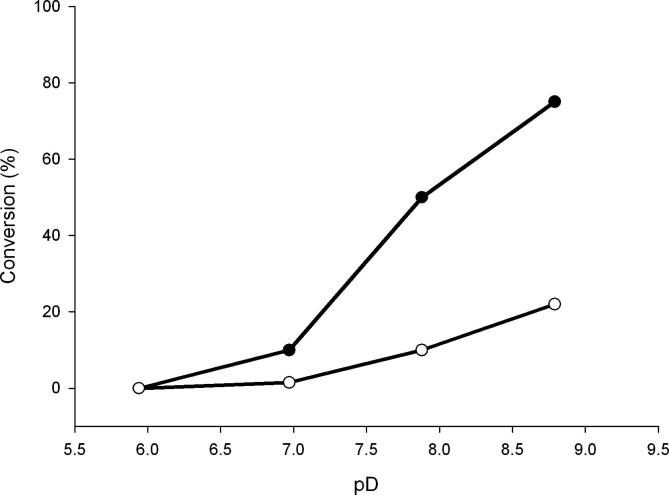
We also examined the effect of pH on the protonation state of C-5 in UDP-GlcUA. Reactions were performed in D2O at pD 5.9, 7.0, 7.9, and 8.8. Conversion of UDP-Glc substrate increased in response to a raise of pD, as one might expect from the known pH dependencies of kcat for wild-type enzyme and E161Q (Fig. 4). Both enzymes show optimum activity at pH 8 or higher.4,10 Under each of the conditions used, neither enzymatic transformation went along with a relative decrease in H-5 signal. We therefore conclude that incorporation of solvent deuterium at C-5 did not take place in the course of reactions catalyzed by wild-type enzyme and E161Q.
Figure 4.
pH dependence of conversion of UDP-Glc by wild-type hUGDH and E161Q mutant. The symbols are filled circle (wild-type enzyme) and empty circle (mutant). The reaction time was 16 h at 25 °C.
In summary, evidence from this work does not support the mechanistic proposal for UGDH (Scheme 2) that the UDP-gluco-hexodialdose intermediate of the enzymatic reaction partly exchanges its proton at C-5 with bulk solvent due to keto-enol tautomerism. The original observation made with bovine liver UGDH that tritium label at C-5 of UDP-Glc was partly washed out during reaction19 was re-examined using an alternative, equally diagnostic approach in which deuterium uptake from solvent was measured. Instead of the bovine liver enzyme, the structurally and mechanistically well-characterized hUGDH, both in wild-type and Glu161→Gln mutated form, was used. There was no deuterium incorporation, measured as relative H-5 signal loss, under a range of conditions (pH, NAD+ concentration) that were chosen to involve change in rate-determining step of the enzymatic reaction. Our observations are therefore consistent with the idea of a concerted mechanism for the human enzyme (Scheme 1) that precludes proton exchange with solvent. They are however also consistent with a sequential mechanism in which the intermediary UDP-gluco-hexodialdose is sequestered at the binding site of the enzyme so that exchange with bulk solvent is prevented effectively. We note however that protons are released from the active site of hUGDH in the course of the first oxidation step, suggesting that there exists probably connection between the catalytic center and solvent during the reaction.4 We also note that chemically, hydration of C-6 aldehyde might be a more likely outcome of contact of UDP-gluco-hexodialdose with water than fast enolization at around neutral pH. It is difficult to envision mechanistic differences between hUGDH and the enzyme from bovine liver that would reconcile the conflicting findings from deuterium incorporation and tritium ‘wash out’ experiments. Accumulation of UDP-gluco-hexodialdose as enzyme-bound intermediate in bovine liver UGDH is a possibility. This would be not consistent however with the proposed kinetic mechanism of the enzyme having thioester hydrolysis as the rate determining step.20
1. Experimental
1.1. Materials
UDP-Glc (sodium salt) was purchased at Carbosynth. UDP-GlcUA (ammonium salt; >98% purity), and NAD+ (sodium salt; 98% purity) were obtained from Sigma–Aldrich.
1.2. Enzymes
Purified preparations of wild-type hUGDH and E161Q mutant were obtained using reported procedures.4,10 Briefly, overexpression of the coding genes was done in Escherichia coli BL21(DE3)-R3. Both enzymes were produced as fusion proteins containing an N-terminal extension, which comprised a solubility enhancement tag, a streptavidin tag, and a tobacco etch virus protease cleavage site. Cell extracts were first purified by affinity chromatography. The N-terminal extension was then removed, and the enzymes were further purified by gel filtration and anion-exchange chromatography. Full details of the purification protocol are given elsewhere.4,10
1.3. Assays
Enzymatic assays were performed in 1 mL of D2O. A 50 mM potassium phosphate buffer at pD 5.9, 7.0, 7.9 or 8.8 was used. The pD was determined as meter reading +0.4. Starting concentrations were 15 mM NAD+, 2 mM UDP-Glc and 0.38 μM wild-type hUGDH or 15 μM E161Q. After addition of all components, the reactions were incubated at 25 °C for 16 h.
For determination of the solvent isotope effect, enzymatic conversions in H2O or D2O were performed in a Beckman Coulter DU800 spectrophotometer using a 50 mM potassium phosphate buffer with pH/pD 7.5. Reaction velocity was measured by NADH absorption (λ = 340 nm).
1.4. In situ proton NMR spectroscopy
All spectra were recorded on a Bruker DRX-600 AVANCE spectrometer (Bruker, Rheinstetten, Germany) at 600.13 MHz (1H). The 1H NMR spectra were measured at 298.2 K with presaturation (1.0 s) and acquisition of 32k data points. After zero filling to 64k data points, spectra were performed with a range of 7200 Hz. Chemical shifts were referenced to external acetone (δH = 2.225 ppm). The reactions were directly made in a 5 mm high precision NMR sample tube (Promochem, Wesel, Germany) to measure in situ 1H NMR spectra. Samples contained 2 mM UDP-Glc, 15 mM NAD+, and 0.084 μM wild-type hUGDH or 33 μM E161Q, as well as 50 mM potassium phosphate buffer in D2O (0.70 mL, 99.9% D, pD 7.5 or 8.3).21,22 Reaction progress was monitored in situ over ca. 12 h in the magnet by recording up to 64 1H NMR spectra in regular intervals.23
1.5. Data analysis
The Topspin 3.0 software from Bruker was used for processing the NMR spectra after data acquisition. For quantitative analysis of the NMR data, a correction value was subtracted from the integral values of each proton to account for background noise.
Acknowledgement
Financial support from the Austrian Science Fund FWF (Project DK Molecular Enzymology W901-B05) is gratefully acknowledged.
References
- 1.Clarkin C.E., Allen S., Kuiper N.J., Wheeler B.T., Wheeler-Jones C.P., Pitsillides A.A. J. Cell. Physiol. 2011;226:749–761. doi: 10.1002/jcp.22393. [DOI] [PubMed] [Google Scholar]
- 2.Egger S., Chaikuad A., Kavanagh K.L., Oppermann U., Nidetzky B. Biochem. Soc. Trans. 2010;38:1378–1385. doi: 10.1042/BST0381378. [DOI] [PubMed] [Google Scholar]
- 3.Campbell R.E., Mosimann S.C., van De Rijn I., Tanner M.E., Strynadka N.C. Biochemistry. 2000;39:7012–7023. [PubMed] [Google Scholar]
- 4.Egger S., Chaikuad A., Kavanagh K.L., Oppermann U., Nidetzky B. J. Biol. Chem. 2011;286:23877–23887. doi: 10.1074/jbc.M111.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha J., Popescu A.O., Borges P., Mil-Homens D., Moreira L.M., Sa´-Correia I., Fialho A.M., Frazão C. J. Bacteriol. 2011;193:3978–3987. doi: 10.1128/JB.01076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y.Y., Ko T.P., Lin C.H., Chen W.H., Wang A.H. J. Struct. Biol. 2011;175:300–310. doi: 10.1016/j.jsb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Kadirvelraj R., Sennett N.C., Polizzi S.J., Weitzel S., Wood Z.A. Biochemistry. 2011;50:5780–5789. doi: 10.1021/bi2005637. [DOI] [PubMed] [Google Scholar]
- 8.Sennett N.C., Kadirvelraj R., Wood Z.A. Biochemistry. 2011;50:9651–9663. doi: 10.1021/bi201381e. [DOI] [PubMed] [Google Scholar]
- 9.Rajakannan V., Lee H.S., Chong S.H., Ryu H.B., Bae J.Y., Whang E.Y., Huh J.W., Cho S.W., Kang L.W., Choe H., Robinson R.C. PLoS One. 2011;6:e25226. doi: 10.1371/journal.pone.0025226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger S., Chaikuad A., Klimacek M., Kavanagh K.L., Oppermann U., Nidetzky B. J. Biol. Chem. 2012;287:2119–2129. doi: 10.1074/jbc.M111.313015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge X., Campbell R.E., van de Rijn I., Tanner M.E. J. Am. Chem. Soc. 1998;120:6613–6614. [Google Scholar]
- 12.Ge X., Penney L.C., van de Rijn I., Tanner M.E. Eur. J. Biochem. 2004;271:14–22. doi: 10.1046/j.1432-1033.2003.03876.x. [DOI] [PubMed] [Google Scholar]
- 13.Ridley W.P., Houchins J.P., Kirkwood S. J. Biol. Chem. 1975;250:8761–8767. [PubMed] [Google Scholar]
- 14.Ordman A.B., Kirkwood S. J. Biol. Chem. 1977;252:1320–1326. [PubMed] [Google Scholar]
- 15.Nelsestuen G.L., Kirkwood S. J. Biol. Chem. 1971;246:3824–3834. [PubMed] [Google Scholar]
- 16.Schiller J.G., Bowser A.M., Feingold D.S. Carbohydr. Res. 1972;21:249–253. doi: 10.1016/s0008-6215(00)82151-5. [DOI] [PubMed] [Google Scholar]
- 17.Pival S.L., Klimacek M., Kratzer R., Nidetzky B. FEBS Lett. 2008;582:4095–4099. doi: 10.1016/j.febslet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Campbell R.E., Tanner M.E. Angew. Chem., Int. Ed. 1997;36:1520–1522. [Google Scholar]
- 19.Prihar H.S., Feingold D.S. FEBS Lett. 1979;99:106–108. doi: 10.1016/0014-5793(79)80259-8. [DOI] [PubMed] [Google Scholar]
- 20.Ordman A.B., Kirkwood S. Biochim. Biophys. Acta. 1977;481:25–32. doi: 10.1016/0005-2744(77)90133-4. [DOI] [PubMed] [Google Scholar]
- 21.Mussini P.R., Mussini T., Rondinini S. Pure Appl. Chem. 1997;69:1007–1014. [Google Scholar]
- 22.Luo D. Huaxue Shijie. 1985;26:24–26. [Google Scholar]
- 23.Brecker L., Ribbons D.W. Trends Biotechnol. 2000;18:197–202. doi: 10.1016/s0167-7799(00)01425-6. [DOI] [PubMed] [Google Scholar]
- 24.Gu X., Glushka J., Yin Y., Xu Y., Denny T., Smith J., Jiang Y., Bar-Peled M. J. Biol. Chem. 2010;295:9030–9040. doi: 10.1074/jbc.M109.066803. [DOI] [PMC free article] [PubMed] [Google Scholar]