Abstract
Background
Bacterial growth in soft tissue and open fractures is a known risk factor for tissue loss and complications in contaminated musculoskeletal wounds. Current care for battlefield casualties with soft tissue and musculoskeletal wounds includes tactical and strategic aeromedical evacuation (AE). This exposes patients to a hypobaric, hypoxic environment. In the present study, we sought to determine whether exposure to AE alters bacterial growth in contaminated complex musculoskeletal wounds and whether supplemental oxygen had any effect on wound infections during simulated AE.
Methods
A caprine model of a contaminated complex musculoskeletal wound was employed. Complex musculoskeletal wounds were created and inoculated with bioluminescent Pseudomonas aeruginosa. Goats were divided into three experimental groups: ground control, simulated aeromedical evacuation (AE), and simulated AE with supplemental oxygen (AE+O2). Simulated AE was induced in a hypobaric chamber pressurized to 8800 feet for 7 hours. Bacterial luminescence was measured using a photon counting camera at three timepoints: preflight (20 hours post surgery), postflight (7 hours from preflight and 27 hours post-surgery), and necropsy (24 hours from preflight and 44 hours post surgery).
Results
There was a significant increase in bacterial growth in the AE group compared to the ground control group measured postflight and at necropsy. Simulated AE induced hypoxia with oxygen saturation less than 93%. Supplemental oxygen corrected the hypoxia and significantly reduced bacterial growth in wounds at necropsy.
Conclusions
Hypoxia induced during simulated AE enhances bacterial growth in complex musculoskeletal wounds which can be prevented with the application of supplemental oxygen to the host.
Keywords: altitude, infection, transport, casualty care
Introduction
Air transportation of injured patients has been a component of casualty care for nearly a century.1 Over the past two decades, aeromedical evacuation (AE) has evolved from relatively simple movement of injured personnel to a complex and efficient system. The current system emphasizes the movement of stabilized (but not necessarily stable) patients over great distances.2 The aircraft environment during AE is notable for imposing hypobaric conditions with a typical cabin altitude up to 8800 feet, which results in a hypoxic environment.3 In commercial flights, previous studies have reported decreased oxygen saturations to levels as low as 80% in both passengers and air crew members.4,5
Many military patients who are transported via the AE system have suffered soft tissue wounds. Due to advances in protective equipment and earlier intervention, fatalities from initial wounding have decreased, but the incidence of extremity injury has increased.6 Complex musculoskeletal wounds (CMW) are the largest category of injuries sustained in combat composing approximately 50% of the injuries incurred during the Global War on Terror.7 These wounds can be difficult to treat, with complications that include infection, ischemia, and tissue loss.
While the benefits of rapid casualty evacuation to higher levels of care are clear, the effect of the hypobaric, hypoxic environment of AE on patients with complex musculoskeletal wounds is unclear. While incompletely understood, it is clear that oxygen tension plays a vital role in wound healing and inflammation.8-12 We hypothesized that the hypobaric, hypoxic environment encountered during AE alters bacterial growth in complex musculoskeletal wounds. We also hypothesized that this effect may be attenuated with the administration of supplemental oxygen.
Materials and Methods
Bacteria
Pseudomonas aeruginosa (ATCC 27317) genetically engineered with a luciferaseluciferin construct luxCDABEPhotorhabdusluminescens (United States Army Institute of Surgical Research), was used for the inoculation and is referred to as Pseudomonas aeruginosa (lux). This bacterium has a stable, heritable luminescence. For each inoculation procedure, stock culture was grown for 18-20 hours, and a dilution of 108 Colony Forming Units (CFU)/ml was obtained, and confirmed by plate count with spread plate technique.
Experimental Animals
All procedures were performed following approval of the protocol by the AFRL 711 HPW/RHDR Institutional Animal Care Use Committee at Brooks City Base, Texas. Fifteen castrated male goats (Capra hircus) (Talley Ranch, Uvalde, Texas) divided into three groups of five subjects each were fasted for 24 hours prior to surgery, and water was withheld for 12 hours prior to surgery. General anesthesia was induced using Ketamine (5.0 – 10.0 mg/kg) IM, then maintained using inhaled isoflurane. An arterial line was placed in the right hind leg in the anterior tibial artery by cutdown.
A complex musculoskeletal injury wound model adapted from that in use at the United States Army Institute of Surgical Research13,14 was utilized to create a contaminated complex extremity wound. Briefly, both hind legs were shaved circumferentially, prepped, and draped. A 5×4 cm area of skin and fascia was removed 1 cm distal from the tibial tubercle, exposing the flexor muscles. A 4×2 cm portion of the flexor muscles along the medial aspect was removed using electrocautery to create a soft tissue defect. Crush injury was induced by clamping the exposed muscle with two Kelly clamps for 1 minute. A 1 cm diameter defect was created in the periosteum along the medial aspect of the tibia, following which a 3mm sterile drill was used to create a cortical injury in the bone at this location, and 1 ml of 108 CFU/ml Pseudomonas aeruginosa (lux) solution was distributed throughout the wound. Then, a sterile swab was used to select a single colony from an agar plate, and swab throughout the wound bed. The wound was then dressed with a wet to dry dressing and self-adhering bandage.
Experimental Design
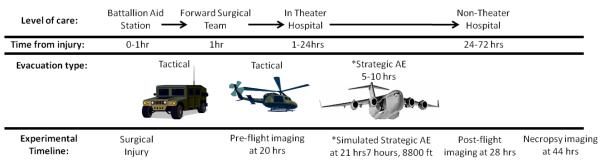
We attempted to mimic the current military AE timeline from initial injury to strategic evacuation from the theater to a non theater hospital (Fig 1). When injury occurs in theater, casualties are rapidly moved to either a battalion aid station (BAS) or to a forward surgical team (FST), usually reaching surgical care within one hour. Following assessment and stabilization at the FST, the patient is air evacuated to a Combat Support or Theater hospital. Within 24 hours following injury, casualties are evacuated from the in-theater hospital to a higher echelon care facility in Europe or the United States. This portion of evacuation typically takes place in a large transport plane. Cabin pressures in these platforms can be equivalent to 4000-8000ft above sea level with flight duration of 5-10 hrs. During strategic AE, any supplemental oxygen given must be either carried with the team or produced at altitude and thus consumes limited resources. Therefore, it is not standard practice to administer supplemental oxygen in the absence of a focused indication.
Figure 1.
Experimental timeline in relation to typical aeromedical evacuation (AE) timeline (*demonstrates strategic AE and simulated strategic AE relationship).
At 21 hours following surgery and inoculation, subjects underwent simulated AE. The first component of the study aimed to determine the effects of simulated AE on bacterial growth in complex musculoskeletal wounds. For this, subjects were divided into two groups; ground control (GD) and simulated AE. The second component of the study examined the effect of supplemental oxygen on bacterial growth in complex musculoskeletal during simulated AE. For this, subjects were divided into three groups; ground control (GD), simulated AE, and simulated AE with supplemental oxygen (AE O2). Simulated AE consisted of a 7 hour “flight” at an altitude of 8800ft using a hypobaric chamber (Brooks City Base, San Antonio, Texas operated by the 711 HPW, USAF). All subjects were placed in slings (Panepinto Inc., Fort Collins, Colorado) for comfort during flight. During transport and flight simulation, goats were awake and without anesthesia or mechanical ventilation. All groups or animals received identical anesthesia and analgesia during both the creation of the wound and during wound imaging. Animals were monitored for the duration of the flight using arterial blood gases and StO2 monitors (Inspectra, Hutchinson Technology, Hutchinson Minnesota, USA). The GD designated subjects underwent transport to the chamber and identical monitoring for a 7 hour period, but did not enter the hypobaric chamber.
Oxygen Supplementation and Monitoring
A 16 French red rubber catheter was placed intranasal prior to flight, and secured to all subject’s forehead to allow administration of supplemental oxygen during the simulated AE. Arterial blood gases were obtained initially at preflight, immediately postflight, and throughout the flight at one hour intervals. These were analyzed using an iSTATVetScan monitor (Abaxis). Supplemental oxygen was administered to the AE O2 subjects via nasal cannula, and was titrated to maintain an oxygen saturation of >93% per arterial blood gas in the AE O2 group.
Photon Imaging and Analysis
Images to assess bioluminescence of the wound bed were taken using a photon counting camera (PCC) (Charge Couple Device [CCD] Imaging System Model C2400; Hamamatsu Photonics, Hamamatsu-City, Japan) at three different time points: 20 hours post surgery, (preflight), 28 hours post-surgery (postflight) and 44 hours post surgery (necropsy). The images were analyzed using Hokawo software with Argus-20 interface software (Hamamatsu Photonics).
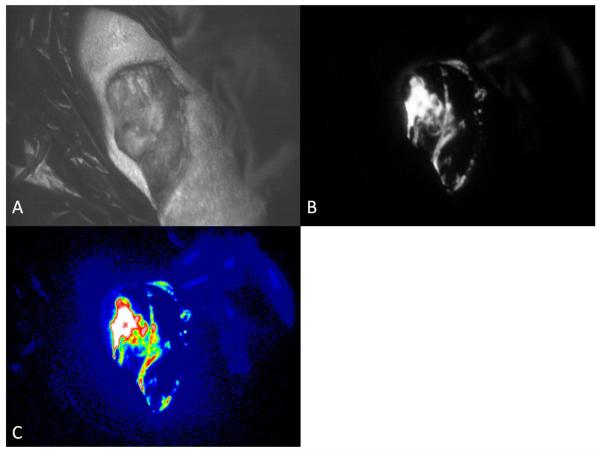
To obtain images, the subject was placed supine on the operating table within a darkroom. The wound was positioned 10cm from the camera lens with a black background around the injured hind extremity. The photon images were analyzed using the interface software. Relative luminescence of the wound bed was obtained as well as spectral images to show areas of highest concentration (Fig 2).
Figure 2.
Images of wound taken using photon counting camera.(A)Brightfield, black and white image for orientation in wound bed. (B) Photon image. Bright areas represent areas of photon emission. (C) Spectral enhancement of photon image. White and red are areas of high concentration, blue and black represent the lowest concentration.
Tissue Oxygenation
Tissue oxygenation (StO2) was measured using a Near Infrared Spectroscopy (NIRS) monitor (Inspectra Model 650, Hutchinson Technology, Hutchinson Minnesota, USA). The monitor sensor was sewn in place using 3-0 silk just lateral to the wound bed following preflight imaging, and was removed prior to post-flight imaging. Baseline readings were obtained prior to flight, directly post-flight, and at one hour intervals during flight.
Data Analysis
Raw data were collected as photon counts from the charge coupled camera, then analyzed with each goat’s photon counts divided by the preflight counts to give a relative change in counts from preflight values. Oxygen saturation and PaO2 were collected from the arterial blood gas analysis. StO2 was collected from the NIRS monitor.
Statistical analysis was accomplished using Sigma Plot 11.0 (Systat Software). Comparison of groups without repeated measures was accomplished using a Mann-Whitney rank sum test. Multiple measures were compared using a one way analysis of variance (ANOVA) with a Student-Newman-Keuls Method for multiple comparisons. Values of p<0.05 were deemed significant.
Results
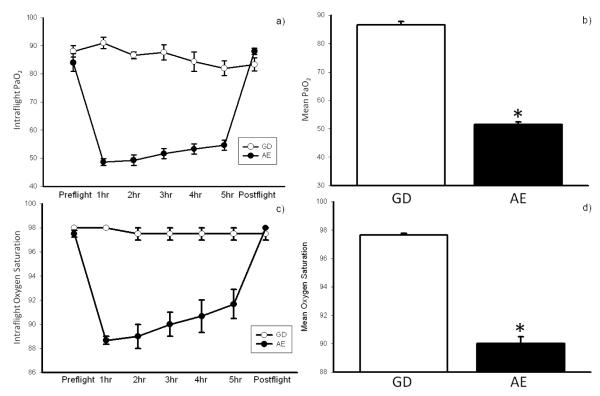
During simulated AE, exposure to the environment was associated with hypoxia as demonstrated by both oxygen saturation and PaO2 values in our subjects (Figure 3). Both oxygen saturation and PaO2 levels decreased shortly after exposure to the hypobaric environment, and returned to baseline immediately following flight. This was consistent with mild hypoxia.
Figure 3.
Mean PO2 (a, b) and oxygen saturation (c, d) in ground control (GD)vs aeromedical evacuation (AE). Oxygenation levels decreased significantly during flight and returned to baseline immediately following flight for the AE group (*p<0.05 compared to GD group).
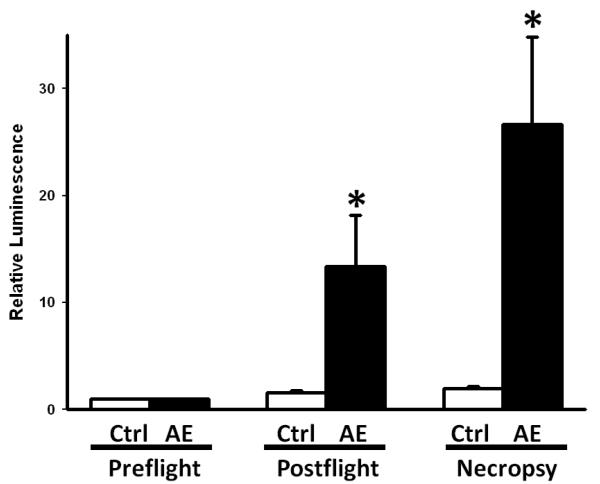
When we examined relative bacterial luminescence in wounds throughout the course of simulated AE, we found a gradual increase in bacterial luminescence, consistent with increased bacterial contamination of the musculoskeletal wound (Figure 4). Relative luminescence was significantly increased in the AE group when compared with GD goats at the post-flight and necropsy time points (Figure 4). This indicates increased bacterial growth within the wound bed when exposed to the hypobaric and hypoxic environment of AE.
Figure 4.
Relative luminescence of the wound bed in ground control (GD) vs aeromedical evacuation (AE). AE luminescence was significantly different than GD in both Postflight and Necropsy groups (p<0.05 compared to GD group).
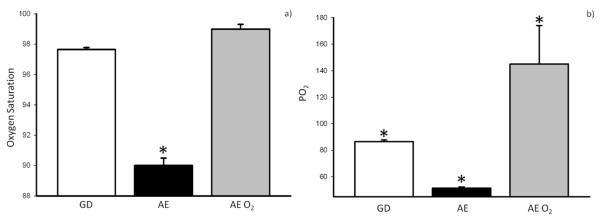
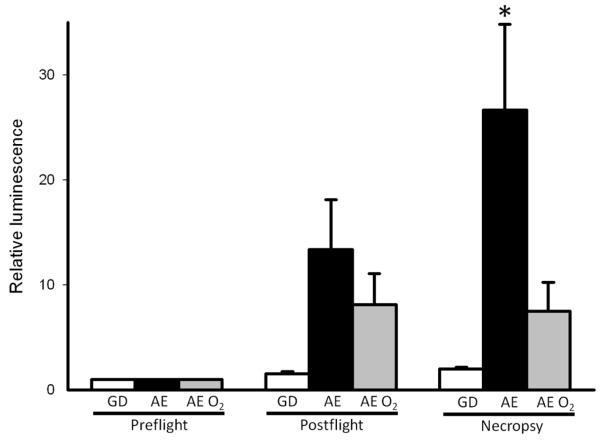
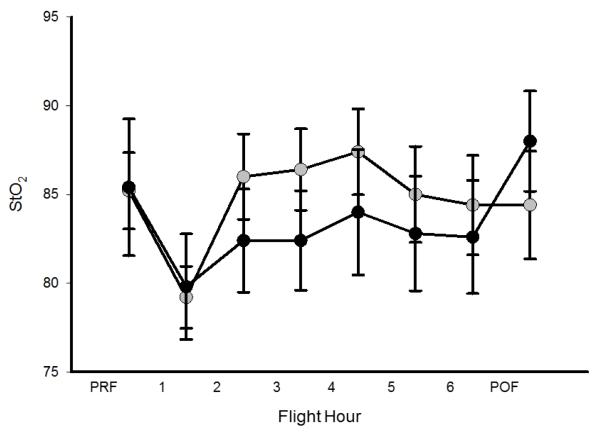
Goats that received supplemental oxygen via nasal cannula (AE O2) had increased oxygen saturation and arterial PaO2 as compared to equivalent goats undergoing simulated AE without oxygen supplementation (Figure 5). When we examined the effect of supplemental oxygen therapy on bacterial luminescence values, we found that the addition of supplemental oxygen attenuated the increased bacterial growth previously seen in the AE group when compared to GD as demonstrated by relative luminescence (Figure 6). We also examined tissue O2 levels under these conditions. These data suggest that even mild elevations in altitude were associated with a trend toward decreasing StO2 levels (Figure 7). This relative decrease in tissue oxygenation was corrected with the addition of supplemental oxygen (Figure 7).
Figure 5.
Mean intra-flight oxygen saturation (a) and PO2 (b) comparing ground control (GD), aeromedical evacuation (AE), and AE + O2 (AE O2). Saturations and PO2 increased significantly in simulated AE with addition of supplemental O2(*p < 0.001 compared to GD and AE O2 groups).
Figure 6.
Relative luminescence of wound bed in ground control (GD), aeromedical evacuation (AE), and AE + O2 (AE O2)..in Preflight, Postflight, and Necropsy groups respectively. Necropsy AE luminescence was significantly different than all other groups (p<0.05 compared to GD and AE O2 groups).
Figure 7.
Comparison of StO2 in aeromedical evacuation (AE; black symbols) and AE + O2 (AE O2; grey symbols) groups. StO2 appears to decrease with simulated AE, and is increased in groups receiving supplemental O2. Preflight=PRF; post-flight=POF.
Discussion
Aeromedical evacuation has been used extensively in the current operations of the Global War on Terror. The impact of the hypobaric and hypoxic environment of AE on patient outcomes with complex musculoskeletal wounds is not well understood. In the present study, we established a model for the evaluation of bacterial growth in complex musculoskeletal wounds exposed to AE. This was accomplished by adapting an accepted wound model which assessed the change in bacterial luminescence over time in a complex musculoskeletal wound.13,14 Increases in luminescence correlated to an increase in the bacterial load of the wound bed. This has previously been shown to be a useful tool in the evaluation of extremity injuries. Simulation of the hypobaric and hypoxic environment of AE was achieved utilizing a hypobaric chamber and parameters consistent with typical maximum cabin pressures.15 A simple intervention was then instituted in the form of supplemental oxygen to determine its effect on the noted increase in luminescence.
Recent military conflicts have resulted in a large burden of complex musculoskeletal wounds.7 This is attributed to advances in body armor and improved survival in the injured population due to a focus on early interventions, including battlefield control of hemorrhage and improved resuscitation. Excellent wound care is essential to good outcomes in extremity injuries, as complications can affect aspects of patient function such as limb salvage, and long term function.16,17 A few previous studies have examined the effect of mild altitude changes on pulmonary and myocardial function with varying conclusions on the importance of oxygen monitoring in patients with chronic conditions.18-20 While important to the overall management of patients during AE, the applicability of these findings to wound management is difficult to assess. To date, there has not been an analysis of hypobaric hypoxia in air travel and its consequences to complex wound outcomes. Our experiments directly examine the effect of hypobaric hypoxia on bacterial growth in complex musculoskeletal wounds and thus have potential implications for medical and wound care during AE.
Goats exposed to the hypobaric hypoxic environment of simulated AE demonstrated decreased oxygenation as measured by arterial blood gases when compared with animals that did not undergo simulated AE. Those same hypoxic subjects experienced increased bacterial growth as measured by wound luminescence when compared with the GD group. Although there are several factors that contribute to the growth of bacteria in wounds, including temperature and glucose levels, our experiments were designed to evaluate the effect of hypobaric hypoxia on bacteria levels in complex wounds. Although the increase in bacterial growth appears to be attributed to simulated AE, it is possible that glucose levels and temperature differed between subjects and represent significant contributing factors to increased bacterial growth. We feel that this is unlikely given the identical treatment that each group received and our direct comparison between AE groups and animals given supplemental oxygen. In this experiment, correction of hypoxia with supplemental oxygen significantly decreased bacterial growth as compared to subjects who underwent simulated AE. These findings strongly suggest that changes in altitude-induced hypoxia correlate with an increase in bacterial growth in a complex musculoskeletal wound. We also found that an increase in bacterial growth during simulated AE is abrogated with the administration of supplemental oxygen.
There are limitations to our study and our findings should be interpreted with caution. In the current study, wet to dry dressings were employed as representative of standard wound care. A recent clinical study describes the initial CCATT experience with negative pressure wound therapy during strategic AE and suggests that this technique may be useful in the care of complex musculoskeletal wounds under these conditions21. The use of alternative wound care techniques, such as negative pressure wound therapy, could potential alter the applicability of our findings. In addition, we employed a photon camera in order to determine the amount of bacteria present in the wounds. Together, the photon counting camera and accompanying interface software quantitation of all photons emitted from within the field of view and have previously been validated by comparison with CFU13. Although we feel that CFU methodology is a valid technique for evaluation of wounds with a homogenous distribution of bacteria, this technique may be inadequate for complex musculoskeletal injuries due to potential sampling errors. Under these conditions, we felt that it was more appropriate to utilize the photon counting technique in order to provide a more complete view of bacterial distribution in the wound and avoid the potential for sampling error. In addition, we noted increased oxygen saturation over time during exposure to the simulated AE environment (Figure 3). This is an interesting finding and suspect that this represents adaptation to acute hypoxic exposure, likely through hyperventilation22.
In the present study, we utilized tissue oxygen monitoring in order to attempt to define the effect of simulated AE on tissue oxygen saturation. Our results suggest that there is a trend toward decreased tissue oxygenation during hypobaric exposure, but this did not reach statistical significance. Tissue oxygen levels have been shown to be critical to wound healing and the pathogenesis of wound infection (reviewed in 23). In the present study, tissue oxygen levels remained above those that have previously been suggested as critical in order to avoid wound infections23, yet our data was consistent with increased bacterial wound growth. The discordance between our findings and this data is unclear. One possible explanation is that our placement of the StO2 monitoring device, which was adjacent to, rather than directly in the wound, led to values that underestimated the degree of tissue hypoxia occurring in the components of the wound.
Our findings have potentially important clinical implications. First, mild changes in altitude induced mild hypoxia. This has been shown previously to occur in other commercial flight settings, but has not previously been studied in the AE environment.4,5 Additionally, those subjects that experienced hypoxia also demonstrated increased bacterial growth in the wound bed, suggesting that the hypoxic, hypobaric environment of AE may complicate wound care and outcomes. When the hypoxia was corrected, increased wound bed bacterial growth was mitigated. Although our data are not conclusive as to an absolute threshold value of PaO2 or SaO2 in order to decrease bacterial growth in wounds we feel that hypoxia should be avoided under these conditions. Flight cabin pressures differ based on many factors, and the level of hypoxia will differ based on the cabin pressure. It could be assumed that the level of hypoxia will also vary based on the severity and character of injury; however, currently the importance of these variables is unknown. In our model, effective intervention appears to be normalization of the hypoxic state. In summary, surveillance of casualty oxygen saturation with pulse oximetry followed by correction of hypoxia with supplemental oxygen as needed may decrease wound complications related to infection in complex musculoskeletal wounds.
Acknowledgments
Support: This work was supported by grants from the United States Air Force (FA7014-09-2-0005) and National Institutes of Health (GM08478)
Footnotes
The views of the authors are their own and do not reflect the views of the United States Air Force or the Department of Defense.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beninati W, Meyer MT, Carter TE. The critical care air transport program. Crit Care Med. 2008;36:S370–6. doi: 10.1097/CCM.0b013e31817e3143. [DOI] [PubMed] [Google Scholar]
- 2.Johannigman JA. Maintaining the continuum of en route care. Crit Care Med. 2008;36:S377–82. doi: 10.1097/CCM.0b013e31817e31e1. [DOI] [PubMed] [Google Scholar]
- 3.Hackett P, Roach RC. High Altitude Medicine. In: Auerbach P, editor. Wilderness Medicine. 5th ed Mosby Elsevier; Philadelphia, PA: 2007. pp. 1–36. [Google Scholar]
- 4.Geertsema C, Williams AB, Dzendrowskyj P, Hanna C. Effect of commercial airline travel on oxygen saturation in athletes. Br J Sports Med. 2008;42:577–81. doi: 10.1136/bjsm.2007.042960. [DOI] [PubMed] [Google Scholar]
- 5.Lee AP, Yamamoto LG, Relles NL. Commercial airline travel decreases oxygen saturation in children. Pediatr Emerg Care. 2002;18:78–80. doi: 10.1097/00006565-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Lin DL, Kirk KL, Murphy KP, McHale KA, Doukas WC. Orthopedic injuries during Operation Enduring Freedom. Mil Med. 2004;169:807–9. doi: 10.7205/milmed.169.10.807. [DOI] [PubMed] [Google Scholar]
- 7.Owens BD, Kragh JF, Jr., Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64:295–9. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 8.Greif R, Akca O, Horn EP, Kurz A, Sessler DI, Outcomes Research Group Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–7. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 9.Ives CL, Harrison DK, Stansby GS. Tissue oxygen saturation, measured by near-infrared spectroscopy, and its relationship to surgical-site infections. Br J Surg. 2007;94:87–91. doi: 10.1002/bjs.5533. [DOI] [PubMed] [Google Scholar]
- 10.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 11.Kuhne HH, Ullmann U, Kuhne FW. New aspects on the pathophysiology of wound infection and wound healing--the problem of lowered oxygen pressure in the tissue. Infection. 1985;13:52–6. doi: 10.1007/BF01660413. [DOI] [PubMed] [Google Scholar]
- 12.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svoboda SJ, Bice TG, Gooden HA, Brooks DE, Thomas DB, Wenke JC. Comparison of bulb syringe and pulsed lavage irrigation with use of a bioluminescent musculoskeletal wound model. J Bone Joint Surg Am. 2006;88:2167–74. doi: 10.2106/JBJS.E.00248. [DOI] [PubMed] [Google Scholar]
- 14.Svoboda SJ, Owens BD, Gooden HA, Melvin ML, Baer DG, Wenke JC. Irrigation with potable water versus normal saline in a contaminated musculoskeletal wound model. J Trauma. 2008;64:1357–9. doi: 10.1097/TA.0b013e31816e3476. [DOI] [PubMed] [Google Scholar]
- 15.Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med. 2008;79:433–9. doi: 10.3357/asem.2272.2008. [DOI] [PubMed] [Google Scholar]
- 16.French DD, Bair MJ, Bass E, Campbell RR, Siddharthan K. Central nervous system and musculoskeletal medication profile of a veteran cohort with blast-related injuries. J Rehabil Res Dev. 2009;46:463–8. doi: 10.1682/jrrd.2008.09.0017. [DOI] [PubMed] [Google Scholar]
- 17.Helgeson MD, Potter BK, Burns TC, Hayda RA, Gajewski DA. Risk factors for and results of late or delayed amputation following combat-related extremity injuries. Orthopedics. 2010;33:669. doi: 10.3928/01477447-20100722-02. [DOI] [PubMed] [Google Scholar]
- 18.Bendrick GA, Nicolas DK, Krause BA, Castillo CY. Inflight oxygen saturation decrements in aeromedical evacuation patients. Aviat Space Environ Med. 1995;66:40–4. [PubMed] [Google Scholar]
- 19.de Vries ST, Kleijn SA, van ’t Hof AW, et al. Impact of high altitude on echocardiographically determined cardiac morphology and function in patients with coronary artery disease and healthy controls. Eur J Echocardiogr. 11:446–50. doi: 10.1093/ejechocard/jep237. [DOI] [PubMed] [Google Scholar]
- 20.de Vries ST, Komdeur P, Aalbersberg S, van Enst GC, Breeman A, van ’t Hof AW. Effects of altitude on exercise level and heart rate in patients with coronary artery disease and healthy controls. Neth Heart J. 2010;18:118–21. doi: 10.1007/BF03091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang R, Dorlac WC, Flaherty SF, et al. Feasibility of negative pressure wound therapy during intercontinental aeromedical evacuation of combat casualties. J Trauma. 2010;69(Suppl 1):S140–5. doi: 10.1097/TA.0b013e3181e452a2. [DOI] [PubMed] [Google Scholar]
- 22.West JB. Respiration in unusual environments. In: West JB, editor. Physiological Basis of Medical Practice. 12th edition Williams and Wilkins; Philadelphia, PA: 1991. pp. 588–590. [Google Scholar]
- 23.Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004 Mar;28(3):312–5. doi: 10.1007/s00268-003-7398-5. [DOI] [PubMed] [Google Scholar]