Abstract
Background
In 2006, the U.S. Food and Drug Administration (FDA) recommended screening of all women with silicone gel breast implants with magnetic resonance imaging (MRI) three years after implantation and every two years thereafter to assess their integrity. The cost for these serial examinations over the lifetime of the breast implants is an added burden to insurance payers and to women. We perform an economic analysis to determine the most optimal screening strategies by considering the diagnostic accuracy of the screening tests, the costs of the tests and subsequent implant removal.
Methods
We determined aggregate/pooled values for sensitivity and specificity of the screening tests ultrasound (US) and MRI in detecting silicone breast implant ruptures from the data obtained from published literature. We compiled costs, based on Medicare reimbursements for 2011, for the following elements: imaging modalities, anesthesia and 3 surgical treatment options for detected ruptures. We used decision tree to compare three alternate screening strategies of US only, MRI only and US followed by MRI in asymptomatic and symptomatic women.
Results
The cost per rupture of screening and management of rupture with US in asymptomatic women was $1,090, whereas in symptomatic women it was $1,622. Similar cost for MRI in asymptomatic women was $2,067, whereas in symptomatic women it was $2,143. Similar cost for US followed by MRI in asymptomatic women was $637, whereas in symptomatic women it was $2,908.
Conclusion
Screening with US followed by MRI was optimal for asymptomatic women and screening with US was optimal for symptomatic women.
Keywords: Silicone breast implants, rupture, Ultrasound, MRI
Introduction
Approximately 3% of women (250,000 – 340,000) in United States get breast implants each year. The majority of implantations are done for augmentation purposes; few are done for reconstruction purposes. Breast augmentation was the top cosmetic surgical procedure performed from 2006–2010.1 Breast reconstruction rose to the top five reconstructive procedures in 2010.1 Among the nearly 300,000 breast augmentations and 93,000 breast reconstructions performed in 2010, 146,000(51%) and 54,450(59%) respectively were done with silicone implants.1 Like any medical device, silicone breast implants have a limited product life. This is especially important considering the young age of many implant recipients. Complications of breast implantation include pain, capsular contracture, and rupture. Rupture is defined as a disruption in the integrity of the implant ranging from focal rupture through pin sized holes to large, visible tears,2 and may result from trauma, deterioration of implant shell with time or manufacturing defect. The resulting leaked silicone gel may remain within the scar tissue capsule as an intra-capsular rupture or may move outside the capsule but remains in the breast tissue as an extra-capsular rupture.3
Silicone implants rupture incidence is estimated to be 8% in asymptomatic women4 and 33% in symptomatic women.5–7 What constituted as symptomatic is unclear, but the literature often defined symptomatic as women presented with symptoms such as pain, capsular contracture, and breast asymmetry. Asymptomatic patients were those who did not present with symptoms but were evaluated due to concerns with regards to the safety of their implants. Holmich et al. reported an incidence rate of 5.3 ruptures/100 silicone implants/ year (95% Confidence Interval:. 4.0–7.0).8 Rupture increases significantly with implant age; a rupture prevalence of 30% at implant age of 5 years, 50% at 10 years and 70% at 17 years has been reported.9 The median age of implant at rupture has been estimated to be 10.8 years (95% C.I. 8.4– 13.9).4,10 Knowledge about the consequences of ruptures is limited. In the late 1990s, Institute of Medicine, and others, reported that leaked silicone gel can cause local symptoms, such as, pain, capsular contracture and breast asymmetry but there was no evidence of systemic disease.11,12 Because symptoms of rupture, if present at all, are minimal, most ruptures are clinically silent or asymptomatic, making the diagnosis of a ruptured breast implant difficult.13 With only 30% sensitivity, physical examination alone is unreliable and difficult to detect a rupture.14
Mammography, ultrasound (US), computed tomography (CT) and MRI have been used for enhanced detection of implant rupture, with varying degrees of accuracy. Mammography is inexpensive, can easily detect free silicone within the breast parenchyma, and is useful in identifying extra-capsular ruptures.15 However, with less than 20% of ruptures being extra-capsular, there is a greater potential to miss the majority of ruptures. Additionally, radiation exposure and possible risk of rupture due to breast compression is a concern. CT is widely available and can detect intracapsular ruptures, but its ability to detect extra-capsular ruptures is limited. Furthermore, CT is costly and has radiation exposure, therefore it is seldom used.12,16 US does not use ionizing radiation, negating this concern. Additionally, it is widely available, relatively inexpensive, and acceptable for patients in whom MRI is contraindicated, due to body size, claustrophobia, or having pacemakers or implanted metal. Nevertheless, US is highly operator dependent and the success of recognizing an intracapsular rupture varies with the experience of the operator.16 MRI demonstrates the highest accuracy in detecting both intra and extra capsular ruptures and has no radiation exposure. But its high cost, limited availability and myriad of contraindications restrict its general usage. Furthermore, the accuracy of MRI varies with the type of coil used; a dedicated breast coil achieves greater accuracy than a body coil or a shoulder coil.16,17 Advances in imaging technology to detect implant rupture must be evaluated in the context of their diagnostic accuracy and the costs of applying these diagnostic tests based on Evidence-based Medicine principles. The current recommendations of using MRI as the sole diagnostic screening tool must be critically examined.
Economic modeling is a form of comparative analysis study that strives to measure the economic values of various interventions. Its application is timely for the current attention on caring for women with silicone breast implant when available screening tools are associated with varying accuracy and cost outcomes. We performed an economic analysis using the metric of cost per rupture detected and compared two commonly applied screening strategies of US and MRI, and a third strategy of US followed by MRI to inform plastic surgeons, policy makers and women as to the costs of detecting silicone implant ruptures.
Methods
Published studies on detecting silicone implant rupture using US and MRI were identified using PubMed. They were categorized based on the imaging technique used, and on sample characteristic such as symptomatic or asymptomatic women. We include only studies that separately evaluated symptomatic and asymptomatic women with silicone gel implants. (Tables 1 and 2).
Table 1.
Ultrasound Studies
| Studies in Symptomatic Women* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Number | Study | Year | # women | # implants | Implant age: mean, (range), y | Subject age: Mean, (range), y | Sample type | US sens % | US speci % | N Sensitivity (TP) | N Specificity (TN) |
| 1. | Debruhl et al26 | 1993 | 74 | 139 | NS | NS | Symptomatic | 70 | 92 | 14□ | 34□ |
| 2. | Caskey et al27 | 1994 | 119 | 221 | NS | NS | Symptomatic | 59 | 59 | 13 | 22 |
| 3. | Ahn et al28 | 1994 | 29 | 59 | 13 (6–19) | 46 (31–72) | Symptomatic | 70 | 92 | 15 | 33 |
| 4. | Reynolds etal29 | 1994 | 13 | 24 | 12 (7–22) | 48.9 (37–63) | Symptomatic | 54 | 64 | 7 | 7 |
| 5. | Everson et al30 | 1994 | 32 | 63 | NS | (29–63) | Symptomatic | 59 | 79 | 13 | 31 |
| 6. | Berg et al31 | 1995 | 282 | 535 | 11.9 (0.7–26) | 47 (29–71) | Symptomatic | 65 | 57 | 26 | 31 |
| 7. | Chung et al32 | 1996 | 98 | 192 | NS | NS | Symptomatic | 74 | 89 | 46 | 116 |
| 8. | Medot et al33 | 1997 | 65 | 122 | 3–25 | 46 (30–65) | Symptomatic | 41.2a | 70a | 7 | 14 |
| 68.7b | 73b | 22 | 39 | ||||||||
| 9. | Ikeda et al34 | 1999 | 30 | 59 | 12 (3–25) | 45 (30–11) | Symptomatic | 67 | 92 | 8 | 8 |
| Study in Asymptomatic Women ** | |||||||||||
| 1. | Scaranelo et al35 | 2004 | 44 | 83 | 11.9 | NS | Asymptomatic | 64 | 76.9 | 16 | 40 |
Symptomatic women were comprised of those who presented with symptoms such as pain, capsular contracture, and breast asymmetry. Often women also presented with other symptoms such as mass in breast, change in firmness and pain in the extremities.
Asymptomatic women were those who did not present with symptoms but were evaluated due to concerns with regards to the safety of their implants.
Number of true positives and true negatives do not add up to number of implants in studies because they come from implants that are only surgically evaluated and also there will be false positives and false negatives for the test.
Sensitivity and specificity with capsular contracture
Sensitivity and specificity without capsular contracture
#women – Number of women in study
#implants- Number of implants in study
TP- True Positives
TN- True Negatives
Y-Years
US- Ultrasound
N-sample size
NS- Not stated
Table 2.
MRI Studies
| Studies in Symptomatic Women* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Study | Year | Number of women | Number of implants | Implant age: mean, (range), y | Subject age, y | Sample type | M | N Sensitivity (TP) | N Specificity (TN) | ||
| RI sens % | MRI speci % | ||||||||||
| 1. | Berg et al36 | 1993 | 33 | 62 | 13.4 (1–22)a | NS | Symptomatic | 58 | 88% | 11□ | 28□ |
| 7.7 (0.5–24)b | |||||||||||
| 2. | Monticciolo et al37 | 1994 | 28 | 38 | NS | NS | Symptomatic | 94 | 100 | 17 | 20 |
| 3. | Gorczycza et al38 | 1994 | 41 | 81 | NS | NS | Symptomatic | 89 | 97 | 16 | 61 |
| 4. | Ahn et al28 | 1994 | 29 | 59 | 13 (6–19) | 46 (31–72) | Symptomatic | 81 | 92 | 17 | 31 |
| 5. | Reynolds et al29 | 1994 | 13 | 24 | 12 (7–22) | 48.9 (37–63) | Symptomatic | 69 | 55 | 9 | 6 |
| 6. | Everson et al30 | 1994 | 32 | 63 | NS | (29–63) | Symptomatic | 95 | 93 | 18 | 37 |
| 7. | De Angelis et al39 | 1994 | 11 | 18 | 10 (2–19) | 42 (18–78) | Symptomatic | 85.7 | 100 | 12 | 4 |
| 8. | Berg et al31 | 1995 | 282 | 535 | 11.9 (0.7–26) | 47 (29–71) | Symptomatic | 98 | 91 | 39 | 49 |
| 9. | Netscher et al7 | 1996 | 81 | 160 | NS | 45.5 (25–64) | Symptomatic | 46.3 | 88.2 | 19 | 105 |
| 10. | Soo et al40 | 1997 | 44 | 86 | NS | NS | Symptomatic | 88 | 92 | 42 | 35 |
| 11. | Ikeda et al34 | 1999 | 30 | 59 | 12 (3–25) | 45 (30–11) | Symptomatic | 100 | 63 | 13 | 10 |
| 12. | Herborn et al41 | 2002 | 25 | 41 | NS | 49 (24–66) | Symptomatic | 86.7 | 88.5 | 13 | 23 |
| Studies in Asymptomatic women ** | |||||||||||
| 1. | Scaranelo et al35 | 2004 | 44 | 83 | 11.9 | NS | Asymptomatic | 64 | 77 | 16 | 40 |
| 2. | Collis et al42 | 2007 | 149 | 298 | 8.8(4.8–13.5) | 41 (23–62) | Asymptomatic | 90 | 43 | 19 | 9 |
Symptomatic women were comprised of those who presented with symptoms such as pain, capsular contracture, and breast asymmetry. Often women also presented with other symptoms such as mass in breast, change in firmness and pain in the extremities.
Asymptomatic women were those who did not present with symptoms but were evaluated due to concerns with regards to the safety of their implants.
Number of true positives and true negatives do not add up to number of implants in studies because they come from implants that are only surgically evaluated and also there will be false positives and false negatives for the test.
Age of ruptured implants
Age of intact implants
#women- Number of women in study
#Implants- Number of implants in study
TP- True Positives
TN- True Negatives
Y-Years
MRI- Magnetic Resonance Imaging
N-sample size
NS-Not Stated
Sensitivity and specificity of the individual studies were used to calculate aggregate values weighted by the respective sample size of the study separately for US and MRI in symptomatic and in asymptomatic women. (Table 3) Scientific data reported in those studies used explantation to confirm the presence of rupture. Prevalence of ruptures in asymptomatic (8%)4 and symptomatic women (33%)5 were obtained from published literature. Predictive probabilities of rupture for positive and negative results were updated using Bayes theorem. (Table 3) Bayes theorem uses the disease prevalence and the test characteristics like sensitivity and specificity to calculate the probability of having or not having the disease given a positive or negative test result. To keep the analysis clear and comprehensible, we used three treatment options for detected ruptures: removal of ruptured implants, removal of ruptured implants with new implant insertion and removal of ruptured implants followed by mastopexy.
Table 3.
Aggregate/ pooled values for sensitivity& specificity of imaging techniques
| Screening Technique | Sample type | Sensitivity % | Specificity % | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| Ultrasound | Symptomatic Women | 82 | 81 | 68 | 90 |
| Asymptomatic women | 64 | 77 | 19 | 96 | |
| MRI | Symptomatic Women | 85 | 90 | 81 | 92 |
| Asymptomatic women | 78 | 71 | 20 | 97 |
Costs Incurred
We compiled costs for imaging services, surgical procedures, and anesthesia from the Medicare Resource Based Relative Value Scale (RBRVS) 2011based on the Current Procedural Terminology (CPT) codes for the rupture treatment. Medicare base rates were obtained and geographically adjusted to account for the national and regional variations in the cost of treatment services for comparative purposes in this study. Medicare RBRVS vary marginally every year; however we used 2011 values to reflect the most recent data. Tables' 4–6 lists the relative value units and Table 7 is the summary of the CPT codes used, the procedures and the Medicare reimbursements for imaging, anesthetic and surgical procedures. The work Geographic Practice Cost Index (GPCI) for all calculations was 1.029, practice expense (PE) GPCI was 1.026, and physician liability (PLI) GPCI was 1.855 for Detroit, MI. The schedule conversion factor for 2011was $33.9764 and the anesthesia conversion factor was $23.19. For example, the reimbursement for ultrasound of breasts was calculated as,
Table 4.
Imaging Relative Value Units
| Procedure | CPT code | Work RVU | Work GPCI | PE RVU | PE GPCI | PLI RVU | PLI GPCI | Total RVU | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Ultrasound Breasts | 76645 | 0.54 | 1.029 | 2.25 | 1.026 | 0.05 | 1.855 | 2.96 |
| 2. | MRI Breasts | 77059 | 1.63 | 1.029 | 21.43 | 1.026 | 0.11 | 1.855 | 23.87 |
Total Imaging RVU = (Work RVU × Work GPCI) + (PE RVU × PE GPCI) + (PLI RVU × PLI GPCI)
Reimbursement = Total RVU × conversion factor
RVU-Relative Value Unit
GPCI- Geographic Practice Cost Index
PE RVU-Practice expense RVU
PLI RVU - Physician Liability RVU
Table 6.
Surgical Relative Value Units
| Procedure | CPT code | Description | Work RVU | Work GPCI | PE RVU | PE GPCI | PLI RVU | PLI GPCI | Total RVU | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Removal of ruptured implant | 19330 | Removal of implant material | 8.54 | 1.029 | 8.35 | 1.026 | 1.66 | 1.855 | 20.43 |
| 2. | Removal of ruptured implant and insert new implants in augmentation | 19325 | Enlarge breast with implant | 8.64 | 1.029 | 8.71 | 1.026 | 1.69 | 1.855 | 20.96 |
| 3. | Removal of ruptured implant and insert new implants in reconstruction | 19342 | Delayed breast prosthesis | 12.63 | 1.029 | 12.19 | 1.026 | 2.44 | 1.855 | 30.02 |
| 4. | Removal of ruptured implant and Mastopexy | 19316 | Suspension of breasts | 11.09 | 1.029 | 9.46 | 1.026 | 2.19 | 1.855 | 25.24 |
Total RVU = (Work RVU × Work GPCI) + (PE RVU × PE GPCI) + (PLI RVU × PLI GPCI)
Medicare reimbursement = [Total RVU + (Anesthesia RVU × Anesthesia GPCI)] × fee schedule conversion factor
RVU-Relative Value Unit
GPCI- Geographic Practice Cost Index
PE RVU-Practice expense RVU
PLI RVU - Physician Liability RVU
Table 7.
CPT codes & Medicare Reimbursements
| Number | Procedure | Code | CPT Descriptions | Medicare Reimbursement |
|---|---|---|---|---|
| Imaging Costs | ||||
| 1. | Ultrasound Breasts | 76645 | Ultrasound, breast(s),real time with image documentation | $100 |
| 2. | MRI both breasts | 77059 | Magnetic resonance imaging, breast bilateral | $810 |
| Anesthesia Costs (General Anesthesia) | ||||
| 1. | Implant removal | 00400 | Anesthesia for the procedures on integumentary system on the extremities, anterior trunk, and perineum, not otherwise specified | $3112 |
| 2. | Implant removal with new implant placement | 00402 | Reconstructive procedures on breast | $5878 |
| 3. | Implant removal with mastopexy | 00402 | Reconstructive procedures on breast | $5186 |
| Surgical Procedure Costs | ||||
| 1. | Implant removal | 19330 | Removal of mammary implant material | $3806 |
| 2. | Implant removal with new implant placement for augmentation | 19325 | Mammoplasty with prosthetic implant | $6590 |
| 3. | Implant removal with new implant placement for reconstruction | 19342 | Delayed insertion of breast prosthesis following mastopexy, mastectomy, or in reconstruction | $6898 |
| 4. | Implant removal with Mastopexy | 19316 | Mastopexy | $5365 |
CPT- Current procedural terminology
Analysis
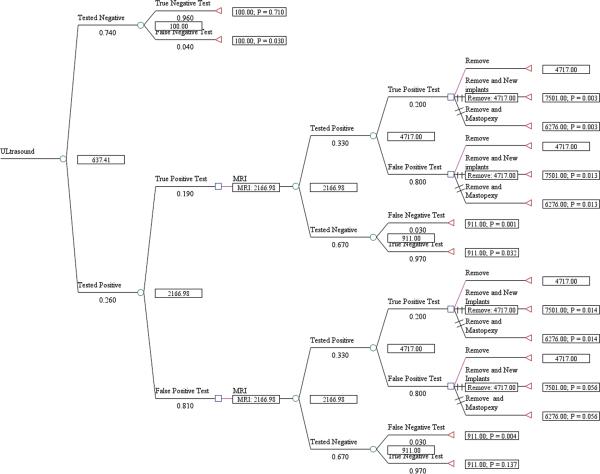
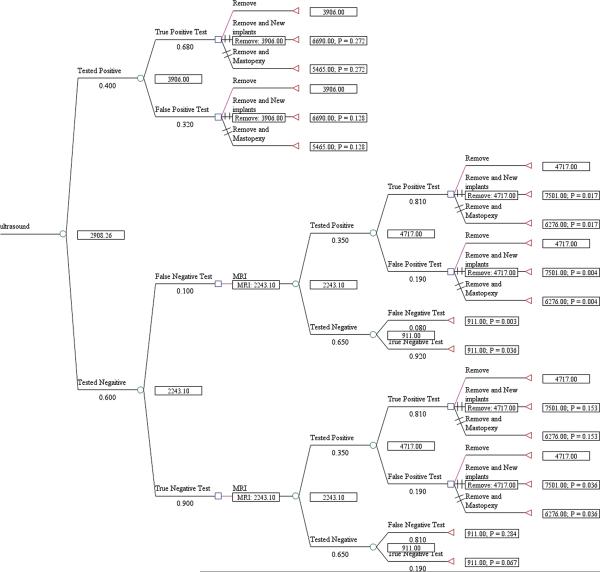
We used TreeAge decision analysis software (TreeAge Pro, version 2011) to construct an expected value decision analysis model for the US and MRI imaging modalities. We created decision trees for US and MRI individually in asymptomatic and symptomatic women and then using US followed by MRI in both populations, which was shown to be a dominant strategy in a prior decision model.18 For example, in our first model, US was shown as a chance node (circle) because the outcomes, positive (suspicious for rupture) or negative (no rupture detected), were not controlled by choice. The test branches (circles) were followed by choice nodes (squares) because the possible outcomes/treatment options were decided. All paths lead to terminal nodes (triangles), true negative or false negative, representing the endpoints of the scenario. The management options for detected rupture remain same across the trees. In the strategy for US followed by MRI, in asymptomatic women, we chose to follow with an MRI for those women tested positive with US for confirmation as these women are symptom free. In the strategy for US followed by MRI in symptomatic women, we chose to do the MRI for those women tested negative with US because these women are symptomatic and a negative test with US necessitates confirmation, for example, an intracapsular rupture. For symptomatic women with positive test with US, they will proceed with implant explantation because of the high probability for rupture. Probabilities were entered for each node and pay-off values (costs) were entered for terminal nodes. The trees were rolled back to obtain the overall expected cost per rupture detected for each strategy. At decision nodes, the non-optimal branches were identified by two slash marks.
Sensitivity Analysis
One way sensitivity analysis was performed by varying the sensitivity and specificity of MRI individually in the strategy of US followed by MRI in both populations to assess the variation in the overall cost per rupture detected. The sensitivity and specificity of MRI may not be same in women who are already tested positive for US as women who are tested with MRI alone, and thus we subjected the MRI test accuracy in sensitivity analysis.
Results
The aggregate sensitivity and specificity of US and MRI (Table 3) were higher in symptomatic women compared to asymptomatic women as suggested by previous studies.19,20 The table also provides the prevalence (obtained from literature), predictive value of a positive test and predictive value of a negative test based on the aggregate sensitivity and specificity values. In asymptomatic women, owing to the low prevalence of rupture (8%), predictive value of a positive test is low for both ultrasound (19%) and for MRI (20%). On the other hand, in symptomatic women, with a prevalence of 33%, predictive value of a positive test was higher for both US (68%) and MRI (81%).
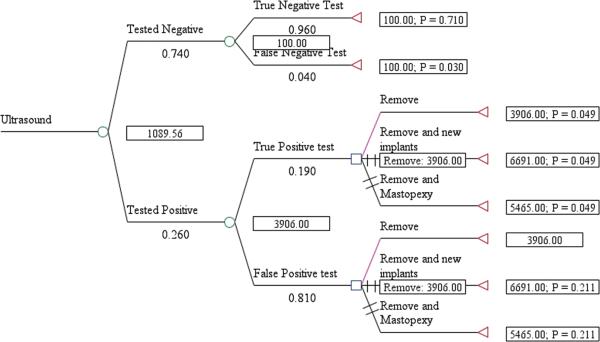
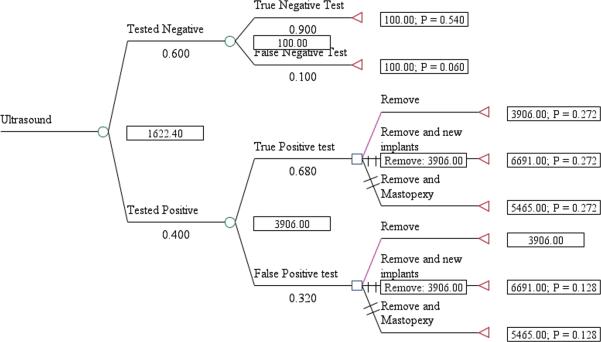
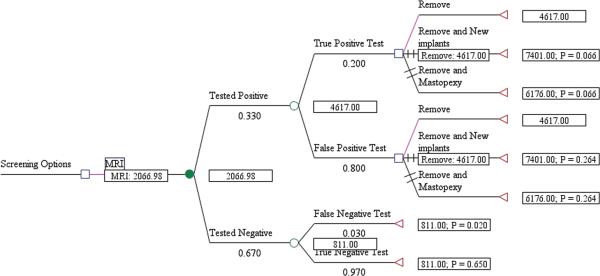
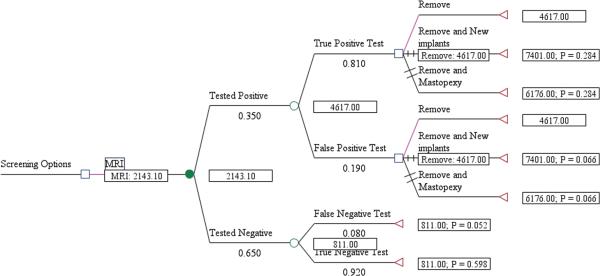
The expected cost per rupture detected for US screening, including management of the rupture, was $1,089 for asymptomatic women (Fig. 1) and $1,622 for symptomatic women (Fig. 2). The cost/rupture for MRI was $2,066 for asymptomatic women (Fig. 3) and $2,143 for symptomatic women (Fig. 4). The expected cost for screening asymptomatic women with US followed by MRI was $637(Fig. 5), and for US followed MRI in symptomatic women was $2,908 (Fig.6). In summary, we found the dominant strategies were US followed by MRI among asymptomatic women ($637) and US for symptomatic women ($1,622).
Figure 1.
Decision analytic model for US in asymtpomatic women
Figure 2.
Decision analytic model for US in symtpomatic women
Figure 3.
Decision analytic model for MRI in asymtpomatic women
Figure 4.
Decision analytic model for MRI in symtpomatic women
Figure 5.
Decision analytic model for US followed by MRI in asymtpomatic women
Figure 6.
Decision analytic model for US followed by MRI in symtpomatic women
Sensitivity Analysis
We varied the sensitivity of MRI from 47% to 98% and specificity from 55% to 95% in the decision tree for US followed by MRI in both groups. The variation ranges were derived from the published studies used in our analysis. US followed by MRI remained the dominant strategy in asymptomatic, and US remained the dominant strategy in symptomatic women.
Discussion
Women who underwent breast augmentation reported improved self-esteem, self-image and highly satisfactory results with the shape, size and feel of their silicone gel implants.21,22 Therefore any unwarranted explantation can result in disfigurement and a huge emotional setback for these women. Additionally, the cost incurred in exploring a false positive case is too high. The national average for surgeon/physician fee in 2010 for breast augmentation was $3,351 (total $992,432,214) and that for removal was $2,288 (total $49, 689,719).1 For highly emotional and resource intensive concern such as silicone breast implants, economic analysis is an effective means to arrive at screening algorithms that impose minimal burden possible on the patient and society, yet yielding accurate results.
The 2006 FDA recommendation to evaluate all silicone breast implants with successive MRI to identify silent ruptures3,23 has raised much controversy in the healthcare community. In our current culture of healthcare cost constraint, it is difficult to justify the use of expensive MRI testing when the consequences of asymptomatic rupture have been shown to be minimal.24 Furthermore, paucity of precise data with regards to MRI accuracy19 and the lack of demonstrable benefit of screening in asymptomatic women may dissuade patients from following the FDA's recommendation. The costs of MRI screening over the lifetime of the average woman, which may not be covered by the patients' insurance, may exceed the costs of initial surgery.23,25 However, if more affordable screening strategy can be supported by scientific data, then more patients and plastic surgeons may be encouraged to participate in these screening efforts to define the true risk of ruptures and health consequences for the silicone gel implants that have garnered resurgent interests in the US and abroad.
Our study shows that screening asymptomatic women with US, followed by MRI screening in US positive patients is the least expensive strategy to detect silent rupture ($637/ rupture detected and managed) . Too low prevalence of rupture (8%) in asymptomatic women results in high false positives with US, therefore screening the US positive women with MRI to confirm the rupture is a good approach in order to prevent the unnecessary exploration of intact implants, and to minimize costs and stress to the women. For screening symptomatic women, our study shows that US alone ($1,622/rupture detected and managed) is the optimal strategy. With the extra-capsular rupture, women tend to present with symptoms such as capsular contracture and breast asymmetry, unlike an intracapsular rupture that is clinically silent and the women's breasts feel normal. Therefore, screening symptomatic women with US alone is ideal because US detects extra-capsular ruptures very well.
As per FDA recommendations, in asymptomatic women, with a cost of $2,067 per rupture detected and managed with MRI, the total cost of detecting and managing all the ruptures that occurred in 2010 alone will be 3 times greater when compared to the total cost incurred with US followed by MRI, (total costs $33,135,828 vs. $8,748,558) as suggested by our study. Similarly, in symptomatic women, with a cost of $2,143 per rupture detected and managed with MRI, the total cost of detecting and managing all the ruptures that occurred in 2010 alone for example, will be 1.3 times greater when compared to the total cost incurred with US alone (total costs $141,706,875 vs. $107,254,750) with a cost of $1,622 per rupture detected and managed. These numbers were obtained taking into account augmentations and reconstructions performed in only 2010, the difference will be even greater when considering total number of women with implants into account. These additional costs will be a huge burden on women with implants and also society as most of the women with silicone implants are younger and therefore not covered by Medicare.
This study has several limitations. Ideally, data for the economic analysis should be obtained from randomized clinical trials, but because none have been conducted so far to detect implant ruptures, we derived data form published literature with silicone gel implants of varying brands. Some of the published studies lack the specific mention whether the study sample comprised of symptomatic or asymptomatic women, because of this concern these studies were excluded. Furthermore, majority of the studies were done in symptomatic women. We found only two studies with MRI and only one study with US done exclusively in asymptomatic women which limits the analysis. Our costs did not include societal costs such as loss of work, which is extremely difficult to capture because variability in job descriptions. Typically, breast implant removal or insertion is done as an outpatient procedure with patient being discharged on the same day from the hospital. Cost of breast implants was not included as the CPT code because cost of implants was not available from the Medicare RBRVS. But including the cost of the implants will not change the comparative analysis of these various screening strategies. In addition, in the decision trees for US followed by MRI, we assumed the sensitivity and specificity of MRI to be same as that for doing MRI alone. Sensitivity and specificity of MRI performed after US (either positive or negative US test) may be different from those performing MRI alone, as the sample population will be different.
The FDA recommends performing serial MRIs to ascertain the implant integrity because of its higher sensitivity and specificity reported in studies. However, a meta-analysis study by Song and Chung et al. revealed that there are several biases in the studies reporting higher accuracy of MRI.19 The diagnostic odds ratio, a measure for overall diagnostic test performance of MRI was found to be 14 times greater in symptomatic samples than asymptomatic samples and the pooled estimate of sensitivity and specificity were 87% and 89.9% respectively. Cher et al. concluded from a meta-analysis study that the positive predictive value (PPV), the probability of having the rupture among those who are tested positive, of MRI was good (>80%) when the prevalence of rupture was high (50%) but the PPV never exceeded 80% when the prevalence of rupture was low (10%). Summary estimates of sensitivity and specificity in that study were 78% (95% CI: 71–83) and 91% (95% CI: 86–94) respectively.20 This meta-analysis included studies in symptomatic women, thus the authors suggested limiting the use of MRI to confirm ruptures in clinically suspected women.
Conclusion
In summary, screening of all women with MRI as recommended by the FDA, would incur substantial costs. Because there is no evidence of the potential benefits of this strategy, we do not find this recommendation to be in the best interest of patients or the healthcare system. Additional data are required to establish with certainty, possible health implications of silicone implant rupture, especially clinical sequela. Furthermore, since their introduction in 1962, breast implants have undergone several changes with regards to their appearance and durability. Clinical trials are underway for the current fifth generation of implants, reportedly more form-cohesive and stable. We therefore suggest a strategy of screening asymptomatic women with US followed by screening with MRI, and a strategy of screening symptomatic women with ultrasound to be optimal and economical. Furthermore, more studies on screening in asymptomatic women are warranted to overcome the dilemma that surgeons often face to arrive at a diagnosis of rupture which ultimately reduce the cost of unnecessary tests and treatment. Such studies also keep plastic surgeons abreast about the evidence-based protocol for the long-term assessment and management of silicone breast implant ruptures.
Table 5.
Anesthesia Relative Value Units
| Procedure | CPT code | Description | Base Units | Time Units | Anesthesia conversion factor | Schedule conversion factor | Anesthesia work fraction | Total RVU | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Removal of ruptured implant | 00400 | Anesthesia for procedures on integumentary system of the extremities, anterior trunk and perineum | 3 | 6 | 23.19 | 33.9764 | 0.7792 | 3.95 |
| 2. | Removal of ruptured implants with new implant insertion | 00402 | Reconstructive procedures on breast | 5 | 12 | 23.19 | 33.9764 | 0.7792 | 7.46 |
| 3. | Removal of ruptured implant with Mastopexy | 00402 | Reconstructive procedures on breast | 5 | 10 | 23.19 | 33.9764 | 0.7792 | 6.59 |
Anesthesia RVU = (Base units+ Time Units) × (Anesthesia conversion factor/ Fee schedule conversion factor) × 0.8252 (Anesthesia work fraction)
CPT- Current Procedural Terminology
RVU-Relative Value Units
Acknowledgments
Supported in part by grants from the National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to Dr. Kevin C. Chung).
Footnotes
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Author Contributions: Study conception and design: Malay, Chung
Analysis and intrepretation of data: Malay, Kim, Chung, Shauver
Manuscript draft: Malay
Critical revision: Shauver, Chung, Kim, Malay
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Accessed October 20, 2011];A.S.P.S National clearinghouse of plastic surgery procedural statistics. Available at http://www.plasticsurgery.org/news-and-resources/statistics.html.
- 2.Brown SL, Silverman BG, Berg WA. Rupture of silicone-gel breast implants: causes, sequelae, and diagnosis. Lancet. 1997 Nov 22;350(9090):1531–1537. doi: 10.1016/S0140-6736(97)03164-4. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed October 20, 2011];2006 guidance for industry and FDA in saline, silicone gel, and alternative breast implants; 8.5 safety assessment - rupture. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/WomensHealthResearch/ucm133361.htm#85.
- 4.Heden P, Nava MB, van Tetering JP, et al. Prevalence of rupture in inamed silicone breast implants. Plast Reconstr Surg. 2006 Aug;118(2):303–308. doi: 10.1097/01.prs.0000233471.58039.30. discussion 309–312. [DOI] [PubMed] [Google Scholar]
- 5.Goodman CM, Cohen V, Thornby J, Netscher D. The life span of silicone gel breast implants and a comparison of mammography, ultrasonography, and magnetic resonance imaging in detecting implant rupture: a meta-analysis. Ann Plast Surg. 1998 Dec;41(6):577–585. doi: 10.1097/00000637-199812000-00001. discussion 585–576. [DOI] [PubMed] [Google Scholar]
- 6.Kessler DA. The basis of the FDA's decision on breast implants. N Engl J Med. 1992 Jun 18;326(25):1713–1715. doi: 10.1056/NEJM199206183262525. [DOI] [PubMed] [Google Scholar]
- 7.Netscher DT, Weizer G, Malone RS, Walker LE, Thornby J, Patten BM. Diagnostic value of clinical examination and various imaging techniques for breast implant rupture as determined in 81 patients having implant removal. South Med J. 1996 Apr;89(4):397–404. doi: 10.1097/00007611-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Holmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003 Jul;138(7):801–806. doi: 10.1001/archsurg.138.7.801. [DOI] [PubMed] [Google Scholar]
- 9.Marotta JS, Widenhouse CW, Habal MB, Goldberg EP. Silicone gel breast implant failure and frequency of additional surgeries: analysis of 35 studies reporting examination of more than 8,000 explants. J Biomed Mater Res. 1999;48(3):354–364. doi: 10.1002/(sici)1097-4636(1999)48:3<354::aid-jbm21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G. Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama. AJR Am J Roentgenol. 2000 Oct;175(4):1057–1064. doi: 10.2214/ajr.175.4.1751057. [DOI] [PubMed] [Google Scholar]
- 11.Noone RB. A review of the possible health implications of silicone breast implants. Cancer. 1997 May 1;79(9):1747–1756. doi: 10.1002/(sici)1097-0142(19970501)79:9<1747::aid-cncr17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.FDA [Accessed October 24, 2011];Update on the Safety of Silicone Gel-Filled Breast Implants. Available at http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/Implants andProsthetics/BreastImplants/UCM260090.pdf.
- 13.Andersen B, Hawtof D, Alani H, Kapetansky D. The diagnosis of ruptured breast implants. Plast Reconstr Surg. 1989 Dec;84(6):903–907. doi: 10.1097/00006534-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Holmich LR, Fryzek JP, Kjoller K, et al. The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg. 2005 Jun;54(6):583–589. doi: 10.1097/01.sap.0000164470.76432.4f. [DOI] [PubMed] [Google Scholar]
- 15.Gorczyca DP, Gorczyca SM, Gorczyca KL. The diagnosis of silicone breast implant rupture. Plast Reconstr Surg. 2007 Dec;120(7 Suppl 1):49S–61S. doi: 10.1097/01.prs.0000286569.45745.6a. [DOI] [PubMed] [Google Scholar]
- 16.Azavedo E, Bone B. Imaging breasts with silicone implants. Eur Radiol. 1999;9(2):349–355. doi: 10.1007/s003300050679. [DOI] [PubMed] [Google Scholar]
- 17.Sinha S, Gorczyca DP, DeBruhl ND, Shellock FG, Gausche VR, Bassett LW. MR imaging of silicone breast implants: comparison of different coil arrays. Radiology. 1993 Apr;187(1):284–286. doi: 10.1148/radiology.187.1.8451430. [DOI] [PubMed] [Google Scholar]
- 18.Chung KC, Greenfield ML, Walters MR. Decision-analysis methodology in the work-up of women with suspected silicone breast implant rupture. Plast Reconstr Surg. 1998;102(3):689–695. doi: 10.1097/00006534-199809030-00011. 1998. [DOI] [PubMed] [Google Scholar]
- 19.Song JW, Kim HM, Bellfi LT, Chung KC. The effect of study design biases on the diagnostic accuracy of magnetic resonance imaging for detecting silicone breast implant ruptures: a meta-analysis. Plast Reconstr Surg. 2011 Mar;127(3):1029–1044. doi: 10.1097/PRS.0b013e3182043630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cher DJ, Conwell JA, Mandel JS. MRI for detecting silicone breast implant rupture: meta-analysis and implications. Ann Plast Surg. 2001 Oct;47(4):367–380. doi: 10.1097/00000637-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Spear SL, Murphy DK, Slicton A, Walker PS. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007 Dec;120(7 Suppl 1):8S–16S. doi: 10.1097/01.prs.0000286580.93214.df. discussion 17S–18S. [DOI] [PubMed] [Google Scholar]
- 22.Murphy DK, Beckstrand M, Sarwer DB. A prospective, multi-center study of psychosocial outcomes after augmentation with natrelle silicone-filled breast implants. Ann Plast Surg. 2009 Feb;62(2):118–121. doi: 10.1097/SAP.0b013e31817f01f8. [DOI] [PubMed] [Google Scholar]
- 23.FDA [Accessed November 2, 2011];FDA Approves Silicone Gel-Filled Breast Implants After In-Depth Evaluation. Avvailable at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108790.htm.
- 24.Holmich LR, Vejborg IM, Conrad C, et al. Untreated silicone breast implant rupture. Plast Reconstr Surg. 2004 Jul;114(1):204–214. doi: 10.1097/01.prs.0000128821.87939.b5. discussion 215–206. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CM, Pusic AL, Kerrigan CL. Silicone breast implants and magnetic resonance imaging screening for rupture: do U.S. Food and Drug Administration recommendations reflect an evidence-based practice approach to patient care? Plast Reconstr Surg. 2008 Apr;121(4):1127–1134. doi: 10.1097/01.prs.0000302498.44244.52. [DOI] [PubMed] [Google Scholar]
- 26.DeBruhl ND, Gorczyca DP, Ahn CY, Shaw WW, Bassett LW. Silicone breast implants: US evaluation. Radiology. 1993 Oct;189(1):95–98. doi: 10.1148/radiology.189.1.8372224. [DOI] [PubMed] [Google Scholar]
- 27.Caskey CI, Berg WA, Anderson ND, Sheth S, Chang BW, Hamper UM. Breast implant rupture: diagnosis with US. Radiology. 1994 Mar;190(3):819–823. doi: 10.1148/radiology.190.3.8115633. [DOI] [PubMed] [Google Scholar]
- 28.Ahn CY, DeBruhl ND, Gorczyca DP, Shaw WW, Bassett LW. Comparative silicone breast implant evaluation using mammography, sonography, and magnetic resonance imaging: experience with 59 implants. Plast Reconstr Surg. 1994 Oct;94(5):620–627. doi: 10.1097/00006534-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds HE, Buckwalter KA, Jackson VP, Siwy BK, Alexander SG. Comparison of mammography, sonography, and magnetic resonance imaging in the detection of silicone-gel breast implant rupture. Ann Plast Surg. 1994 Sep;33(3):247–255. doi: 10.1097/00000637-199409000-00003. discussion 256–247. [DOI] [PubMed] [Google Scholar]
- 30.Everson LI, Parantainen H, Detlie T, et al. Diagnosis of breast implant rupture: imaging findings and relative efficacies of imaging techniques. AJR Am J Roentgenol. 1994 Jul;163(1):57–60. doi: 10.2214/ajr.163.1.8010248. [DOI] [PubMed] [Google Scholar]
- 31.Berg WA, Caskey CI, Hamper UM, et al. Single- and double- lumen silicone breast implant integrity: prospective evaluation of MR and US criteria. Radiology. 1995 Oct;197(1):45–52. doi: 10.1148/radiology.197.1.7568852. [DOI] [PubMed] [Google Scholar]
- 32.Chung KC, Wilkins EG, Beil RJ, Jr., et al. Diagnosis of silicone gel breast implant rupture by ultrasonography. Plast Reconstr Surg. 1996 Jan;97(1):104–109. doi: 10.1097/00006534-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Medot M, Landis GH, McGregor CE, et al. Effects of capsular contracture on ultrasonic screening for silicone gel breast implant rupture. Ann Plast Surg. 1997 Oct;39(4):337–341. doi: 10.1097/00000637-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda DM, Borofsky HB, Herfkens RJ, Sawyer-Glover AM, Birdwell RL, Glover GH. Silicone breast implant rupture: pitfalls of magnetic resonance imaging and relative efficacies of magnetic resonance, mammography, and ultrasound. Plast Reconstr Surg. 1999 Dec;104(7):2054–2062. doi: 10.1097/00006534-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Scaranelo AM, Marques AF, Smialowski EB, Lederman HM. Evaluation of the rupture of silicone breast implants by mammography, ultrasonography and magnetic resonance imaging in asymptomatic patients: correlation with surgical findings. Sao Paulo Med J. 2004;122(2):41–7. doi: 10.1590/S1516-31802004000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg WA, Caskey CI, Hamper UM, Anderson ND, Chang BW, Sheth S, et al. Diagnosing breast implant rupture with MR imaging, US, and mammography. Radiographics. 1993;13(6):1323–36. doi: 10.1148/radiographics.13.6.8290727. [DOI] [PubMed] [Google Scholar]
- 37.Monticciolo DL, Nelson RC, Dixon WT, Bostwick J, 3rd, Mukundan S, Hester TR. MR detection of leakage from silicone breast implants: value of a silicone-selective pulse sequence. AJR Am J Roentgenol. 1994;163(1):51–6. doi: 10.2214/ajr.163.1.8010247. [DOI] [PubMed] [Google Scholar]
- 38.Gorczyca DP, Schneider E, DeBruhl ND, Foo TK, Ahn CY, Sayre JW, et al. Silicone breast implant rupture: comparison between three-point Dixon and fast spin-echo MR imaging. AJR Am J Roentgenol. 1994;162(2):305–10. doi: 10.2214/ajr.162.2.8310916. [DOI] [PubMed] [Google Scholar]
- 39.DeAngelis GA, de Lange EE, Miller LR, Morgan RF. MR imaging of breast implants. Radiographics. 1994;14(4):783–94. doi: 10.1148/radiographics.14.4.7938768. [DOI] [PubMed] [Google Scholar]
- 40.Soo MS, Kornguth PJ, Walsh R, Elenberger C, Georgiade GS, DeLong D, et al. Intracapsular implant rupture: MR findings of incomplete shell collapse. J Magn Reson Imaging. 1997;7(4):724–30. doi: 10.1002/jmri.1880070419. [DOI] [PubMed] [Google Scholar]
- 41.Herborn CU, Marincek B, Erfmann D, Meuli-Simmen C, Wedler V, Bode-Lesniewska B, et al. Breast augmentation and reconstructive surgery: MR imaging of implant rupture and malignancy. Eur Radiol. 2002;12(9):2198–206. doi: 10.1007/s00330-002-1362-x. [DOI] [PubMed] [Google Scholar]
- 42.Collis N, Litherland J, Enion D, Sharpe DT. Magnetic resonance imaging and explantation investigation of long-term silicone gel implant integrity. Plast Reconstr Surg. 2007;120(5):1401–6. doi: 10.1097/01.prs.0000279374.99503.89. [DOI] [PubMed] [Google Scholar]