Abstract
Background
The increased morbidity and mortality associated with coagulopathy and thrombocytopenia after trauma are well-described. However, few studies have assessed platelet function after injury.
Methods
Blood samples were prospectively collected from 101 critically-injured trauma patients on arrival to the emergency department and serially after admission to a Level I urban trauma ICU from November 2010 to October 2011, and functionally assayed for responsiveness to adenosine diphosphate, thrombin receptor-activating peptide (TRAP), arachidonic acid (AA), and collagen using multiple electrode impedance aggregometry.
Results
Of 101 enrolled patients, 46 (45.5%) had below-normal platelet response to at least one agonist (‘platelet hypofunction’) on admission, and 92 patients (91.1%) had platelet hypofunction at some time during their ICU stay. Admission platelet hypofunction was associated with low Glasgow coma score (GCS) and a nearly 10-fold higher early mortality. Logistic regression identified admission GCS (odds ratio 0.819, p=0.008) and base deficit (odds ratio 0.872, p=0.033) as independent predictors of platelet hypofunction. Admission AA and collagen responsiveness were significantly lower in patients who died (p<0.01), while admission platelet counts were similar (p=0.278); Cox regression confirmed TRAP, AA, and collagen responsiveness as independent predictors of in-hospital mortality (p<0.05). Receiver operator characteristic analysis identified admission AA and collagen responsiveness as negative predictors of both 24-hour (AA AUC: 0.874, collagen AUC: 0.904) and in-hospital mortality (AA AUC: 0.769, collagen AUC: 0.717).
Conclusions
In this prognostic study, we identify clinically significant platelet dysfunction after trauma in the presence of an otherwise reassuring platelet count and standard clotting studies, with profound implications for mortality. Multiple electrode impedance aggregometry reliably identifies this dysfunction in injured patients, and admission arachidonic acid and collagen responsiveness are sensitive and specific independent predictors of both early and late mortality.
Level of evidence
Level I
Keywords: Platelets, impedance aggregometry, multiple electrode aggregometry
BACKGROUND
Platelets play a pivotal role in hemostasis after injury.1 Recent evidence identifies that admission platelet counts are inversely correlated with early mortality and transfusion in critically injured trauma patients, even for platelet counts well into the normal range.2 Quantitative platelet deficits also predict progression of intracranial hemorrhage and mortality after traumatic brain injury.3 While the increased morbidity and mortality associated with enzymatic coagulopathy after trauma is well-described, near-total impairment of clot formation can also occur as a result of platelet dysfunction despite the presence of normal-range coagulation studies and platelet count.4 Thorough study of platelet dysfunction has been hindered by the technical complexity of existing platelet function assays; however, recent advances in impedance-based platelet aggregometry allow for rapid, point-of-care assessment of platelet function.5
Impedance aggregometry assays platelet aggregation via electrical resistance across sets of silver-coated copper electrodes immersed in whole blood; non-thrombogenic resting platelets are activated using specific platelet agonists, causing platelets to aggregate on the charged surface and increasing impedance in proportion to the degree of platelet activation.6 This principle underlies the recently-developed Multiplate® multiple electrode aggregometer, which uses disposable test cells containing duplicate pairs of sensor wires to measure platelet aggregation in response to agonists of interest in citrated, heparinized, or hirudin-anticoagulated whole blood.5 Impedance aggregometry has been cross-validated with single platelet counting, turbidimetric platelet aggregation, vasodilator stimulated phosphoprotein (VASP) phosphorylation, and light aggregometry5, 7, 8 in normal controls and in monitoring clopidogrel and aspirin effects; however, only preliminary investigations exist using impedance aggregometry to characterize platelet dysfunction related to trauma.9
Therefore, the purpose of this study was to prospectively quantify platelet function using multiple electrode aggregometry in order to identify previously undetected platelet dysfunction in trauma patients. We further sought to relate any observed dysfunction to outcomes after severe injury.
METHODS
Blood samples were prospectively collected from 101 critically-injured trauma patients on arrival and at 6, 12, 24, 48, 72, 96, and 120h after admission to a Level I urban trauma ICU from November 2010 to October 2011. Admission samples were collected via initial placement of a 16G or larger peripheral IV; subsequent samples were collected via indwelling arterial catheters. Standard laboratory vacuum-sealed tubes containing 3.2% (0.109M) sodium citrate were used for all draws. A total of 376 samples were analyzed, with median 3 (interquartile range 2–4) samples per patient. Demographics, resuscitation data, clinical laboratory results, and outcomes were collected in parallel. Informed consent was obtained from all patients, as approved by the University of California Committee on Human Research.
Platelet function was assessed at point of care using the Multiplate® multiple electrode aggregometer (Verum Diagnostica GmbH; Munich, Germany) immediately after sample collection. Briefly, 0.3mL of whole blood was diluted in warmed normal saline containing 3mM CaCl2 and incubated for 3 minutes at 37°C with continuous stirring in a Multiplate® test cell. Each test cell contains two sets of 3mm silver-coated copper wires, across which electrical resistance is measured at 0.57 second intervals. Platelet activation was induced by adenosine diphosphate (ADP, final concentration 6.5µM; via P2 receptors), thrombin receptor activating peptide-6 (TRAP, final concentration 32µM; via PAR receptors), arachidonic acid (AA, final concentration 0.5mM; via the cyclooxygenase pathway), or collagen (final concentration 3.2µg/mL; via GpIa/IIa and GpVI receptors). Platelet adhesion to the electrodes was detected as increasing electrical impedance, measured by duplicate sets of sensor wires in each test cell. Agonist responses are reported as area under the aggregation curve in units (U) over a 6-minute measurement period. Reference ranges for citrated whole blood were provided by the manufacturer based on studies of healthy controls.
Data are presented as mean ± standard deviation, median (interquartile range [IQR]), or percentage; univariate comparisons were made using Student’s t-test for normally distributed data, Wilcoxon rank-sum testing for skewed data, and Fisher’s exact test for proportions. Logistic regression was performed to identify predictors of platelet hypofunction. Kaplan-Meier time-to-event analysis was used to assess differences in mortality; Cox proportional hazards regression was used to identify adjusted predictors of mortality. Nonparametric receiver-operator characteristic (ROC) analysis was performed to characterize the ability of continuous agonist responses to classify binary outcomes. An alpha of 0.05 was considered significant. All analysis was performed by the authors using Stata version 12 (StataCorp; College Station, TX).
RESULTS
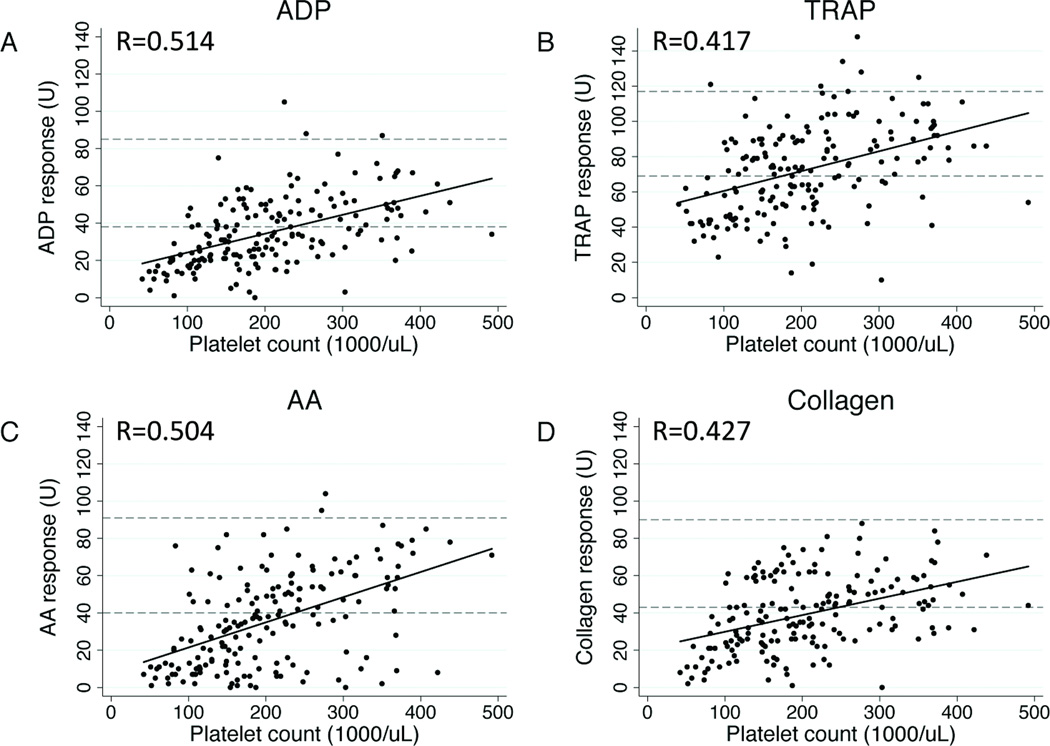
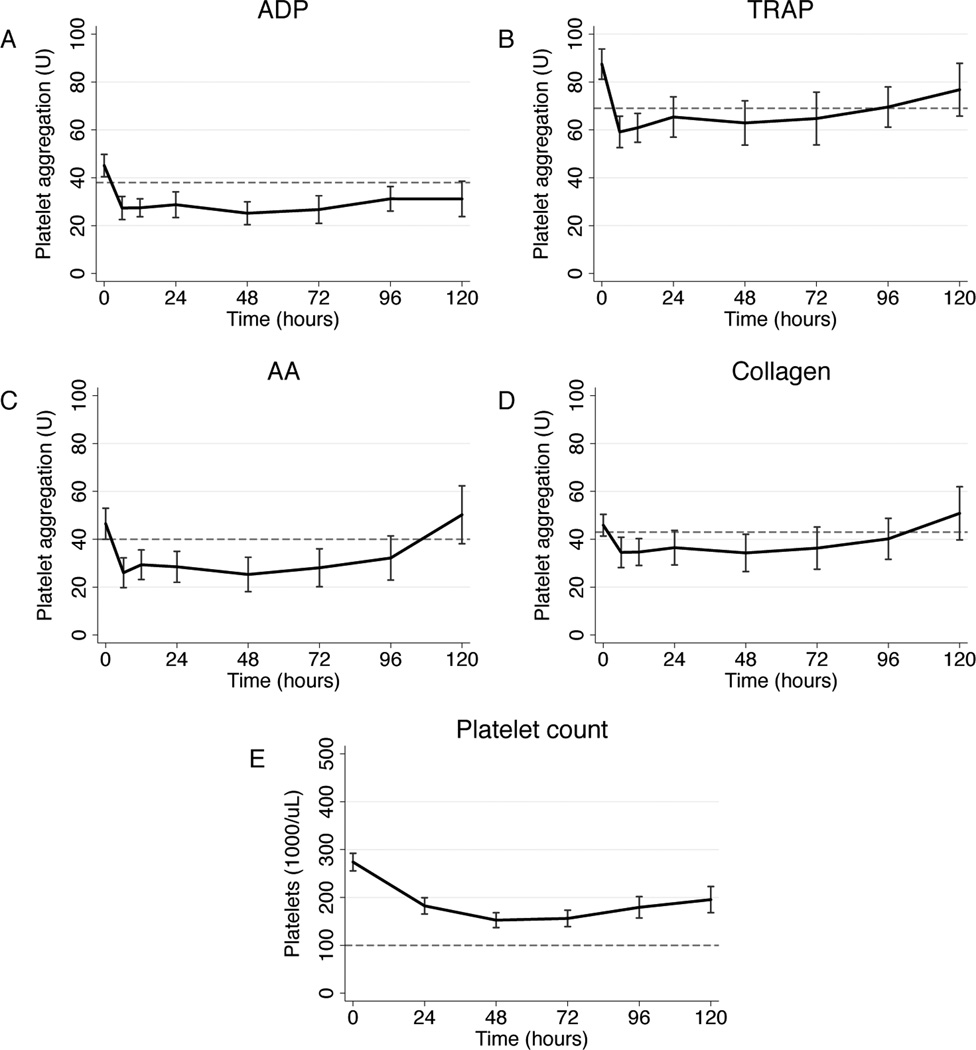
Our 101-patient study population had mean age 41.3±19.3y and mean injury severity score 23.2±5.4; there was 31.0% penetrating and 61.2% brain injury. Mean platelet responsiveness to ADP, TRAP, AA, and collagen on admission were in the low-normal range according to manufacturer-provided reference values (Table 1). Notably, the mean admission platelet count was 274.4±85.4*103/µL, with no admission platelet count below 140*103/µL (Table 1). Significant correlations between agonist response and platelet count were observed for all agonists, with linear correlation between platelet response extending well into the clinically ‘normal’ platelet range (Fig 1). Platelet responsiveness was then longitudinally evaluated from ICU admission to ICU discharge or 120h. For all agonists, mean platelet responsiveness fell sharply to below the normal range by 6h (Fig 2). TRAP and collagen responsiveness returned to the low-normal range by 24h, while ADP and AA (Fig 2a and b) responsiveness remained significantly impaired until 96h (ADP; Fig 2c) and 120h (AA; Fig 2d), respectively. Mean platelet count remained above 100*103/µL for the entirety of ICU stay (Fig 2e).
Table 1.
Admission platelet agonist responses and platelet counts.
| Admission values (N=78) |
Observed range | Normal range | |
|---|---|---|---|
| ADP (U) | 44.6 ± 20.4 | 0–105 | 38–85 |
| TRAP (U) | 86.6 ± 27.0 | 10–170 | 69–117 |
| AA (U) | 44.3 ± 28.3 | 0–104 | 40–91 |
| Collagen (U) | 44.7 ± 19.7 | 0–88 | 43–90 |
| Platelets (*103/µL) | 274.4 ± 85.4 | 140–605 | 150–400 |
Values are presented as mean ± standard deviation, with measurements of area under the curve expressed as units (U) or platelet counts. ADP = adenosine diphosphate, TRAP = thrombin receptor-activating peptide, AA = arachidonic acid.
Figure 1.
Scatter plots showing correlation between platelet count and adenosine diphosphate (ADP; a), thrombin receptor-activating peptide (TRAP; b), arachidonic acid (AA; c), and collagen (d) responsiveness using matched data from all time points collected. Manufacturer-provided normal ranges indicated by reference lines at the lower (5th percentile) and upper (95th percentile) boundaries. Rho values for each pairwise correlation given at bottom right of each graph, with all associated p < 0.001.
Figure 2.
Platelet adenosine diphosphate (ADP; a), thrombin receptor-activating peptide (TRAP; b), arachidonic acid (AA; c), and collagen (d) responsiveness as area under the aggregation curve in units (U) over time. Platelet count measurements (e) are shown for comparison. Data points are mean values, with capped bars representing 95% confidence intervals; dotted lines represent the lower bound (5th percentile) of normal values for each measurement.
Using the lower bound (5th percentile) of manufacturer-provided reference ranges, 46 patients (45.5%) had below-normal platelet response to at least one agonist on admission; 92 patients (91.1%) had a below-normal response at some time during their ICU stay. Of 42 patients with confirmed prehospital medication data, 4 patients were taking aspirin and 1 patient was taking clopidogrel (Plavix®) at the time of injury. Patients taking aspirin had significantly lower admission AA responsiveness (5.8±3.3 vs. 48.0±26.1 U, p<0.001) and a trend towards lower collagen responsiveness (24.5±18.6 vs. 46.7±18.1 U, p=0.092), but did not differ significantly in responsiveness to other agonists or by admission platelet count (all p>0.400). Similarly, a single patient known to be taking Plavix® had an admission ADP response of 27 U (below the 25th percentile in the study population) and a below-normal AA response of 38 U (normal range: 40–91 U). For all subsequent analysis, patients known to be taking aspirin or Plavix® were excluded unless otherwise noted.
We then dichotomized the study population into 39 patients (42.9%) with a below-normal response to any agonist on admission (“platelet hypofunction”) compared to 52 patients (57.1%) with all normal-range responses (“normal function”). Platelet hypofunction was associated with low admission GCS (p=0.007), higher mechanical ventilation requirements (p=0.040), and a nearly 10-fold higher early mortality (p=0.009; Table 2). Logistic regression identified base deficit (odds ratio 0.872, p=0.033) and GCS (odds ratio 0.819, p=0.008) as independent predictors of admission platelet hypofunction; platelet count was not a significant predictor of hypofunction (p = 0.150). Analysis was repeated in an intention-to-treat fashion including patients taking aspirin and Plavix®: odds ratios and significance were similar for base deficit and GCS, with older age identified as an additional significant predictor (odds ratio 1.041, p=0.032).
Table 2.
Patient characteristics by platelet hypofunction on admission.
| Platelet hypofunction (N=39) |
Normal function (N=52) |
P-value | |
|---|---|---|---|
| Age (y) | 44.4 ± 20.7 | 36.7 ± 16.5 | 0.060 |
| BMI (kg/m2) | 26.3 ± 5.7 | 25.6 ± 4.9 | 0.543 |
| Blunt injury | 65.7% | 70.2% | 0.811 |
| ISS | 22.8 ± 14.3 | 25.4 ± 15.3 | 0.513 |
| GCS* | 7 (3–10) | 13 (6–15) | 0.007 |
| Temperature (°C) | 35.7 ± 0.7 | 35.8 ± 0.8 | 0.539 |
| Prehospital IVF (mL) | 250 (50–1000) | 250 (50–750) | 0.711 |
| pH | 7.23 ± 0.20 | 7.31 ± 0.14 | 0.178 |
| Base deficit | −6.9 ± 6.4 | −3.9 ± 5.6 | 0.072 |
| INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.875 |
| PTT (sec) | 28.6 (26.3–31.5) | 26.4 (25.3–31.1) | 0.322 |
| Hematocrit (%) | 40.4 ± 5.5 | 39.7 ± 4.8 | 0.553 |
| Platelet count (*103/µL) | 257.7 ± 75.3 | 285.7 ± 88.5 | 0.125 |
| RBC / 24h | 0 (0–1) | 0 (0–4) | 0.688 |
| FFP / 24h | 0 (0–2) | 0 (0–2) | 0.795 |
| Plts / 24h | 0 (0–0) | 0 (0–0) | 0.405 |
| Hospital days | 6 (2–27) | 10 (6.5–20) | 0.090 |
| ICU days | 3.5 (1–14) | 3 (2–14) | 0.436 |
| Vent-free days / 28d* | 12 (0–26) | 26 (7.5–27) | 0.040 |
| Multiorgan failure | 31.7% | 27.3% | 0.656 |
| 24h mortality* | 20.0% | 2.1% | 0.009 |
| In-hospital mortality | 34.3% | 14.6% | 0.062 |
Data are presented as mean ± standard deviation or median (interquartile range).
BMI = body mass index, ISS = injury severity score, GCS = Glasgow coma score, IVF = intravenous fluid, INR = international normalized ratio, PTT = partial thromboplastin time, RBC = red blood cell units, FFP = fresh frozen plasma units, Plt = platelet units, ICU = intensive care unit.
p < 0.05 by Student’s t, Mann-Whitney, or Fisher’s exact testing.
In order to identify patient factors related to differential agonist responses, the study population was dichotomized by age (≥55 vs. <55 years old), admission base deficit (≤−6 vs. >−6), traumatic brain injury (AIS-head ≥3 vs. <3), and admission GCS (≥8 vs. <8). Older patients had significantly lower responsiveness to TRAP (74.5±27.2 vs. 91.9±27.2 U, p=0.030) and AA (26.8±23.5 vs. 51.8±26.2 U, p=0.001); ADP (p=0.074) and collagen (p=0.375) responsiveness did not differ by age. Patients in shock as defined by admission base deficit had significantly lower responsiveness to collagen (34.7±20.5 vs. 50.8±16.7 U, p=0.011), with no differences in ADP, TRAP, or AA responsiveness (p>0.200). No significant differences were observed in traumatic brain injury as identified by AIS score (all p>0.500); however, patients with lower admission GCS had lower responsiveness to ADP (38.6±20.7 vs. 48.6±20.3 U, p=0.016) and collagen (35.5±20.1 vs. 47.2±18.3 U, p=0.008), with no differences in TRAP or AA (p>0.300). Admission platelet count did not statistically differ by age, base deficit, AIS-head score, or GCS (all p>0.100).
Agonist responses were then examined for differences by mortality. In patients who died in-hospital at any time, admission AA and collagen responses were significantly lower than survivors (AA: 22.4±24.3 vs. 48.8±25.6 U, p=0.001; collagen: 29.6±21.4 vs. 47.0±17.6 U, p=0.008), while admission platelet count did not differ significantly (p=0.278). In order to account for the contribution of other patient and injury characteristics, Cox proportional hazards regression was used to adjust for age, GCS, base deficit, and platelet count. In multivariate analysis, low TRAP (hazard ratio 0.980, p=0.047), AA (hazard ratio 0.968, p=0.003), and collagen responses (hazard ratio 0.955, p=0.031) were independent predictors of in-hospital mortality. These results were unchanged when including patients taking aspirin or Plavix®.
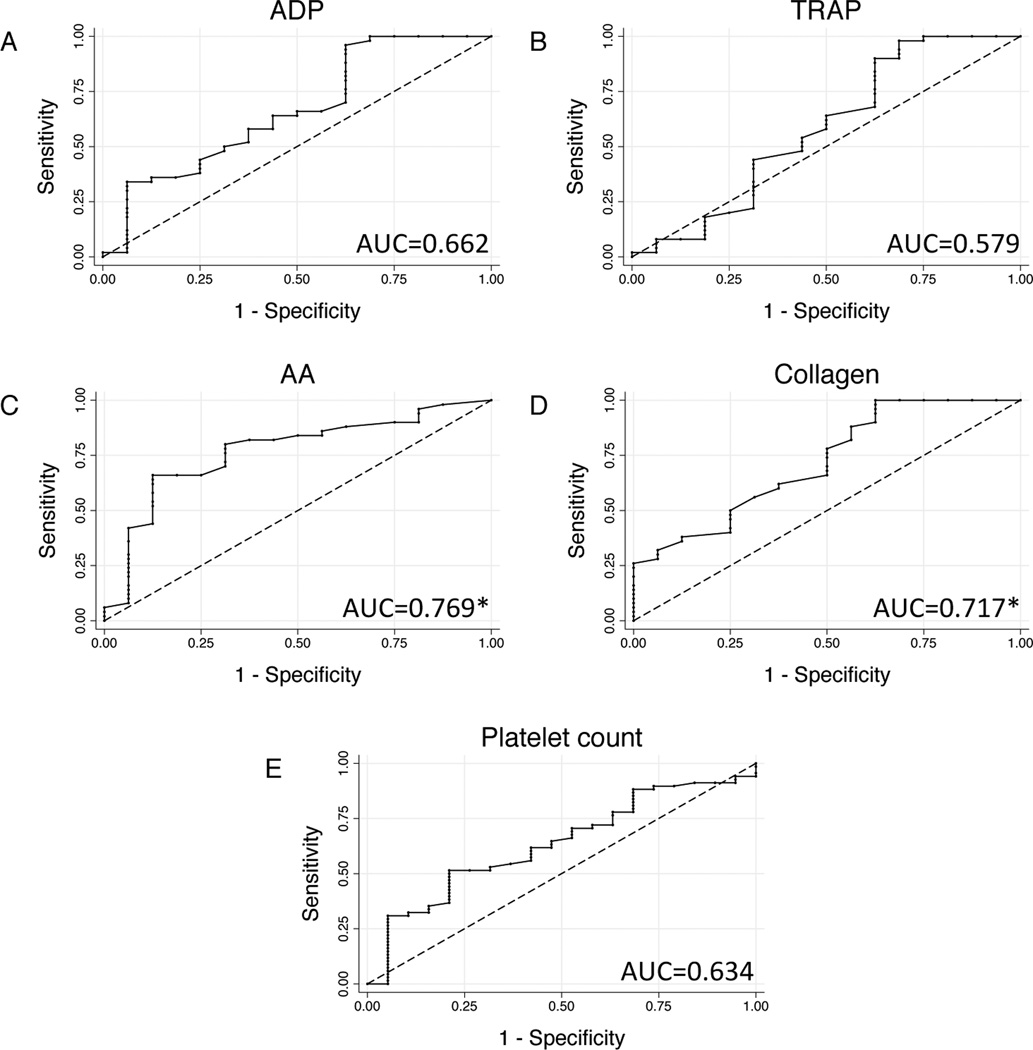
In order to establish the utility of admission platelet function testing, non-parametric ROC analysis was used to identify clinically relevant cut-off values for admission agonist responsiveness as predictors of in-hospital mortality. Based on ROC curves (Fig 4), AA (area under the curve 0.769) and collagen responses (area under the curve 0.717) were robust predictors of mortality; in contrast, admission ADP response, TRAP response, and platelet count did not statistically differ from chance. Specifically, an admission AA response ≥35 U had 80.0% sensitivity and 68.8% specificity for in-hospital mortality (negative likelihood ratio 0.291), correctly classifying mortality in 77.3% of patients; admission collagen response ≥20 U had 96.0% sensitivity and 37.5% specificity (negative likelihood ratio 0.107), correctly classifying 81.8% of patients.
Figure 4.
Receiver-operator characteristic curves using admission platelet responsiveness to adenosine (ADP; a), thrombin receptor-activating peptide (TRAP; b), arachidonic acid (AA; c), and collagen (d) as predictors of in-mortality. Curve for admission platelet count (e) is shown for comparison. Area under the receiver-operator characteristic curve (AUC) values given at upper left in each graph. *AUCs for which 95% confidence intervals differ significantly from chance.
DISCUSSION
Here we report a prospective, impedance aggregometry-based analysis of platelet dysfunction after trauma. Using the Multiplate® multiple electrode aggregometer, we serially assayed platelet activation to ADP, TRAP, AA, and collagen in 101 critically-injured patients on arrival and then serially for the remainder of their ICU stay. Despite uniformly normal admission platelet counts, platelet hypofunction was strikingly common, occurring in 45.5% of patients on admission and 91.1% at some time during their ICU stay; mean responsiveness to some agonists remained abnormal for up to 120h after admission. We identified severe base deficit and low GCS as multivariate predictors of admission platelet hypofunction. Using Cox proportional hazards regression, we demonstrated that admission platelet hyporesponsiveness to TRAP, AA, and collagen were independent predictors of mortality when adjusted for other patient and injury characteristics. Using ROC analysis to identify the most informative agonist responses, admission AA and collagen were found to be significant predictors of in-hospital mortality.
Platelet dysfunction after trauma has been systematically described in only three other studies. Jacoby et al. used the PFA-100 platelet function analyzer (which measures shear-induced occlusion of an aperture in an agonist-impregnated cartridge) and flow cytometric markers of platelet activation (platelet microparticles, P-selection, and activated glycoprotein IIb/IIIa) to prospectively assess platelet function in 100 trauma patients.10 In this study, significantly impaired collagen/epinephrine closure times were observed in 6 non-survivors at later time point, although admission values were statistically similar to 94 survivors; similarly, closure times were impaired in 22 patients with significant head injury (AIS-head score ≥4) compared to 78 patients without head injury at 24h, with no difference on admission. This parallels our finding that platelet dysfunction is associated with mortality, and that brain injury is a significant predictor of platelet dysfunction. This study found no differences in non-survivors or brain-injured patients on admission based on aperture closure time, although platelet microparticle levels were significantly higher on admission in these populations; this indicates that alterations in platelet function present on arrival are not reliably detected by PFA-100 aggregation. We here identify that impedance aggregometry is sensitive to these early differences, and that poor admission AA- and collagen-induced responsiveness are associated with later mortality. Impedance aggregometry appears superior to PFA-100 aggregation in identifying platelet dysfunction on arrival, potentially allowing better triage and earlier targeted therapy.
Solomon et al. recently reported a retrospective study of impedance aggregometry responses to ADP, TRAP, and collagen on admission in 163 trauma patients.9 The incidence of platelet hypofunction in their study was notably lower than that reported here. They found platelet hyporesponsiveness to ADP in 13.9% of patients, TRAP in 13.7%, and collagen in 5.6%: comparatively, we identified hyporesponsiveness to ADP in 30.7%, TRAP in 18.7%, and collagen in 34.7%. Similarly, they identified only weak correlation between platelet count and agonist responsiveness, but did not report statistical significance. Many of these differences are likely attributable to differences in study population: their population had median ISS of 18 and overall mortality of 12.3%, compared to our median ISS of 25 and mortality rate of 22.7%. As no adjusted analysis was performed, the absence of additional findings may be due to the predominance of milder injury in their population. Their finding that ADP and TRAP responses were significantly lower in 7 patients with ISS ≥50 compared to 113 patients with ISS ≤25 supports this explanation. Despite these differences, the association of platelet dysfunction with mortality in their study parallels the unadjusted and multivariate analysis presented here.
Nekludov et al. reported a smaller experience using thromboelastography (TEG®)-based platelet mapping™ to evaluate platelet response to ADP and AA in 30 trauma patients compared to controls.11 TEG®-based platelet mapping™ measures the maximal amplitude of clot formation in heparin- and reptilase-treated whole blood in response to ADP and AA, comparing it to kaolin-activated maximal amplitude to generate an agonist-specific percentage of platelet inhibition.12 The authors found significantly impaired AA responsiveness in brain-injured patients compared to trauma patients without brain injury as well as controls, but were unable to detect platelet dysfunction in 10 trauma patients without brain injury. While platelet mapping has been correlated with light aggregometry results,13 to our knowledge no direct comparison of TEG®-based platelet mapping™ and impedance aggregometry exists to facilitate comparison of results. However, while we describe broader impaired AA responsiveness in patients with and without brain injury, our finding that low admission GCS is a multivariate predictor of platelet hypofunction parallels the findings of Nekludov et al, pointing to important associations between platelet dysfunction and brain injury. These data have clear clinical implications for identifying patients at risk for intracranial hemorrhage progression.
The mechanisms underlying trauma-associated platelet dysfunction are poorly understood. One potential mechanism is suggested by Jacoby and colleagues, who identified that flow cytometric markers of platelet activation were elevated in trauma patients, despite impaired functional aperture closure times.10 In non-trauma studies, prolonged circulation of activated but hypofunctional platelets has been observed for up to 96 hours after activation.14 These data mirror our finding that platelet function falls within 6h of admission, and remains suppressed for up to 120h after injury. Taken together, this suggests that immediate platelet activation in response to tissue injury may induce a prolonged refractory state, in which a fraction of activated platelets remain in circulation but are dysfunctional. In light of the critical role played by platelets in the cell-based model of coagulation, this platelet hypofunction may correlate with functionally impaired thrombin generation even in the absence of classical explanations for coagulopathy (such as clotting factor depletion or hyperfibrinolysis), or may partially mediate the effects of hypothermia, hemodilution, and acidosis on clot formation. The ability to identify this state and assess the impact of targeted therapies would allow better guidance for the conduct of resuscitation and operative intervention.
Several limitations exist that are important for interpretation of this study. Similar to other platelet function studies, ours remains an initial, single-center experience; further work is needed to confirm and extend these findings. Although previous studies have cross-validated impedance aggregometry with several other assays of platelet function,5, 7, 8 these studies were performed in healthy controls or were designed to detect antiplatelet medication effects. The ‘normal’ ranges derived from these studies may not be ideal measures of platelet hypofunction in the setting of trauma. Point of care instrument use in a busy trauma center poses additional challenges in sample handling and evaluation of results that need to be addressed before these results be clinically applied. Finally, although physiological relevance is suggested by prospective correlation of platelet function with later stent thrombosis in the cardiovascular literature,15 further study is required to confirm that platelet aggregation in a laboratory test cell is a meaningful surrogate for hemostatic function in the bleeding trauma patient.
These results highlight two important clinical issues. First, while impairment of platelet response to ADP and AA have been characterized in response to clopidogrel and aspirin, respectively,16 there is no a priori sense of which agonists are relevant in the setting of trauma. Given the wide availability of over-the-counter medications with antiplatelet effects and the known variability of platelet function in the population at large,17 one could posit that the dysfunction of AA and collagen pathways seen here may be the result of an occult medication-related effect, as opposed to a trauma-related phenomenon. However, the observed hyporesponsiveness to TRAP argues that this platelet dysfunction is related to injury, as neither thrombin generation nor platelet activation downstream of thrombin receptors are affected by COX-pathway blockade.18 The data presented here provide evidence for a specific platelet dysfunction induced by traumatic injury, but careful additional in vitro and clinical characterization are required to elucidate the mechanism and to validate trauma-relevant agonists, ‘normal’ ranges, and indications for clinical action.
Second, while we clearly identify the grave prognosis associated with platelet hypofunction, few therapeutic options exist to address it, calling into question the clinical utility of identifying an untreatable pathology. Studies of platelet function-targeted therapy have been impeded by a lack of well-validated assays with which to demonstrate efficacy. However, the recent development of new functional analyzers has fostered a growing literature on several potential pro-platelet therapies, including studies of desmopressin19 and tranexamic acid20 in reversing platelet dysfunction in cardiac surgical patients. Ongoing in vitro aggregometry studies and further prospective clinical studies will provide a platform for the evaluation of novel pro-platelet agents, adding to the clinical armamentarium for treatment of trauma-associated platelet dysfunction.
Here we demonstrate that clinically significant platelet dysfunction after trauma exists in the presence of an otherwise reassuring platelet count and clotting studies, with profound implications for mortality. Impedance aggregometry reliably identifies this dysfunction in injured patients, and admission AA and collagen responses are significant predictors of both early and late mortality. The significance of low GCS as an independent predictor of platelet hypofunction highlights the importance of further investigation into the link between traumatic brain injury and platelet dysfunction. The clinical availability of rapid, point-of-care platelet function testing will lead to improved triage, more appropriately targeted therapy, and better outcomes after trauma.
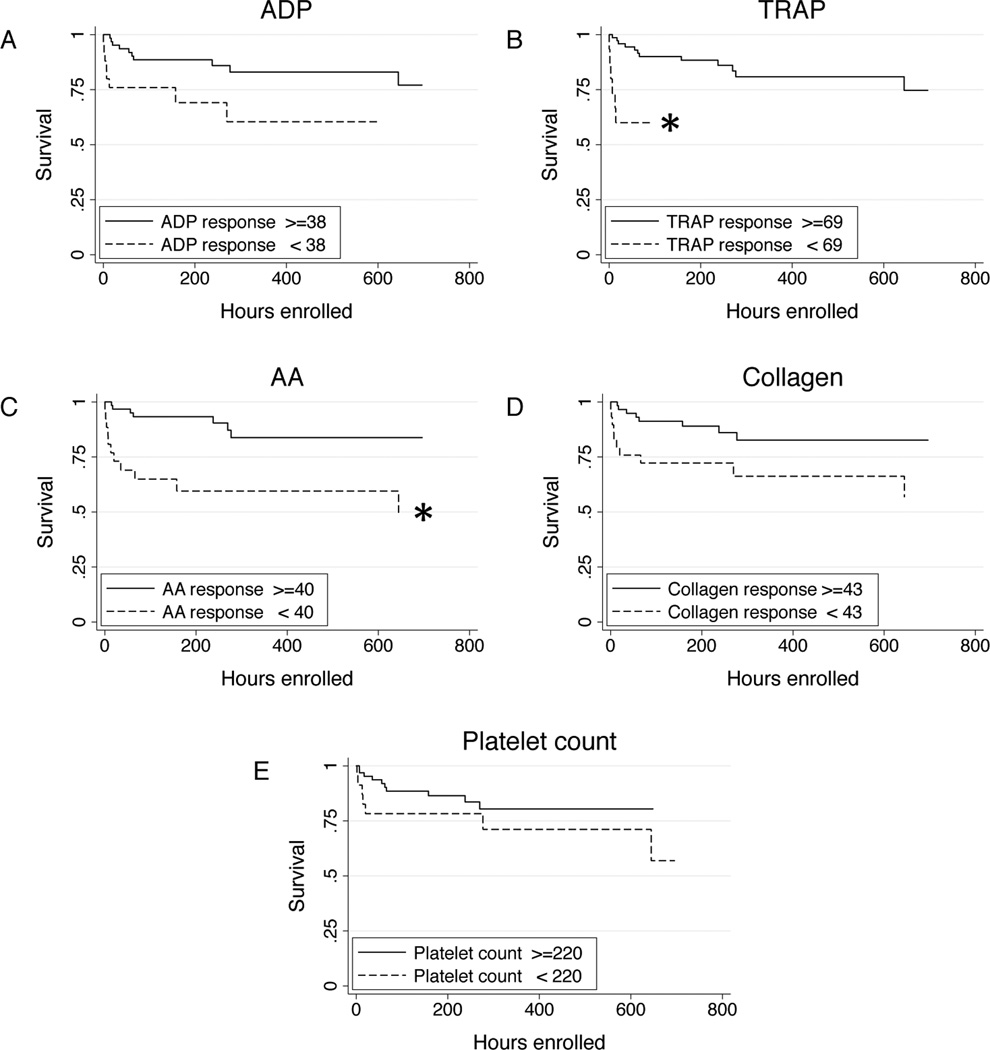
Figure 3.
Kaplan-Meier 30-day survival curves showing survival differences between patients with below-normal admission platelet responsiveness to adenosine diphosphate (ADP; a), thrombin receptor-activating peptide (TRAP; b), arachidonic acid (AA; c), and collagen (d). Survival curves for patient admission platelet counts below the 25th percentile (e) are shown for comparison. *p < 0.05 by log-rank test.
ACKNOWLEDGEMENTS
Funding: Supported in part by NIH T32 GM-08258-20 (MEK) and NIH GM-085689 (MJC)
The authors acknowledge technical support from the Multiplate® instrument distributor (DiaPharma Group, Inc.; West Chester, OH) and the helpful technical assistance of Pamela Rahn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The Multiplate® device was loaned and reagents provided by the distributor (DiaPharma Group, Inc; West Chester, OH) for this investigator-initiated study. There are no direct financial relationships between the authors and manufacturer.
Meetings: None
AUTHOR CONTRIBUTIONS
MEK and MJC prepared the manuscript, performed all data analysis, and take full responsibility for the data as presented. BJR and MFN made significant contributions to study design and implementation. RCM, IMC, MDG, and LAC performed all clinical data collection.
Contributor Information
Matthew E Kutcher, Email: matthew.kutcher@ucsfmedctr.org.
Brittney J Redick, Email: redickb@sfghsurg.ucsf.edu.
Ryan C McCreery, Email: mccreeryr@sfghsurg.ucsf.edu.
Ian M Crane, Email: cranei@sfghsurg.ucsf.edu.
Molly D Greenberg, Email: greenbergm@sfghsurg.ucsf.edu.
Leslie M Cachola, Email: cacholal@sfghsurg.ucsf.edu.
Mary F Nelson, Email: nelsonm@sfghsurg.ucsf.edu.
Mitchell Jay Cohen, Email: mcohen@sfghsurg.ucsf.edu.
REFERENCES
- 1.Davenport RA, Brohi K. Coagulopathy in trauma patients: importance of thrombocyte function? Curr Opin Anaesthesiol. 2009;22(2):261–266. doi: 10.1097/ACO.0b013e328325a6d9. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Call MS, Margaret Knudson M, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 Suppl 3):S337–S342. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnuriger B, Inaba K, Abdelsayed GA, et al. The impact of platelets on the progression of traumatic intracranial hemorrhage. J Trauma. 2010;68(4):881–885. doi: 10.1097/TA.0b013e3181d3cc58. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 5.Toth O, Calatzis A, Penz S, et al. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96(6):781–788. [PubMed] [Google Scholar]
- 6.Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3(2):135–158. doi: 10.1016/0160-5402(80)90024-8. [DOI] [PubMed] [Google Scholar]
- 7.Seyfert UT, Haubelt H, Vogt A, et al. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18(3):199–206. doi: 10.1080/09537100600944277. [DOI] [PubMed] [Google Scholar]
- 8.Siller-Matula JM, Gouya G, Wolzt M, et al. Cross validation of the Multiple Electrode Aggregometry. A prospective trial in healthy volunteers. Thromb Haemost. 2009;102(2):397–403. doi: 10.1160/TH08-10-0669. [DOI] [PubMed] [Google Scholar]
- 9.Solomon C, Traintinger S, Ziegler B, et al. Platelet function following trauma. A Multiple Electrode Aggregometry study. Thromb Haemost. 2011;106(2):322–330. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby RC, Owings JT, Holmes J, et al. Platelet activation and function after trauma. J Trauma. 2001;51(4):639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nekludov M, Bellander BM, Blomback M, et al. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007;24(11):1699–1706. doi: 10.1089/neu.2007.0322. [DOI] [PubMed] [Google Scholar]
- 12.Bochsen L, Wiinberg B, Kjelgaard-Hansen M, et al. Evaluation of the TEG platelet mapping assay in blood donors. Thromb J. 2007;5:3. doi: 10.1186/1477-9560-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft RM, Chavez JJ, Bresee SJ, et al. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J Lab Clin Med. 2004;143(5):301–309. doi: 10.1016/j.lab.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Wun T, Paglieroni T, Holland P. Prolonged circulation of activated platelets following plasmapheresis. J Clin Apher. 1994;9(1):10–16. doi: 10.1002/jca.2920090104. [DOI] [PubMed] [Google Scholar]
- 15.Sibbing D, Braun S, Morath T, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53(10):849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Maridakis V, O'Neill EA, et al. A randomized clinical trial comparing point-of-care platelet function assays and bleeding time in healthy subjects treated with aspirin or clopidogrel. Platelets. 2011 doi: 10.3109/09537104.2011.604806. [DOI] [PubMed] [Google Scholar]
- 17.Can MM, Tanboga IH, Turkyilmaz E, et al. The risk of false results in the assessment of platelet function in the absence of antiplatelet medication: comparison of the PFA-100, multiplate electrical impedance aggregometry and verify now assays. Thromb Res. 2010;125(4):e132–e137. doi: 10.1016/j.thromres.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong PC, Truss NJ, Ali FY, et al. Aspirin and the in vitro linear relationship between thromboxane A2-mediated platelet aggregation and platelet production of thromboxane A2. J Thromb Haemost. 2008;6(11):1933–1943. doi: 10.1111/j.1538-7836.2008.03133.x. [DOI] [PubMed] [Google Scholar]
- 19.Weber CF, Dietrich W, Spannagl M, et al. A point-of-care assessment of the effects of desmopressin on impaired platelet function using multiple electrode whole-blood aggregometry in patients after cardiac surgery. Anesth Analg. 2010;110(3):702–707. doi: 10.1213/ANE.0b013e3181c92a5c. [DOI] [PubMed] [Google Scholar]
- 20.Weber CF, Gorlinger K, Byhahn C, et al. Tranexamic acid partially improves platelet function in patients treated with dual antiplatelet therapy. Eur J Anaesthesiol. 2011;28(1):57–62. doi: 10.1097/EJA.0b013e32834050ab. [DOI] [PubMed] [Google Scholar]