Abstract
Context:
Evidence suggests that a number of microRNA (miRNA) are aberrantly expressed in endometrial disorders with potential posttranscriptional regulation of their specific target genes, including ovarian steroid receptors.
Objectives:
Our objective was to assess the endometrial expression of miR-98 and miR-181a and their respective target genes, progesterone (P4) receptor membrane component 1 (PGRMC1) and P4 receptor (PGR).
Design, Setting, and Patients:
We evaluated tissue expression and in vitro regulation at an academic university medical center in endometrial biopsies and endometrial tissues from follicular and luteal phases with and without exposure to hormonal therapies and grade I–III endometrial cancer (n = 52).
Interventions:
Interventions included endometrial biopsies and in vitro transfection.
Main Outcome Measures:
We evaluated expression and function of miR-98 and miR-181a.
Results:
Aberrant expression of miR-98 and miR-181a is associated with endometrial transition from normal into cancerous states, which to some extent is influenced by hormonal milieu, and exhibited an inverse relationship with PGMRC1 and PGR expression, respectively. Treatments of Ishikawa cells with 17β-estradiol, P4, or medroxyprogesterone acetate had limited effects on miR-98, miR-181a, and PGRMC1 expression, whereas 17β-estradiol treatment increased PGR expression. In Ishikawa cells, gain of function of miR-98 repressed PGRMC1 and CYP19A1, and miR-181a repressed PGR, DDX3X, and TIMP3 at mRNA and protein levels through direct interactions with their respective 3′-untranslated regions and CCNE1 through miR-181a-induced DDX3X repression, with miR-98 reducing the rate of cell proliferation as compared with controls.
Conclusion:
miR-98 and miR-181a through their regulatory functions on PGRMC1, PGR, CYP19A1, TIMP3, and DDX3X expression may influence a wide range of endometrial cellular activities during normal menstrual cycle and transition into disease states, including endometrial cancer.
MicroRNA (miRNA) have emerged as key posttranscriptional regulators with an estimated 30–50% of protein-coding genes as their potential targets (1–3) regulating a wide range of normal cellular activities, including cell growth, differentiation, apoptosis, inflammation, and tissue turnover (1–5). Conversely, aberrant expression of a number of miRNA have been closely associated with various disorders, specifically cancers, whereby acting as oncogenes and/or tumor suppressors, they mediate malignant cellular transformation and tumorigenesis (1–7). Expression profiling and next-generation sequencing have also identified the expression of a large number of miRNA in human endometrium, with altered expression in endometriosis and endometrial cancer (8–12).
Human endometrium undergoes dynamic cyclic changes throughout the reproductive years under the control of ovarian steroids; however, decline in their biosynthesis after menopause or exposure to excess estrogens causes cellular and molecular changes resulting in endometrial atrophy or pathological disorders such as endometriosis and endometrial cancer (13–21). Ovarian steroids mediate their actions through estrogen receptors (ESR) and progesterone (P4) receptor (PGR) isoforms, whose expression is highly regulated at transcriptional and translational levels and undergo cyclic variations in the endometrium during normal and disease states (14, 20, 21). The ligand-activated ESR and PGR through genomic pathways that involve interactions with their respective response elements on the promoter of specific target genes or interaction with other transcription factors, as well as nongenomic pathways, regulate the expression of many genes to implement their diverse biological actions (22–26).
Considerable evidence exists regarding the role of P4 and the stability of PGR isoform expression in genesis of endometrial disorders, specifically endometrial cancer where a decline in relative expression of PGR has been associated with poorly differentiated tumors (20, 27–31). Several miRNA are predicted to target the stability of PGR expression, and to date, miR-181a has been experimentally validated to target PGR expression in MCF7 breast cancer cell line (7). Additionally, let7i/miR-98 has been identified to target the expression of PGR membrane component 1 (PGRMC1), in SKOV-3, an ovarian cancer cell line (32). As a component of a multiprotein complex, PGRMC1 has been considered to mediate the nongenomic action of P4, and its expression has been demonstrated in a number of cells and tissues, including uterus, placenta, ovary, and ovarian cancer (33, 34). Although miR-98 and miR-181a have been experimentally validated to target the expression of PGRMC1 and PGR, respectively (7, 32), evidence suggests that miRNA expression and regulation of their target genes occurs in a cell- and tissue-specific manner (35, 36). As such and due to limited information regarding the regulatory function of miRNA on their target genes in the endometrium, the objective of this study was to investigate the temporal expression of miR-98 and miR-181a in normal endometrium biopsies from throughout the menstrual cycle, endometrial tissues from the follicular and luteal phase of the menstrual cycle, including those who were exposed to hormonal therapy, and peri-postmenopausal period as well as endometrial cancer at different stages of the disease. Using Ishikawa cells as an in vitro model, we also assessed the hormonal regulation of miR-98 and miR-181a and validated PGRMC1 and PGR as well as cytochrome P450 aromatase (CYP19A1), tissue inhibitor of matrix metalloproteinase 3 (TIMP3), and DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, X-linked (DDX3X) as their direct targets and their influence on the rate of cell proliferation.
Materials and Methods
Tissue collection
A total of 52 endometrial tissues of normal biopsies (n = 15) and endometrial tissues from women undergoing hysterectomies for benign (n = 20) and endometrial cancer (n = 17) were used in this study. The endometrial biopsies were collected with the aid of a suction curette (Pipelle; Laboratorie CCD, Paris, France) from women with ages ranging from 22–37 yr who were requesting permanent surgical sterilization (tubal ligation) under informed consent approved by the University of Florida Institutional Review Board previously. All women reported normal menstrual cycles and were not taking any hormonal medications for the last 3 months before collection of the biopsies and based on their last menstrual period were from midproliferative (n = 2), late-proliferative (n = 1), early-secretory (n = 5), midsecretory (n = 3), and late-secretory (n = 4) phases of the menstrual cycle. Endometrial tissues were collected from women with ages ranging from 23–67 yr (median = 42.5 yr) undergoing hysterectomy for symptomatic leiomyomas, pelvic pain, or pelvic organ prolapse at the University of Florida-affiliated Shands Hospital. Based on patients' last menstrual period, the endometrial tissues were from follicular (n = 3) and luteal (n = 5) phases of the menstrual cycle and peri-postmenopausal period (n = 5) and from patients who were exposed to hormonal therapies, including GnRH agonist (GnRHa) (n = 2) and Depo-Provera (n = 5). Endometrial tumors were obtained from the University of Florida Tissue Bank and, based on their histological typing/surgical staging according to International Federation of Gynecology and Obstetrics, the tumors were from grade I (n = 4), II (n = 9), and III (n = 4). The patients' ages ranged from 40–90 yr (median = 65 yr). All the endometrial tissues and endometrial tumors were collected with previous approval from the Institutional Review Board. After collection, the endometrial tissue samples were snapped frozen and kept in liquid nitrogen until further analysis.
RNA isolation and analysis
Total RNA was extracted from a small portion of the endometrial tissues using Trizol (Invitrogen, Carlsbad, CA), and their quantity and quality were assessed using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Two micrograms (for mRNA) or 10 ng (for miRNA) of total RNA was reverse transcribed using random primers or specific stem-loop primers for miRNA expression (Applied Biosystems, Foster City, CA). Real-time PCR was carried out using TaqMan reagents. Reactions were incubated for 10 min at 95 C followed by 40 cycles of 15 sec at 95 C and 1 min at 60 C, and the level of mRNA and miRNA expression was determined using the Applied Biosystems 7300 Detection System with 18S rRNA and RNU6B used for normalization, respectively. All reactions were run in triplicate, and relative expression was analyzed with comparative cycle threshold method (2−ΔΔCT) according to the manufacturer's guidelines.
Cell culture and transfection
Ishikawa cells were cultured in DMEM/F12 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum and 1% (vol/vol) of antibiotic and antimycotic solution. All the supplies for cell culture were purchased from Sigma-Aldrich (St. Louis, MO), Invitrogen, and Fisher Scientific (Pittsburgh, PA). The cells were seeded at various densities in six-well plates or 10-cm petri dishes and cultured in antibiotic/antimycotic-free media until reaching 70% confluence and then transfected with 50 nm pre-miR-98 and pre-miR-181a or pre-miR negative control 1 (PreNC) (Ambion/Applied Biosystems, Foster City, CA) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. After transfection, the cells were cultured for an additional 96 h, and total RNA and protein were isolated and subjected to real-time PCR and Western blot analysis.
Luciferase reporter assay
Ishikawa cells were seeded in six-well plates and transfected with pre-miR-98, pre-miR-181a, or PreNC as described above. After 24 h, the medium was changed and cells were transfected with dual luciferase reporter plasmid containing 3′-untranslated region (UTR) of PGR (a gift from Dr. Vagner) (7), TIMP3 (a gift from Dr. Ghoshal) (37), and PGRMC1 and DDX3X (Genecopoeia, Inc., Rockville, MD) using Lipofectamine 2000. Because the PGR 3′-UTR reporter vector and TIMP3 3′-UTR reporter vector did not contain the renilla luciferase (pRL) gene, they were cotransfected with pRL-TK plasmid (Promega, Madison, WI). Firefly and renilla luciferase activities were assayed after 48 h of transfection using a dual-luciferase reporter assay system (Promega) with some modification according to recommendation from GeneCopoeia. Relative luciferase activities were calculated by normalization of firefly luciferase activity to renilla luciferase activity to correct for transfection efficiencies in each sample, and the level of induction was compared with cells transfected with PreNC.
Western blot analysis
Total protein was isolated from Ishikawa cells, and after centrifugation, supernatants were collected and protein concentrations were determined by standard method (Pierce BCA protein assay kit; Fisher). Aliquots of 30 μg total protein were subjected to Western blot analysis and transferred to polyvinylidene difluoride membrane. The membranes were probed with primary antibodies (dilution 1:200) generated against PGRMC1, PGR, TIMP3, DDX3X, CYP19A1, and CCNE1 (Sigma Chemical Co., St. Louis, MO; Cell Signaling Technology, Danvers, MA; and Abcam Inc., San Francisco, CA) and α-tubulin (dilution 1:2000) was used for normalization and loading control. The membranes were exposed to horseradish peroxidase-labeled secondary antiserum (Santa Cruz Biotechnology, Santa Cruz, CA), and immunoreactive proteins were detected with the ECL Western blotting substrate (Fisher, Thermo Scientific, Inc., Waltham, MA).
Ovarian steroid treatments
Ishikawa cells were cultured in six-well plates as above for 24 h, washed, and incubated in phenol red-free media with charcoal-stripped fetal bovine serum for an additional 24 h and then treated with 17β-estradiol (E2), P4, or medroxyprogesterone acetate (MPA) (Sigma) at 10−8 m concentration for 6, 24, and 48 h. Total RNA was isolated and subjected to quantitative RT-PCR as described above.
Cell proliferation assay
Ishikawa cells were transfected with pre-miR-181a, pre-miR-98, or PreNC, and the rate of their proliferation was determined 1–3 d after transfection, using CellTiter 96 cell proliferation assay (Promega) according to the manufacturer's instructions.
Statistical analysis
All the real-time PCR were carried out in triplicate. The in vitro experiments were performed in three independent cell cultures in duplicate. Where appropriate, the results are expressed as mean ± sem and statistically analyzed using either nonparametric Student's t test for two-group comparisons or ANOVA with post hoc test for multiple comparisons, with P < 0.05 considered significant.
Results
miR-98 and miR-181a are aberrantly expressed in endometrial disorder
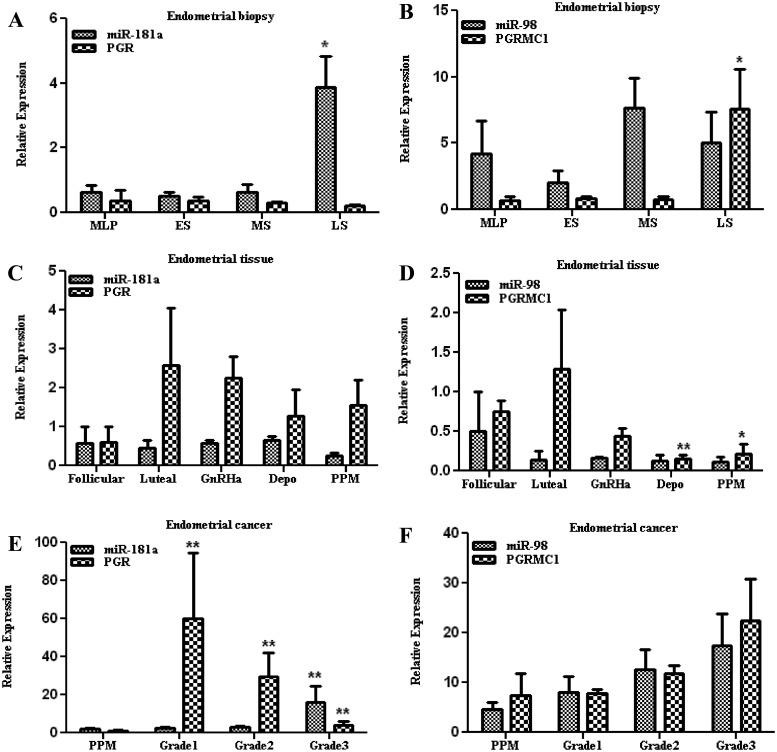
We first determined the temporal expression of miR-98 and miR-181a as well as their respective targets, PGRMC1 and PGR, in endometrial biopsies from throughout the normal menstrual cycle and endometrial tissues from follicular and luteal phases of the menstrual cycle and peri-postmenopausal period and from women who were exposed to GnRHa and Depo-Provera as well as grade I–III endometrial cancer. As shown in Fig. 1, the relative level of miR-98, miR-181a, and PGR expression was lower and did not significantly change in endometrial biopsies from throughout the menstrual cycle, with the exception of increased PGRMC1 expression in the late-secretory phase compared with other stages (Fig. 1, A and B). Endometrial tissues from follicular and luteal phases and peri-postmenopausal period and those who were exposed to GnRHa and Depo-Provera expressed similar levels of miR-181a as detected in the endometrial biopsies; however, these levels were to some extent inversely related with the expression of PGR, which was expressed at higher levels (Fig. 1C). In contrast, miR-98 was expressed at lower levels in endometrial tissues from follicular and luteal phases and peri-postmenopausal period and specifically in tissues exposed to GnRHa and Depo-Provera; however, these levels did not correlate with PGRMC1, whose expression did not change compared with endometrial biopsies (Fig. 1D). In endometrial tumors, the expression of miR-181a increased from grade I to grade III compared with the peri-postmenopausal period, with a concurrent decline in PGR expression that was inversely related with the expression of miR-181a (Fig. 1E). In contrast, the expression of miR-98 and PGRMC1 increased in all tumor grades without any correlation in their expression (Fig. 1F). The results indicated that miR-98, miR-181a, and their target genes PGMRC1 and PGR are coexpressed with a possible influence of hormonal milieu and disease state on their expression.
Fig. 1.
Relative expression of miR-98, miR-181a, PGR, and PGRMC1 in endometrial biopsies (A and B) from mid-late proliferative (MLP), early-secretory (ES), midsecretory (MS), and late-secretory (LS) phases of the menstrual cycle and endometrial tissues (C and D) from follicular and luteal phases of the menstrual cycle and peri-postmenopausal period (PPM) from women who were exposed to GnRHa and Depo-Provera (Depo) as well as endometrial cancer (E and F) grades I, II, and III. During the calculation of expression value, one of the samples from MLP, follicular phase, and PPM was taken as control for endometrial biopsies, endometrial tissues, and endometrial cancer, respectively. The values are reported as mean ± sem and analyzed using nonparametric t test and ANOVA. *, P < 0.05; **, P < 0.005, indicating significant difference from the control.
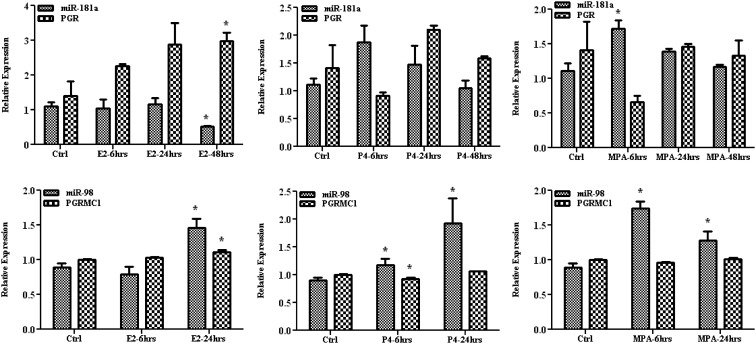
PGR, miR-98, and miR-181a are hormonally regulated
We next analyzed the influence of ovarian steroids on the expression of miR-98 and miR-181a using the Ishikawa cell line as an in vitro model. As shown in Fig. 2, treatments with E2, P4, and MPA did not significantly alter the expression of miR-181a and PGR, with the exception of a significant time-dependent inhibition of miR-181a and an increase in PGR expression by E2 treatment (P < 0.05). The expression of miR-98 was moderately increased by E2 and P4 treatments after 24 h and by MPA after 6 h; however, these treatments did not significantly alter the expression of PGRMC1 at the time points examined (Figs. 2). The results suggest that the expression of mature miR-98 and miR-181a may be influenced by hormonal milieu, possibly mediated through a mechanism previously reported in a breast cancer cell line (38).
Fig. 2.
The effect of E2, P4, and MPA on the expression of miR-98, miR-181a, PGR, and PGRMC1 in Ishikawa cells compared with untreated control [control (Ctrl)], after 6, 24, and 48 h of treatments as determined by quantitative RT-PCR. The results are presented as mean ± sem of three independent experiments and analyzed using nonparametric Student's t test. *, P < 0.05, indicating significantly different from untreated control.
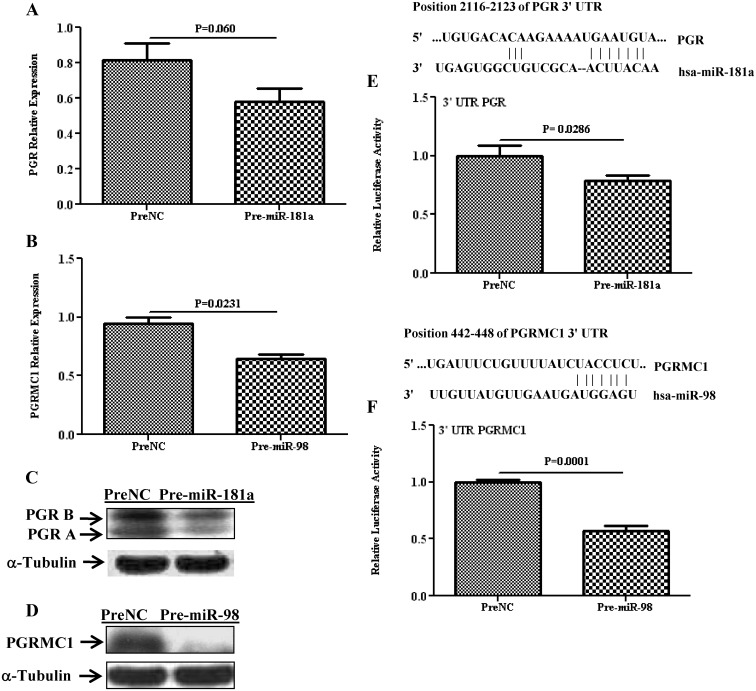
PGR and PGRMC1 are direct targets of miR-181a and miR-98
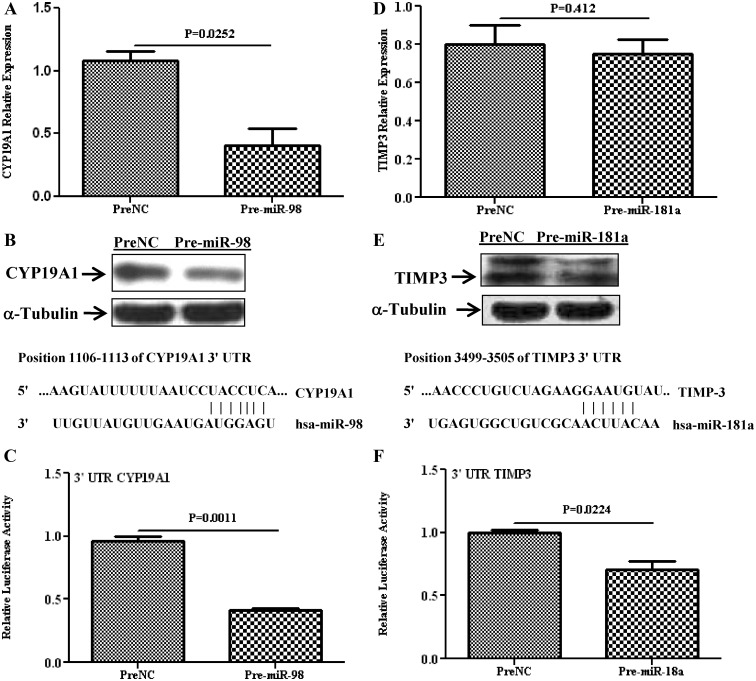
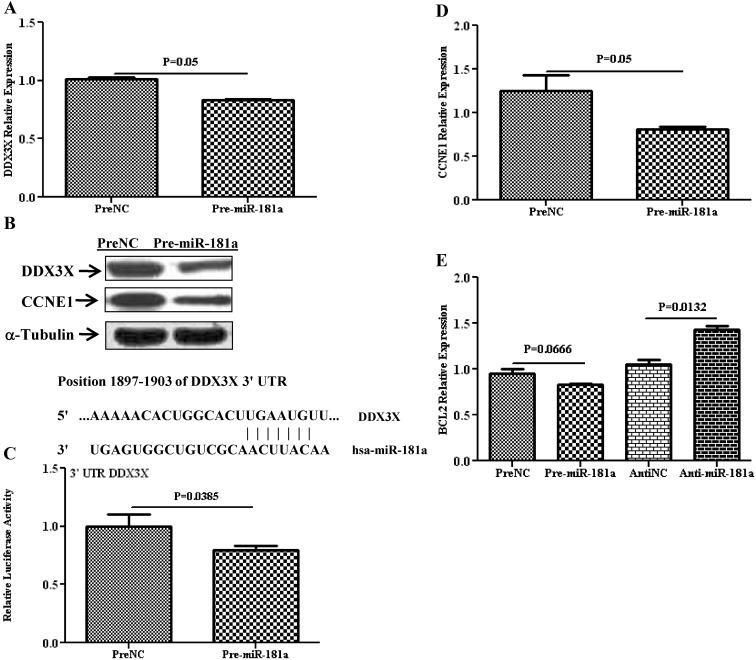
Several hundred genes have been predicted as targets of miR-181a and miR-98, including PGR and PGRMC1, whose expression has been experimentally validated as direct targets of miR-181a, and miR-98 in MCF7 and SKOV-3 cell lines, respectively. However, miRNA expression and regulatory function of their target genes has been shown to occur in a cell- and tissue-specific manner. As such, using Ishikawa cells, we showed that gain of function of miR-181a and miR-98 repressed the expression of PGR (P = 0.06; Fig. 3A) and PGRMC1(P = 0.023; Fig. 3B), respectively, at the transcriptional and translational level (Figs. 3, C and D). The regulatory function of miR-181a and miR-98 on PGR and PGRMC1 expression occurred through direct interaction with their respective 3′-UTR as demonstrated by luciferase reporter assay (P = 0.028 and 0.0001; Fig. 3, E and F). Gain of function of miR-98 decreased the level of CYP19A1 at the mRNA (P = 0.025; Fig. 4A) and protein (Fig. 4B) level and was validated as the direct target of miR-98 (P = 0.001; Fig. 4C). Similarly, gain of function of miR-181a repressed the expression of TIMP3 (P = 0.412; Fig. 4D) and DDX3X (P = 0.05; Fig. 5A) at the mRNA and protein level (Figs. 4E and 5B) through direct interactions with their respective 3′-UTR as revealed by luciferase reporter assay (P = 0.022 and 0.038, Figs. 4F and 5C).
Fig. 3.
Relative expression of PGR and PGRMC1 in Ishikawa cells after overexpression of miR-181a and miR-98 (pre-miR-transfected) as compared with cells transfected with PreNC determined by quantitative RT-PCR (A and B) and Western blot analysis (C and D). Firefly luciferase assay with pGL3-TK modified construct and pZEX-MT01 construct carrying a 3′-UTR fragment of PGR (E) and PGRMC1 (F), respectively, in which Ishikawa cells were cotransfected with, respectively, firefly luciferase construct, pre-miR-181a, or pre-miR-98 or PreNC. The ratio of firefly to renilla was determined and reported as relative luciferase activity and was compared with levels in PreNC, which was set at 1. The results present as mean ± sem of three sets of independent experiments performed in duplicate and analyzed using nonparametric Student's t test with P values presented as indicated by corresponding line. Sequence alignment of miR-181a and miR-98 seed regions and PGR and PGRMC1 mRNA target site at their 3′-UTR with the coordinated positions are shown at the top of each graph.
Fig. 4.
Relative expression of CYP19A1 and TIMP3 in Ishikawa cells after overexpression of miR-98 and miR-181a (pre-miR-transfected), respectively, as compared with PreNC determined by quantitative RT-PCR (A and D) and Western blot analysis (B and E). Firefly luciferase assay with pZEX-MT01 construct and pIS0 construct carrying a 3′-UTR fragment of CYP19A1 (C) and TIMP3 (F), respectively, in Ishikawa cells cotransfected with firefly luciferase construct, pre-miR-98 or pre-miR-181a or preNC. The ratio of firefly to renilla was determined and reported as relative luciferase activity and was compared with levels in PreNC, which was set at 1. The results present mean ± sem of three sets of independent experiments performed in duplicate and analyzed using nonparametric Student's t test with P values presented as indicated by corresponding line. Sequence alignment of miR-181a and miR-98 seed regions and CYP19A1 and TIMP3 mRNA target site at their 3′-UTR with the coordinated positions are shown at the top of each graph.
Fig. 5.
Relative expression of DDX3X and CCNE1 in Ishikawa cells after overexpression of miR-181a (pre-miR-181a), or PreNC determined by quantitative RT-PCR (A and D) and Western blot analysis (B). C, Firefly luciferase assay with pZEX-MT01 construct carrying a 3′-UTR fragments of DDX3X in Ishikawa cells cotransfected with firefly luciferase reporter, pre-miR-181a, or PreNC. The ratio of firefly to renilla was determined and reported as relative luciferase activity compared with empty vector with levels in PreNC independently set at 1. The results present mean ± sem of three sets of independent experiments performed in duplicate and analyzed using nonparametric Student's t test with P values presented as indicated by corresponding line. Sequence alignment of miR-181a seed region and DDX3X mRNA target site at 3′-UTR with the coordinated positions is shown at the top of each graph. E, Relative expression of BCL2 in Ishikawa cells after overexpression of miR-181a (Pre-miR-181a) as compared to its control (PreNC) and depletion of miR-181a (Anti-miR-181a) as compared to its control (AntiNC) by quantitative RT-PCR.
We also found that loss of function of miR-181a in Ishikawa cells increased the BCL2 mRNA expression (P = 0.013; Fig. 5E) whereas gain of function inhibited cyclin E1 (CCNE1) expression at the mRNA (P = 0.05, Figs. 5D) and protein (Fig. 5B) level. BCL2 and CCNE1 are well established as PGR- and DDX3X-regulated genes, respectively, and their expression could be indirectly regulated through miR-181a-induced regression of PGR and DDX3X. miR-181a has also been predicted to target the expression of fms-related tyrosine kinase 1 (FLT1), stanniocalcin 2 (STC2), splicing factor (SF1), and SMAD family member 7 (SMAD7); however, gain of function of miR-181a in Ishikawa cells had a limited effect on their expression, at least at the transcriptional level (data not shown).
Gain of function of miR-98 and miR-181a had a limited effect on cell proliferation
Because miR-98, and miR-181a have been shown to regulate cell proliferation, we examined the consequence of their gain of function on the rate of proliferation of Ishikawa cells. As shown in Supplemental Fig. 1, A and B (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), gain of function of miR-98 decreased (P < 0.05), whereas miR-181a had no significant effect on the rate of cell proliferation after 3 d treatment.
Discussion
Using normal endometrial biopsies from throughout the menstrual cycle and endometrial tissues from benign and cancerous conditions, we demonstrated that miR-181a and miR-98 expression undergoes significant temporal alteration during transition from normal into cancerous states. The expression of miR-181a and miR-98 to some extent exhibited an inverse relationship with the expression of PGR and PGRMC1, respectively, with a significant decline in PGR and a concurrent increase in PGRMC1 expression with advanced stage of endometrial cancer. Although the endometrial expression of PGRMC1 has not been previously reported, the expression of PGR (PGR-A and PGR-B) has been well documented, with a loss of both isoforms linked to early events in endometrial tumor progression, whereas loss of PGR-B is associated with the development of poorly differentiated tumors, through which P4 inhibited the cancer cells' growth and invasiveness (27, 39, 40). However, assessing the functional relevance of miR-98 and miR-181a based on the expression of their target genes at the tissue level is complex, specifically in the endometrium, which undergoes significant molecular and cellular changes throughout the menstrual cycle in response to hormonal milieu and progression into benign and cancerous states. As such, using Ishikawa cells, which has been extensively used as an in vitro model, we validated PGR and PGRMC1 as direct targets of miR-181a and miR-98, respectively, confirming previous results obtained in MCF7 (7) and SKOV-3, an ovarian cancer cell line (32). In addition to miR-98, let7i has also been shown to target PGRMC1 expression, which has one highly and one poorly conserved binding site in its 3′-UTR (32).
The significance of miR-98 and miR-181a expression and regulatory actions on PGRMC1 and PGR expression in the endometrium relates to the essential role of PGR and PGRMC1 in mediating the genomic and nongenomic signaling of P4 (32). More importantly, our observation that CYP19A1 (aromatase) is a direct target of miR-98 further implicates miR-98 regulatory function in the local estrogen biosynthesis, which has been documented in endometrial disorders such as endometriosis and endometrial cancer (13, 41). Furthermore, miR-181a differential regulatory function on PGR isoforms expression, specifically PGR-B, may contribute to the alteration of PGR-A to PGR-B ratios, which is critical in P4-mediated signaling and the progression of endometrial cancer (20, 27–31). Interestingly, gain of function of miR-181a in Ishikawa cells repressed the expression of both PGR isoforms, but more effectively PGR-B as seen on Western blot analysis, whereas in MCF7 cells, miR-181a and miR-26a equally down-regulated both isoforms (7). Although the role of P4 and PGR in pathogenesis of breast cancer is controversial, in endometrial cancer, P4 deficiency has been associated with an increased risk of endometrial cancer (20, 30). In ovariectomized mice, ip injection of Ishikawa cells expressing PGR-A, PGR-B, or both isoforms, only cells expressing PGR-B were found to proliferate and migrate in response to MPA treatment (20, 39, 42, 43). As such, aberrant endometrial expression of miR-181a and miR-98 through their regulatory functions on PGR, CYP19A1, and PGRMC1 expression may play an important role in influencing the balance of E2-induced PGR and PGR-mediated genomic and nongenomic actions, specifically during transition into disease states.
We extended our investigation and provided additional evidence for direct regulatory function of miR-181a on TIMP3 and DDX3X expression and indirectly the expression of CCNE1 through DDX3X regulation. Because TIMP3, DDX3X, and CCNE1 play a central role in regulating matrix metalloproteinase proteolytic activities and cell cycle progression, respectively, their regulation by miR-181a may influence the outcome of endometrial tissue turnover and regeneration during normal and cellular invasion and metastasis in endometrial cancer progression. Although miR-98 and miR-181a has been predicted to target the expression of many genes, only the expression of IL-10; members of the Bcl-2 family Bcl-2-L11/Bim, Mcl-1, and Bcl-2; p27, k-ras, MYC, Fas, and HMGA2; and Src homology 2-containing protein (CIS), a member of the suppressors of cytokine signaling family; and enhancer of Zeste homolog 2 (EZH2) have been experimentally validated (44–52). Our results also indicated that Bcl2, a well-established P4-regulated gene, is regressed after gain of function of miR-181a in Ishikawa cells. Although STC2, SMAD7, FLT1, and SF1 are among some predicted targets of miR-181a, our preliminary results indicate that overexpression of miR-181a did not alter their expression at least at the transcriptional level. In addition to the interaction with 3′-UTR of target genes, a few recent studies have demonstrated the potential interaction of miRNA with coding regions of genes, such as miRNA-181a down-regulation of a number of zinc finger genes due to the presence of multiple binding sites located within their genes (48).
Regarding the influence of hormonal milieu on the expression of miR-98 and miR-181a at the tissue level and/or after treatment of Ishikawa cells with E2, P4, or MPA, we did not observe significant alterations in their expression, in contrast to PGR expression at the tissue level (benign and cancer) and Ishikawa cells treated with E2. However, P4 treatment of SKOV-3 has been reported to decrease PGRMC1 expression, although increasing let-7i (32), with E2 repressing miR-181a expression in MCF7 cells (7). Although E2-induced PGR expression is well established in many steroid-sensitive cells and tissues, including the endometrium and Ishikawa cells, promoters of both PGR isoforms lack a classical estrogen response element (53). In addition, E2 trans-activation of PGR appeared to occur in a cell-line-specific manner, and acting through ESR1 increased PGR-B promoter activity involving the AF1 domain in HeLa and Ishikawa cells, and AF2 in BT20 cells, in contrast to SK-BR-3 cells in which both ESR repressed PGR-B promoter activity, which was enhanced by cotransfection of steroid receptor coactivator 1 (53). These observations as well as other reports suggest that estrogen regulation of miRNA may occur at the level of their biosynthesis rather than maturation (38, 54, 55), implying a need for detailed investigation of the complex regulatory action of ovarian steroids on miRNA expression.
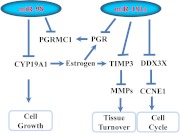
In summary, we demonstrated that miR-98 and miR-181a are expressed in the endometrium during the normal menstrual cycle and altered during transition into disease states, where either directly or through indirect mechanism they target the expression of PGRMC1, PGR, CYP19A1, TIMP3, and DDX3X. Based on these findings, we hypothesize that miR-98 and miR-181a, by regulating the endometrial stability of these and possibly other target genes, play a central regulatory function influencing a wide range of P4-mediated cellular activities during the normal menstrual cycle, menopausal transition, and progression into benign and cancerous states (Fig. 6).
Fig. 6.
Schematic presentation of miR-98 and miR-181a regulatory function on specific target genes, including PGR, PGRMC1, CYP19A1, TIMP3, and DDX3X and the regulatory interactions among their products in endometrial cells. The expression and regulatory interactions among these gene products regulated by miR-98 and miR-181a may influence various cellular activities that result in tissue turnover, cell cycle progression, and growth.
Supplementary Material
Acknowledgments
This work was supported by Grants HD37432 and HD58664 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: There is no potential conflict of interest to disclose.
Footnotes
- CYP19A1
- Cytochrome P450 aromatase
- DDX3X
- DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, X-linked
- E2
- 17β-estradiol
- ESR
- estrogen receptor
- GnRHa
- GnRH agonist
- miRNA
- microRNA
- MPA
- medroxyprogesterone acetate
- PGR
- progesterone receptor
- PGRMC1
- PGR membrane component 1
- PreNC
- pre-miR negative control 1
- TIMP3
- tissue inhibitor of matrix metalloproteinase 3
- UTR
- untranslated region.
References
- 1. Bartel DP. 2009. MicroRNA: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Djuranovic S, Nahvi A, Green R. 2011. A parsimonious model for gene regulation by miRNA. Science 331:550–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fabian MR, Sonenberg N, Filipowicz W. 2010. Regulation of mRNA translation and stability by microRNA. Annu Rev Biochem 79:351–379 [DOI] [PubMed] [Google Scholar]
- 4. Almeida MI, Reis RM, Calin GA. 2011. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat Res 717:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Hagen JW, Lai EC. 2008. microRNA control of cell-cell signaling during development and disease. Cell Cycle 7:2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM. 2008. MicroRNA and their target messenger RNA associated with endometrial carcinogenesis. Gynecol Oncol 110:206–215 [DOI] [PubMed] [Google Scholar]
- 7. Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Bénès V, Roché H, Dalenc F, Auboeuf D, Millevoi S, Vagner S. 2009. Widespread estrogen-dependent repression of microRNA involved in breast tumor cell growth. Cancer Res 69:8332–8340 [DOI] [PubMed] [Google Scholar]
- 8. Creighton CJ, Benham AL, Zhu H, Khan MF, Reid JG, Nagaraja AK, Fountain MD, Dziadek O, Han D, Ma L, Kim J, Hawkins SM, Anderson ML, Matzuk MM, Gunaratne PH. 2010. Discovery of novel microRNA in female reproductive tract using next generation sequencing. PLoS ONE 5:e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hawkins SM, Buchold GM, Matzuk MM. 2011. The roles of small RNA pathways in reproductive medicine. Mol Endocrinol 25:1257–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam EW, Shah K, Brosens JJ. 2012. The diversity of sex steroid action: the role of micro-RNA and FOXO transcription factors in cycling endometrium and cancer. J Endocrinol 212:13–25 [DOI] [PubMed] [Google Scholar]
- 11. Ohlsson Teague EM, Print CG, Hull ML. 2010. The role of microRNA in endometriosis and associated reproductive conditions. Hum Reprod Update 16:142–165 [DOI] [PubMed] [Google Scholar]
- 12. Pan Q, Chegini N. 2008. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med 26:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bulun SE, Simpson ER. 2008. Aromatase expression in women's cancers. Adv Exp Med Biol 630:112–132 [DOI] [PubMed] [Google Scholar]
- 14. Critchley HO, Saunders PT. 2009. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 16:191–199 [DOI] [PubMed] [Google Scholar]
- 15. Di Cristofano A, Ellenson LH. 2007. Endometrial carcinoma. Annu Rev Pathol 2:57–85 [DOI] [PubMed] [Google Scholar]
- 16. Eliassen AH, Hankinson SE. 2008. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol 630:148–165 [PubMed] [Google Scholar]
- 17. Geisler JP, Buller E, Manahan KJ. 2008. Estrogen receptor α and β expression in a case matched series of serous and endometrioid adenocarcinomas of the ovary. Eur J Gynaecol Oncol 29:126–128 [PubMed] [Google Scholar]
- 18. Gründker C, Günthert AR, Emons G. 2008. Hormonal heterogeneity of endometrial cancer. Adv Exp Med Biol 630:166–188 [DOI] [PubMed] [Google Scholar]
- 19. Hawkins SM, Matzuk MM. 2008. The menstrual cycle: basic biology. Ann NY Acad Sci 1135:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JJ, Chapman-Davis E. 2010. Role of progesterone in endometrial cancer. Semin Reprod Med 28:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mylonas I, Jeschke U, Shabani N, Kuhn C, Kunze S, Dian D, Friedl C, Kupka MS, Friese K. 2007. Steroid receptors ERα, ERβ, PR-A and PR-B are differentially expressed in normal and atrophic human endometrium. Histol Histopathol 22:169–176 [DOI] [PubMed] [Google Scholar]
- 22. Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. 2010. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol 24:346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheskis BJ, Greger JG, Nagpal S, Freedman LP. 2007. Signaling by estrogens. J Cell Physiol 213:610–617 [DOI] [PubMed] [Google Scholar]
- 24. Ellmann S, Sticht H, Thiel F, Beckmann MW, Strick R, Strissel PL. 2009. Estrogen and progesterone receptors: from molecular structures to clinical targets. Cell Mol Life Sci 66:2405–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCormack O, Harrison M, Kerin MJ, McCann A. 2007. Role of the progesterone receptor (PR) and the PR isoforms in breast cancer. Crit Rev Oncog 13:283–301 [DOI] [PubMed] [Google Scholar]
- 26. Zhao C, Dahlman-Wright K, Gustafsson JA. 2008. Estrogen receptor β: an overview and update. Nucl Recept Signal 6:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanekamp EE, Gielen SC, De Ruiter PE, Chadha-Ajwani S, Huikeshoven FJ, Burger CW, Grootegoed JA, Blok LJ. 2005. Differences in invasive capacity of endometrial cancer cell lines expressing different progesterone receptor isotypes: possible involvement of cadherins. J Soc Gynecol Investig 12:278–284 [DOI] [PubMed] [Google Scholar]
- 28. Leslie KK, Stein MP, Kumar NS, Dai D, Stephens J, Wandinger-Ness A, Glueck DH. 2005. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol Oncol 96:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryan AJ, Susil B, Jobling TW, Oehler MK. 2005. Endometrial cancer. Cell Tissue Res 322:53–61 [DOI] [PubMed] [Google Scholar]
- 30. Schindler AE. 2009. Progestogen deficiency and endometrial cancer risk. Maturitas 62:334–337 [DOI] [PubMed] [Google Scholar]
- 31. Sorosky JI. 2008. Endometrial cancer. Obstet Gynecol 111:436–447 [DOI] [PubMed] [Google Scholar]
- 32. Wendler A, Keller D, Albrecht C, Peluso JJ, Wehling M. 2011. Involvement of let-7/miR-98 microRNA in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep 25:273–279 [PubMed] [Google Scholar]
- 33. Ahmed IS, Rohe HJ, Twist KE, Mattingly MN, Craven RJ. 2010. Progesterone receptor membrane component 1 (Pgrmc1): a heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J Pharmacol Exp Ther 333:564–573 [DOI] [PubMed] [Google Scholar]
- 34. Peluso JJ. 2011. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids 76:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih IeM, Zhang Y, Wood W, 3rd, Becker KG, Morin PJ. 2008. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One 3:e2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. 2009. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood 113:396–402 [DOI] [PubMed] [Google Scholar]
- 37. Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. 2010. TGFβ-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 29:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshimoto N, Toyama T, Takahashi S, Sugiura H, Endo Y, Iwasa M, Fujii Y, Yamashita H. 2011. Distinct expressions of microRNA that directly target estrogen receptor α in human breast cancer. Breast Cancer Res Treat 130:331–339 [DOI] [PubMed] [Google Scholar]
- 39. Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL. 2004. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab 89:1429–1442 [DOI] [PubMed] [Google Scholar]
- 40. Dai D, Wolf DM, Litman ES, White MJ, Leslie KK. 2002. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res 62:881–886 [PubMed] [Google Scholar]
- 41. Dassen H, Punyadeera C, Kamps R, Delvoux B, Van Langendonckt A, Donnez J, Husen B, Thole H, Dunselman G, Groothuis P. 2007. Estrogen metabolizing enzymes in endometrium and endometriosis. Hum Reprod 22:3148–3158 [DOI] [PubMed] [Google Scholar]
- 42. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. 2000. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- 43. Hanekamp EE, Kühne LM, Grootegoed JA, Burger CW, Blok LJ. 2004. Progesterone receptor A and B expression and progestagen treatment in growth and spread of endometrial cancer cells in nude mice. Endocr Relat Cancer 11:831–841 [DOI] [PubMed] [Google Scholar]
- 44. Cuesta R, Martínez-Sánchez A, Gebauer F. 2009. miR-181a regulates cap-dependent translation of p27(kip1) mRNA in myeloid cells. Mol Cell Biol 29:2841–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, Pertsemlidis A. 2009. 2009 miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res 7:1234–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. 2007. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW, Chen XM. 2009. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol 183:1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang S, Wu S, Ding J, Lin J, Wei L, Gu J, He X. 2010. MicroRNA-181a modulates gene expression of zinc finger family members by directly targeting their coding regions. Nucleic Acids Res 38:7211–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H, Cao X, Wang Q. 2011. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 585:1963–1968 [DOI] [PubMed] [Google Scholar]
- 50. Ouyang YB, Lu Y, Yue S, Giffard RG. 2012. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alajez NM, Shi W, Hui AB, Bruce J, Lenarduzzi M, Ito E, Yue S, O'Sullivan B, Liu FF. 2010. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis 1:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang S, Tang Y, Cui H, Zhao X, Luo X, Pan W, Huang X, Shen N. 2011. Let-7/miR-98 regulate Fas and Fas-mediated apoptosis. Genes Immun 12:149–154 [DOI] [PubMed] [Google Scholar]
- 53. Flötotto T, Niederacher D, Hohmann D, Heimerzheim T, Dall P, Djahansouzi S, Bender HG, Hanstein B. 2004. Molecular mechanism of estrogen receptor (ER)α-specific, estradiol-dependent expression of the progesterone receptor (PR) B-isoform. J Steroid Biochem Mol Biol 88:131–142 [DOI] [PubMed] [Google Scholar]
- 54. Fujiyama-Nakamura S, Yamagata K, Kato S. 2011. Hormonal repression of miRNA biosynthesis through a nuclear steroid hormone receptor. Adv Exp Med Biol 700:43–55 [DOI] [PubMed] [Google Scholar]
- 55. Nothnick WB, Healy C, Hong X. 2010. Steroidal regulation of uterine miRNA is associated with modulation of the miRNA biogenesis components Exportin-5 and Dicer1. Endocrine 37:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.