Abstract
Context:
ACTH-producing neuroendocrine tumor (NET) of the thymus is a rare cause of Cushing's syndrome (CS). The literature consists mainly of isolated case reports.
Patients:
We studied 12 cases (eight males and four females) diagnosed between 1986 and 2010 with CS and thymic NET who underwent surgical resection.
Main Outcome Measures:
We measured time from onset of CS to diagnosis of thymic NET, tumor size, histological grade, time to recurrence, and survival and performed a meta-analysis of other published cases of CS associated with thymic NET.
Results:
Eleven of 12 patients presented with classic features of CS at a median age of 21 yr (range, 7–51). Four were children. The 24-h urine free cortisol was greater than 16-fold of normal, and biochemical testing was consistent with ectopic ACTH production in all 11. Another patient presenting with pulmonary embolus had a thymic mass and was later diagnosed with CS. All patients underwent thymectomy, and nine of 10 tumors exhibited positive ACTH immunochemistry. Median tumor diameter was 5 cm (range, 1–11.5). Six patients recurred 20–28 months after surgery with metastases to mediastinal lymph nodes (n = 5), bone (n = 5), liver (n = 1), parotid gland (n = 1), and breast (n = 1). Four of five patients treated with radiation therapy also received chemotherapy. All recurrent patients received ketoconazole; four later underwent bilateral adrenalectomy. Six recurrent patients died 22–90 months (median, 57) after thymectomy. At last review, six patients were alive 14–90 months (median, 49) after thymectomy. These data are similar to those from the meta-analysis.
Conclusions:
Thymic ACTH-producing NET is an aggressive disease that should be considered in CS with ectopic ACTH secretion, particularly in younger patients.
An association between thymic masses and Cushing's syndrome (CS) was first described in 1931 (1), although these tumors were originally thought to be of epithelial origin. It was not until the 1970s that neuroendocrine thymic tumors were first recognized as a pathological entity distinct from thymoma by Rosai and Higa (2).
The minority of neuroendocrine thymic tumors that secrete ACTH are generally aggressive (3). In 2000, Moran and Suster (3) examined histological specimens from 80 patients with neuroendocrine thymic tumors, five of whom had CS. They defined a simplified grading system and correlated it with the clinical course in 50 patients. Overall, the patients had a significant degree of cellular atypia and a relatively poor prognosis. Therefore, they recommended that the term thymic neuroendocrine carcinoma be used rather than thymic carcinoid (3) to distinguish them from pulmonary and other carcinoids, which tend to have a less aggressive course (4).
To date, the literature on neuroendocrine tumors (NET) of the thymus, and in particular those associated with CS, consists mainly of single case reports. Therefore the presentation, diagnostic process, and clinical course are not well characterized. To learn more about ACTH-producing thymic NET, we performed a retrospective analysis of patients treated at the National Institutes of Health (NIH) from 1982 until 2011. We also performed a meta-analysis of previous publications describing cases of ACTH-producing thymic NET, to put our experience into context.
Patients and Methods
We reviewed records of patients with ACTH-secreting neuroendocrine thymic tumors admitted to the NIH Clinical Center between 1982 and 2011. Patients participated in protocols approved by the Investigational Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute. Adult patients and parents of children provided written informed consent. Patients who presented before 2005 were included in a report of the varying presentations of ectopic CS (5).
Diagnostic evaluation
Plasma electrolytes, fasting glucose, diurnal serum cortisol (0800 and 2400 h), plasma ACTH, and 24-h urine free cortisol (UFC) were measured. Most patients underwent 8-mg overnight high-dose dexamethasone suppression test (6), ovine CRH stimulation test (7), and/or inferior petrosal sinus sampling (IPSS) with administration of CRH (8). Thymic vein cannulation was not performed because false positives may occur with other sources of ACTH, including bronchial carcinoid (9).
After biochemical confirmation of ectopic ACTH secretion, imaging studies were performed to identify the source, including computed tomography (CT) with contrast and T1- and T2-weighted magnetic resonance imaging scans of the neck, chest, abdomen, and pelvis (5). Whole body octreotide and [18F]flurodeoxyglucose or [18F]l-3,4-dihydroxyphenylalanine (F-Dopa) positron emission tomography (PET) scans were performed in patients who presented after 2005, as previously described (10, 11). The time from the initial features of CS, from the history, to diagnosis of thymic NET was calculated.
When a tumor was not identified initially, patients were treated with steroidogenesis inhibitors or the glucocorticoid receptor antagonist, mifepristone, and invited for imaging studies every 6–12 months.
Operative approach and postsurgical course
Once a thymic mass was identified, surgery was performed via median sternotomy or thoracotomy. Mediastinal lymph nodes were resected when enlarged on preoperative imaging or found at the time of surgery. En-bloc resections of pleura, pericardium, or cervical lymph nodes were completed to remove all gross disease. Patients with a postoperative serum cortisol level below 5 μg/dl (138 nmol/liter) were considered to be in remission (5). Patients were followed at the NIH and by contact with the local physician. The time to any recurrence was noted.
Pathological examination
The largest dimension of the tumor was noted from the gross pathology. Hematoxylin and eosin stains from all cases were examined by a single pathologist (C.A.M.) and graded histologically based on mitotic index and degree of cellular atypia (3). Low grade was defined as mild cellular atypia with a mitotic index of less than 3 per 10 high-powered fields (HPF), intermediate grade was defined as moderate cellular atypia with a mitotic index of 4–10 per 10 HPF, and high grade was severe cellular atypia with a mitotic index greater than 10 per 10 HPF (3).
Assays
RIA (12), immunoradiometric assay (Nichols Institute Diagnostics, San Clemente, CA), or chemiluminescence immunoassay (Siemens Healthcare Diagnostics, Deerfield, IL) was used to quantify plasma ACTH. RIA and immunochemiluminometric assays were used for measurement of serum and urine cortisol. These assays had similar characteristics and normal ranges (13, 14).
Meta-analysis
A meta-analysis was performed of the English language literature since 1980 to characterize patients with both thymic NET and CS. Case reports and series were identified from the following PubMed search on May 17, 2011: (Thymic ACTH carcinoid*) OR (thymic carcinoid AND Cushing*). Any report of neuroendocrine thymic tumor associated with CS that provided the age and sex was included. In articles published after 2001, if the ages and sexes were not specified, we contacted the authors and included these data if provided. Patients were considered to have ACTH-producing NET based on pathology or the presence of ectopic CS and abnormal thymic imaging. Care was taken to avoid duplication of patients reported more than once. The following were recorded if available: treatments (surgical, radiation therapy, chemotherapy), evidence of metastases, time to any recurrence, time to death, or length of follow-up. Subgroup analysis on males and females was performed, and data were compared using an unpaired Student's t test. P < 0.05 was considered to be statistically significant.
Results
Presentation and biochemical testing of NIH patients
Eleven patients (seven males and four females) presented with classical features of CS at relatively young ages (range, 7–51 yr; median, 21) (Table 1). Four were aged 7–17 yr. Another male patient (case 12) was diagnosed with CS at age 45, 4 yr after resection of a thymic NET. Two patients were smoking cigarettes at the time of diagnosis, and the remaining 10 were lifelong nonsmokers.
Table 1.
Biochemical data of the 11 patients evaluated for CS before resection of ACTH-producing thymic NET
| Case no. | Age (yr) | Baseline plasma ACTH (pg/ml) | Baseline UFC (μg/24 h) | 8-mg Dex suppression test (% cortisol reduction from pre- to posttest) | CRH stimulation (ovine) |
IPSS: maximum ratio of IPS ACTH to peripheral ACTH | |
|---|---|---|---|---|---|---|---|
| Mean % rise in ACTH at 15 and 30 min | Mean % rise in cortisol at 30 and 45 min | ||||||
| 1 | 7 | 41 | 9,388 | 10 | 1 | 21 | 1.2 |
| 2 | 13 | 222 | 1,785 | 16 | −11 | 3 | 1.2 |
| 3 | 14 | NA | 9,806 | 24 | −10 | −2 | NA |
| 4 | 17 | 87 | 777 | NA | NA | NA | 1.4 |
| 5 | 20 | 127 | 9,130 | NA | 14 | 4 | 1.2 |
| 6 | 21 | 149 | 18,390 | NA | 34 | NA | 1.6 |
| 7 | 24 | 362 | 8,613 | −21 | 1 | 3 | 2.3 |
| 8 | 27 | 333 | 3,580 | 6 | −3 | 12 | 1.2 |
| 9 | 40 | 149 | 8,200 | 11 | 1 | 12 | 1.4 |
| 10 | 50 | 59 | 2,890 | −38 | 5 | 28 | 1.6 |
| 11 | 51 | 385 | 2,800 | −2 | 3 | −7 | 1.4 |
| Median | 21 | 149 | 5,890 | 8 | 1 | 4 | 1.4 |
UFC, Normal ranges, 20–90 μg/24 h (cases 1, 3, 5, 9, 11), 24–108 (case 7), 8–77 (case 8), and 3–45 (cases 2, 4, 10). Dex suppression, 8 mg dexamethasone given at 2400 h; CRH, 1 μg/kg ovine CRH; IPSS, after peripheral injection of 1 μg/kg ovine CRH; NA, not available. To convert ACTH from pg/ml to pmol/liter, multiply by 0.22. To convert 24-h UFC from μg to pmol, multiply by 2.76. Boldface indicates median values.
Preoperative features noted in the patients with CS included central obesity, depression, easy bruising, proximal muscle weakness, and facial fullness. Five were noted to be hyperpigmented. Nine of the 11 patients were hypertensive, of whom six were taking antihypertensive medication; the median blood pressure was 145/74 mm Hg (ranges: systolic, 130–173; diastolic, 74–107). Six patients had active infections, including fungal foot infection, shingles, impetigo, herpes zoster, and epididymitis. Nine patients were hypokalemic (median potassium, 2.9 mmol/liter; range, 2.1–3.8). The median fasting blood glucose was 136 mg/dl (7.5 mmol/liter), and the range was 80–207 mg/dl (4.4–11.5 mmol/liter). Six patients were diabetic, of whom two were taking medications for diabetes. One patient (case 9) suffered from a myocardial infarction during diagnostic evaluation, but the others did not have known cardiovascular disease. Serum calcium was normal in all, and there was no personal or family history of multiple endocrine neoplasia type 1 (MEN-1).
CS was confirmed biochemically 0–41 months (median, 10) after retrospective recall of first symptoms. In all cases, 24-h UFC was highly elevated (16–104 times the upper limit of normal; median, 50 times; Table 1), and the physiological diurnal variation in serum cortisol was absent. Plasma ACTH was not suppressed [median morning value, 186 pg/ml (41 pmol/liter); range, 41–1957 pg/ml (9–431 pmol/liter); normal, <46 pg/ml (10 pmol/liter)], ruling out primary adrenal pathology.
After administration of peripheral CRH, the mean rise in plasma ACTH at 15 and 30 min was less than 35% in all 11 cases (Table 1). In nine patients, the mean rise in serum cortisol at 30 and 45 min was less than 20%. No patient suppressed morning cortisol to less than 69% after 8 mg overnight dexamethasone. Ten patients underwent IPSS, and none had a ratio of petrosal to peripheral ACTH level greater than two before or three after CRH administration. Apart from the cortisol response to CRH in two cases, these findings were consistent with an ectopic source of ACTH production (7, 15, 16).
Case 5 presented with features of CS at the age of 20 yr, including weight gain, pigmented striae, easy bruisability, and difficulty concentrating. These symptoms waxed and waned over 2 yr, corresponding with changes in UFC, which ranged from normal [<90 μg (248 nmol)/24 h] to 9130 μg/24 h (25,200 nmol/24 h), consistent with cyclic CS. Differential biochemical testing, performed when she was hypercortisolemic, indicated ectopic ACTH production.
Case 12 presented with shortness of breath and chest pain at the age of 45 yr. Chest CT showed a pulmonary infarct and a thymic mass, which was resected and stained for ACTH. The patient was diagnosed with CS 4 yr after thymectomy, when UFC was first measured and found to be 1141 μg/24 h (3149 pmol/24 h) [normal, 3–45 μg/24 h (8–124 pmol/24 h)]. With hindsight, features of CS including muscle weakness, irritability, diabetes, and hypertension were present before thymectomy but were not florid. This patient noticed increased pigmentation 4 yr before thymectomy.
Imaging studies
Chest CT imaging with contrast was performed in all 11 patients 5–55 months (median, 10) after the onset of CS. A thymic tumor was identified in nine of the 11 cases on the initial CT (Fig. 1). In one case, the tumor extended to the superior vena cava, with internal jugular vein thrombus. This patient was subsequently anticoagulated pre- and postoperatively without further clot progression. A tumor was seen in seven of eight patients who underwent chest magnetic resonance imaging, all of whom had positive CT findings. In the two cases that were initially CT negative, a thymic tumor was identified on next CT imaging 33 and 36 months later. Positive uptake corresponding with the CT lesion was observed on octreotide imaging in two of four patients, on [18F]Fluorodeoxyglucose PET imaging in three of three patients, and on [18F]l-3,4-dihydroxyphenylalanine (F-Dopa) PET in none of one.
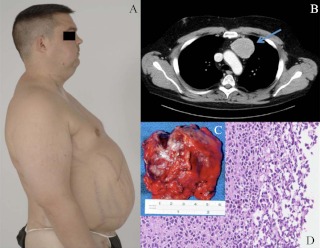
Fig. 1.
Images from case 8. A, Clinical photography illustrating Cushing's syndrome; B, CT chest with contrast (the arrow indicates the thymic tumor); C, thymic tumor macropathology; D, thymic NET histology, hematoxylin and eosin staining (magnification, ×20).
Initial treatment
Preoperatively, nine patients received medical treatment for CS with ketoconazole (n = 6), octreotide (n = 1), mifepristone (n = 1), or aminoglutethimide and metyrapone (n = 1). All patients underwent thymectomy—7 to 72 months (median, 21) after the onset of CS features (patients 1–11) or 4 yr before the diagnosis of CS (patient 12). Ten patients underwent thymectomy via median sternotomy; thymectomy via thoracotomy was performed for patients 3 and 9. Patient 2 underwent sternotomy with clamshell extension and en-bloc resection of bilateral cervical lymph nodes. En-bloc resection of pericardium was required for patients 4 and 12 and resection of pleura for patient 6.
In the postoperative period, four patients received adjuvant fractionated mediastinal radiation therapy, three were also treated with adjuvant chemotherapy (etoposide and cisplatin, and 5-flurouracil), and one received capecitabine as a radiosensitizer (Table 2).
Table 2.
Summary of clinical course in patients with ACTH-producing thymic NET
| Case no. | Age (yr) | Sex | Maximal tumor size (cm) | No. of thymic tumors | Resected lymph nodes | Masaoka clinical stage | T | N | M | Histological grade | Adjuvant therapy | Recurrence of CS (months) | Subsequent metastases | Further therapy | Alive/dead (months postsurgery) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | F | 1 | 1 | 0/0 | I | T1 | NX | M0 | Well-diff | Alive (90) | ||||
| 2 | 13 | M | 11.5 | 2 | 17/17 | IVb | T3 | N3 | M0 | Mod-diff | Persistent | Ba | BA (em) | Dead (22) | |
| 3 | 14 | F | nk | nk | nk | nk | nk | nk | nk | Mod-diff | Yes (36) | c, a, m LN, Ba | S, S, R | Dead (90) | |
| 4 | 17 | M | 6 | 1 | 20/20 | IVb | T3 | N1 | M0 | Mod-diff | Alive (23) | ||||
| 5 | 20 | F | 2 | 1 | 0/1 | IVa | T3 | N0 | M0 | Well-diff | Alive (14) | ||||
| 6 | 21 | M | 5 | 1 | 1/3 | IVb | T2 | N1 | M0 | Mod-diff | R | Yes (24) | nk | C | Dead (72) |
| 7 | 24 | F | 2.5 | 3 | 3/3 | IVb | T2 | N1 | M0 | Mod-diff | Yes (25) | mLN, breast | S, R, C, BA | Alive (58) | |
| 8 | 27 | M | 8 | 1 | 0/0 | III | T3 | NX | M0 | Mod-diff | C, R(G) | Yes (28) | L, mLNa, Ba | C, BA | Dead (56) |
| 9 | 40 | M | 5 | 2 | 2/12 | IVb | T2 | N1 | M0 | Mod-diff | Yes (nk) | mLN | S, BA | Dead (57) | |
| 10 | 50 | M | 2 | 1 | 0/2 | I | T1 | N0 | M0 | Well-diff | Alive (40) | ||||
| 11 | 51 | M | 8 | 1 | 1/1 | IVb | T3 | N1 | M0 | Mod-diff | C, R | Yes (20) | cLN, Ba, La, Par | S, S, BA | Dead (50) |
| 12 | 45 | M | nk | nk | nk | IVb | T3 | N1 | M0 | Mod-diff | C, R | Yes (48)b | c, a, m LN, Ba | R, S, C | Alive (62) |
Age indicates age at time of presentation. Sex: M, male, F, female. Resected lymph nodes indicates the number of lymph nodes positive for NET/number of lymph nodes resected. Masaoka clinical staging: stage I, completely encapsulated tumor; stage II, invasion of tumor into the surrounding tissues; stage III, invasion into surrounding organs such as the pericardium or great vessels; stage IVa, pleural or pericardial invasion; and stage IVb, lymphogenous or hematogenous metastases. Yamakawa-Masaoka TNM staging: T, tumor; T1, completely encapsulated; T2, macroscopic invasion to the surrounding tissue or invasion of the capsule; T3, invasion into neighboring organs; N, nodes; NX, not sampled; N1, metastasis to anterior mediastinal lymph nodes; M, metastasis, M0, no hematogenous metastasis; M1, hematogenous metastasis. Histological grade: well-diff, well-differentiated neuroendocrine carcinoma (low grade, conventional carcinoid); mod-diff, moderately differentiated neuroendocrine carcinoma (intermediate grade, atypical carcinoid). Subsequent metastases: m,c,a LN, mediastinal, cervical, axillary lymph nodes; B, bone; L, liver; Par, parotid gland. Adjuvant and further therapy: S, surgery to remove recurrence of metastasis; R, radiation therapy (adjuvant dose, 54–60 cGy); R(G), radiation therapy with gemcitabine as a radiosensitizer; C, chemotherapy; BA, surgical bilateral adrenalectomy; BA (em), bilateral adrenalectomy by embolization; nk, not known.
Inferred from imaging without pathology.
Patient 12 was first diagnosed with CS 48 months after surgery.
Pathology
The surgical specimens contained areas of necrosis and hemorrhage (Fig. 1). The maximal tumor diameter was 1–11.5 cm (median, 5) (Table 2), with a single tumor in seven patients and evidence of multifocality in three. The Masaoka stages were I (n = 2), III (n = 1), IVa (n = 1), and IVb (n = 7) (Table 2), where stage I represents completely encapsulated tumor; stage II, invasion of tumor into the surrounding tissues; stage III, invasion into surrounding organs such as the pericardium or great vessels; stage IVa, pleural or pericardial invasion; and stage IVb, lymphogenous or hematogenous metastases (17). Table 2 shows the tumor (T), lymph nodes (N), and metastasis (M) classification of Yamakawa-Masaoka for each case (18).
On histopathological examination, nine tumors (2.5–11.5 cm diameter) were moderately differentiated neuroendocrine carcinoma (intermediate grade, atypical carcinoid), and three tumors (1–2 cm in diameter) were well-differentiated neuroendocrine carcinoma (low grade, conventional carcinoid) (3). Nine of 10 tumors examined stained positively for ACTH.
Postoperative course
One patient (case 2) had persistent CS, with elevated postoperative serum cortisol and evidence of residual tumor on CT. The remaining 10 patients with known CS all had clinical resolution of their Cushing's features. Postoperative biochemical data were available for nine of these patients, all of whom had biochemical remission with postoperative morning serum cortisol below 5 μg/dl (138 nmol/liter) (5).
Seven of the 12 patients developed recurrent or metastatic disease, including the four patients treated adjuvantly. Symptoms of recurrent CS occurred 20–36 months (median, 25) after surgery. Recurrent or metastatic tumor was not seen on imaging studies until a median of 33 months after surgery (range, 12–49 months), although, with the exception of patient 11 who developed a palpable facial mass, imaging studies were not performed until after diagnosis of recurrent CS.
Among the seven patients with recurrence, six had imaging and/or biopsy evaluations. Metastases were histologically confirmed in lymph nodes (n = 6), as well as breast (case 7), liver (case 8), and parotid gland (case 11). CT revealed probable metastases in the lung (n = 1) and bone (n = 5), but these lesions were not confirmed with biopsy.
Recurrence within the mediastinum (patient 12) and metastasis in the parotid gland (patient 11) were treated with excision before diagnosis or recurrence of CS, respectively. Patient 7 developed recurrence of CS with elevated UFC 13 months after thymectomy, but no definitive source was detected on imaging studies. Thirty-two months after thymectomy, enlarged mediastinal lymph nodes were found on chest CT and were surgically excised. Pathology showed metastatic NET that stained for ACTH; however, there was no resolution of CS after surgery, suggesting more extensive disease.
Other treatments of recurrence included fractionated mediastinal radiation therapy in three patients and systemic chemotherapy including cisplatin-etoposide or cisplatin-gemcitabine in four. The patient with hepatic metastases (patient 8) also received an intrahepatic infusion of melphalan. Case 12 was treated with a cyclin-dependent kinase inhibitor (PHA-848125AC) as part of a phase II trial, after failure of conventional chemotherapy. There was no evidence of tumor shrinkage or biochemical control of CS after any of these treatments.
All recurrent patients developed CS and were treated with ketoconazole and/or metyrapone. These medical therapies tended to be effective initially but became less so in advancing disease. Therefore, four of seven recurrent patients underwent bilateral adrenalectomy for symptomatic relief of CS.
Overall, six patients died between 22 and 90 months after thymectomy. At last follow-up, six patients were alive 14–90 months after thymectomy.
Meta-analysis
The PubMed search yielded 124 results; 66 articles published between 1980 and 2011 met our criteria (Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). These described 92 cases of ACTH-producing thymic NET associated with CS. Three of these cases were noted to have MEN-1, and in one of these a spontaneous novel germline nonsense mutation (W423X) was reported (19). Cyclic CS was noted in two cases. Seven of the 92 cases (8%) were children younger than 18 yr, and 52 cases (56%) presented between the ages of 18 and 40.
Treatment was described in 69 cases, of which 53 underwent surgical thymectomy or debulking. Most patients who did not have thymectomy had advanced disease or had undergone bilateral adrenalectomy. Four patients underwent thymectomy after bilateral adrenalectomy. The longest time interval between remission after thymectomy to recurrence was 8 yr (20); the longest interval without recurrence was 20 yr (21). Overall, the presentation and outcome of these cases were similar to those in our series (Table 3).
Table 3.
Comparison of meta-analysis and NIH cohort
| Meta-analysis | NIH series | |
|---|---|---|
| Total no. of patients | 92 | 12 |
| Median age in yr (range) | 34 (2.5–75) | 21 (8–51) |
| Sex ratio (males:females) | 1.5:1 | 2:1 |
| Median tumor diameter in cm (range) | 5 (0.4–25) | 5 (1–11.5) |
| No. of patients with follow-up | 53 | 12 |
| Median time to recurrence of CS in months (range) | 18 (2–98) | 25 (20–36) |
| Location of metastases | LN, bone, lung, pancreasa | LN, bone, liver, breast, parotid |
| No. alive | 28 (52%) | 6 (50%) |
| Median time alive after surgery in months (range) | 20 (0–238) | 49 (14–90) |
| No. dead | 26 (48%) | 6 (50%) |
| Median time to death in months (range) | 35 (1–105) | 57 (22–90) |
LN, lymph nodes.
Pancreatic metastases found in one case at autopsy.
The ratio of male to female cases was 1.5:1 in the meta-analysis and 2:1 in our series, suggesting a slight male preponderance. In the meta-analysis, gender was not a statistically significant variable with respect to outcomes, including age of presentation, death, time to death, or duration of survival (P > 0.05 for all comparisons). However, the maximal tumor diameter from preoperative imaging or pathology was significantly greater in males than females (maximal tumor diameter: males, 7.7 ± 1.1 cm; females, 3.9 ± 0.9 cm; P = 0.01).
Discussion
We have described the presentation, treatment, and outcomes of 12 patients with ACTH-producing thymic NET. To our knowledge, this is the largest reported case-series of this rare disease. A meta-analysis showed similar presentations and outcomes, suggesting that this series is representative. Eleven of our cases presented with florid CS, and biochemical testing pointed clearly to an ectopic source. In one case, there was clinical and biochemical evidence of cyclic CS. A thymic tumor was identified from initial chest imaging (CT or plain x-ray) in nine of 11 cases and was identified on second chest imaging in the remaining two cases. Thus, in contrast with bronchial carcinoid, which may remain occult for many years (5), thymic ACTH-producing NET can usually be identified on initial or relatively early repeat chest imaging. Thymic imaging should be interpreted carefully, especially in younger patients in whom a thymic remnant may still be present (22).
The young age of our patients is striking. The median age at presentation was only 21 yr, and four were children of ages 8–17. This is in keeping with the slightly older median age at presentation in our meta-analysis of 35 yr, and the previously reported peak incidence in the second to fourth decades of life (23). The youngest patient previously reported to have ACTH-producing thymic NET presented with CS at age 2.5 yr (24). Because most children presenting with ACTH-dependent CS will have pituitary Cushing's disease (25), an ectopic and in particular a thymic source might easily be overlooked. Pediatric patients could then be exposed to futile transsphenoidal surgery and delayed identification of a thymic tumor.
It is interesting that hyperpigmentation was noted in the presentation in six of our 12 cases, which may reflect elevated plasma ACTH concentrations. In case 12, hyperpigmentation was noted years before diagnosis of a thymic tumor or CS. A previous report showed that 25% of patients with ectopic CS from all sources are hyperpigmented (26). Other authors also have reported hyperpigmentation in ACTH-producing thymic NET (27–29). This may be due to increased α-melanocyte stimulating hormone, which like ACTH is a product of proopiomelanocortin (POMC) processing. Shorter ACTH peptides have been detected from thymic NET (30), suggesting altered POMC expression and processing that may be associated with greater melanocyte stimulating hormone receptor stimulation and pigmentation. We speculate that modified POMC and ACTH processing may explain the early increased pigmentation but delayed presentation of CS in case 12.
No patient in our series and only three of the 91 patients in the meta-analysis had clinical or genetic evidence of MEN-1. In contrast, 30% of thymic NET not associated with CS are found in patients with MEN-1 (31). This increased incidence is seen almost exclusively in males who are usually smokers and may be lethal (32). It is not clear why thymic NET associated with MEN-1 are less likely than sporadic tumors to secrete ACTH. Nonetheless, as Ghazi et al. (33) point out in their recent case report, the possibility of MEN-1 should be considered in all patients with thymic NET.
All of our patients underwent surgical thymectomy, as did the majority of other reported cases. It is widely agreed that total thymectomy is the initial treatment of choice for thymic NET (23). Pass et al. (34) argued that surgery should include aggressive lymph node dissection to put CS in remission and to gain control of the underlying disease. Anterior mediastinal lymph node sampling may also enable more accurate clinical staging (35). The midline sternotomy approach is recommended because this enables excision of the entire thymus, perithymic fat, and other affected mediastinal structures and the sampling of lymph nodes (36).
The optimal timing and need for nonsurgical treatments such as radiation and chemotherapy are less clear. Because the patients treated with these modalities often have more aggressive disease, it is difficult to assess their efficacy. Ruffini et al. report that they and others have had favorable outcomes in advanced cases of thymic NET treated with pre- and postoperative radiation and chemotherapy (23, 37), although not all of these patients exhibited CS. They note that postoperative radiation therapy may reduce the risk of local relapse but has little effect on long-term survival. Unfortunately, these tumors appear to be resistant to standard chemotherapy regimens, and there is no drug regime proven to have a consistent response. Further work is needed to understand better the biology of thymic NET to improve systemic therapies.
The two patients with stage I disease had no evidence of recurrence over 37 and 50 months. The majority of our patients had stage IVb disease because they had evidence of lymph node metastases, and overall they tended to have a worse outcome. The Masaoka staging system was designed for thymoma, which rarely metastasizes, rather than thymic carcinoma or thymic NET (17). A high proportion of thymic NET may be stage IVb, and therefore the TNM classification system may be more useful (18). In our series, the three patients with well-differentiated rather than moderately differentiated histological grade appeared to have a better prognosis (Table 2), although the numbers are too small to draw any formal conclusion.
From the case series and the meta-analysis, metastases were reported in mediastinal and cervical lymph nodes, bone, lung, liver, parotid, and breast and from autopsy in the pancreas (38). Thus, in addition to local recurrence and mass effects, ACTH-producing thymic NET is prone to metastasize.
Thymic NET associated with CS have poorer outcomes than those without CS (39). It is unclear whether this is due to metabolic complications associated with CS or to inherently more aggressive tumor behavior. All patients with tumor recurrence in our series developed CS again, and an important part of their management was control of hypercortisolism, either with ketoconazole and/or metyrapone or bilateral adrenalectomy. Treatment with mitotane could also be considered, although due to its significant side effect profile (40), bilateral adrenalectomy may be preferable.
Although some patients in our series and in the meta-analysis had rapidly progressing disease, others demonstrated a more indolent course. In the meta-analysis, the longest reported time interval between initial surgical resection and recurrence was 8 yr (19). Therefore, all patients with a history of ACTH-producing thymic NET should have careful extended clinical follow-up with interval biochemical testing.
In summary, thymic ACTH-producing NET is an aggressive disease that should be considered in CS due to ectopic ACTH secretion, particularly in younger patients. The current first-line treatment is surgical resection, with or without adjuvant radiation therapy. In recurrent patients, CS can be controlled pharmacologically or with bilateral adrenalectomy. Further research is needed to develop a successful systemic medical therapy for this challenging disease.
Supplementary Material
Acknowledgments
We thank the patients, their families, and their local physicians for making this work possible. We are also grateful to the staff of the Mark O. Hatfield Clinical Center for their assistance with this study.
This work was supported by the intramural programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CS
- Cushing's syndrome
- CT
- computed tomography
- HPF
- high-powered fields
- IPSS
- inferior petrosal sinus sampling
- MEN-1
- multiple endocrine neoplasia type 1
- NET
- neuroendocrine tumor
- PET
- positron emission tomography
- POMC
- proopiomelanocortin
- UFC
- urine free cortisol.
References
- 1. Leyton O, Turnbull HM, Bratton AB. 1931. Primary cancer of the thymus with pluriglandular disturbance. J Path Bact 34:635–660 [Google Scholar]
- 2. Rosai J, Higa E. 1972. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 29:1061–1074 [DOI] [PubMed] [Google Scholar]
- 3. Moran CA, Suster S. 2000. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 114:100–110 [DOI] [PubMed] [Google Scholar]
- 4. de Perrot M, Spiliopoulos A, Fischer S, Totsch M, Keshavjee S. 2002. Neuroendocrine carcinoma (carcinoid) of the thymus associated with Cushing's syndrome. Ann Thorac Surg 73:675–681 [DOI] [PubMed] [Google Scholar]
- 5. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. 2005. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab 90:4955–4962 [DOI] [PubMed] [Google Scholar]
- 6. Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB., Jr 1994. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab 78:418–422 [DOI] [PubMed] [Google Scholar]
- 7. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB., Jr 1993. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab 77:1308–1312 [DOI] [PubMed] [Google Scholar]
- 8. Kaltsas GA, Giannulis MG, Newell-Price JD, Dacie JE, Thakkar C, Afshar F, Monson JP, Grossman AB, Besser GM, Trainer PJ. 1999. A critical analysis of the value of simultaneous inferior petrosal sinus sampling in Cushing's disease and the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab 84:487–492 [DOI] [PubMed] [Google Scholar]
- 9. Doppman JL, Pass HI, Nieman LK, Miller DL, Chang R, Cutler GB, Jr, Chrousos GP, Jaffe GS, Norton JA. 1992. Corticotropin-secreting carcinoid tumors of the thymus: diagnostic unreliability of thymic venous sampling. Radiology 184:71–74 [DOI] [PubMed] [Google Scholar]
- 10. Pacak K, Ilias I, Chen CC, Carrasquillo JA, Whatley M, Nieman LK. 2004. The role of [(18)F]fluorodeoxyglucose positron emission tomography and [(111)In]-diethylenetriaminepentaacetate-D-Phe-pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin-secreting tumors causing Cushing's syndrome. J Clin Endocrinol Metab 89:2214–2221 [DOI] [PubMed] [Google Scholar]
- 11. Zemskova MS, Gundabolu B, Sinaii N, Chen CC, Carrasquillo JA, Whatley M, Chowdhury I, Gharib AM, Nieman LK. 2010. Utility of various functional and anatomic imaging modalities for detection of ectopic adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab 95:1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orth DN. 1978. Adrenocorticotropic hormone (ACTH). In: Behrman HR, ed. Methods of hormone radioimmunoassay. New York, Academic Press; 254–284 [Google Scholar]
- 13. Kao M, Voina S, Nichols A, Horton R. 1975. Parallel radioimmunoassay for plasma cortisol and 11-deoxycortisol. Clin Chem 21:1644–1647 [PubMed] [Google Scholar]
- 14. Murphy BE. 1968. Clinical evaluation of urinary cortisol determinations by competitive protein-binding radioassay. J Clin Endocrinol Metab 28:343–348 [DOI] [PubMed] [Google Scholar]
- 15. Lindsay JR, Nieman LK. 2005. Differential diagnosis and imaging in Cushing's syndrome. Endocrinol Metab Clin North Am 34:403–421, x [DOI] [PubMed] [Google Scholar]
- 16. Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. 1991. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med 325:897–905 [DOI] [PubMed] [Google Scholar]
- 17. Masaoka A, Monden Y, Nakahara K, Tanioka T. 1981. Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485–2492 [DOI] [PubMed] [Google Scholar]
- 18. Yamakawa Y, Masaoka A, Hashimoto T, Niwa H, Mizuno T, Fujii Y, Nakahara K. 1991. A tentative tumor-node-metastasis classification of thymoma. Cancer 68:1984–1987 [DOI] [PubMed] [Google Scholar]
- 19. Takagi J, Otake K, Morishita M, Kato H, Nakao N, Yoshikawa K, Ikeda H, Hirooka Y, Hattori Y, Larsson C, Nogimori T. 2006. Multiple endocrine neoplasia type I and Cushing's syndrome due to an aggressive ACTH producing thymic carcinoid. Intern Med 45:81–86 [DOI] [PubMed] [Google Scholar]
- 20. Sugiura H, Morikawa T, Itoh K, Ono K, Okushiba S, Kondo S, Katoh H. 2000. Thymic neuroendocrine carcinoma: a clinicopathologic study in four patients. Ann Thorac Cardiovasc Surg 6:304–308 [PubMed] [Google Scholar]
- 21. Doi M, Sugiyama T, Izumiyama H, Yoshimoto T, Hirata Y. 2010. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J 57:1061–1069 [DOI] [PubMed] [Google Scholar]
- 22. Hanson JA, Sohaib SA, Newell-Price J, Islam N, Monson JP, Trainer PJ, Grossman A, Besser GM, Reznek RH. 1999. Computed tomography appearance of the thymus and anterior mediastinum in active Cushing's syndrome. J Clin Endocrinol Metab 84:602–605 [DOI] [PubMed] [Google Scholar]
- 23. Ruffini E, Oliaro A, Novero D, Campisi P, Filosso PL. 2011. Neuroendocrine tumors of the thymus. Thorac Surg Clin 21:13–23, v [DOI] [PubMed] [Google Scholar]
- 24. McCaughey ES, Walker V, Rolles CJ, Scheurmier NI, Hale AC, Rees LH. 1987. Ectopic ACTH production by a thymic carcinoid tumour. Eur J Pediatr 146:590–591 [DOI] [PubMed] [Google Scholar]
- 25. Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr, Nieman LK, Chrousos GP. 1994. Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med 331:629–636 [DOI] [PubMed] [Google Scholar]
- 26. Imura H, Matsukura S, Yamamoto H, Hirata Y, Nakai Y. 1975. Studies on ectopic ACTH-producing tumors. Clinical and biochemical features of 30 cases. Cancer 35:1430–1437 [DOI] [PubMed] [Google Scholar]
- 27. Brown LR, Aughenbaugh GL, Wick MR, Baker BA, Salassa RM. 1982. Roentgenologic diagnosis of primary corticotropin-producing carcinoid tumors of the mediastinum. Radiology 142:143–148 [DOI] [PubMed] [Google Scholar]
- 28. Wang WQ, Ye L, Bi YF, Zhao HY, Sun SY, Tang ZY, Zhao YJ, Fang WQ, Chen ZY, Chen KM, Jin XL, Ning G. 2006. Six cases of ectopic ACTH syndrome caused by thymic carcinoid. J Endocrinol Invest 29:293–297 [DOI] [PubMed] [Google Scholar]
- 29. Reddy KS, Shriraam M, Bhansali A. 2007. Vanishing nail pigmentation. Ectopic Cushing's syndrome due to thymic carcinoid with secondary diabetes mellitus and hypertension. J Assoc Physicians India 55:226. [PubMed] [Google Scholar]
- 30. Lowry PJ, Rees LH, Tomlin S, Gillies G, Landon J. 1976. Chemical characterization of ectopic ACTH purified from a malignant thymic carcinoid tumor. J Clin Endocrinol Metab 43:831–835 [DOI] [PubMed] [Google Scholar]
- 31. Teh BT, Zedenius J, Kytölä S, Skogseid B, Trotter J, Choplin H, Twigg S, Farnebo F, Giraud S, Cameron D, Robinson B, Calender A, Larsson C, Salmela P. 1998. Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg 228:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferolla P, Falchetti A, Filosso P, Tomassetti P, Tamburrano G, Avenia N, Daddi G, Puma F, Ribacchi R, Santeusanio F, Angeletti G, Brandi ML. 2005. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab 90:2603–2609 [DOI] [PubMed] [Google Scholar]
- 33. Ghazi AA, Dezfooli AA, Mohamadi F, Yousefi SV, Amirbaigloo A, Ghazi S, Pourafkari M, Berney D, Ellard S, Grossman AB. 2011. Cushing's syndrome secondary to a thymic carcinoid tumor due to multiple endocrine neoplasia type 1. Endocr Pract 17:e92–e96 [DOI] [PubMed] [Google Scholar]
- 34. Pass HI, Doppman JL, Nieman L, Stovroff M, Vetto J, Norton JA, Travis W, Chrousos GP, Oldfield EH, Cutler GB., Jr 1990. Management of the ectopic ACTH syndrome due to thoracic carcinoids. Ann Thorac Surg 50:52–57 [DOI] [PubMed] [Google Scholar]
- 35. Kondo K, Monden Y. 2003. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 76:1859–1864; discussion 1864–1865 [DOI] [PubMed] [Google Scholar]
- 36. Rena O, Filosso PL, Maggi G, Casadio C. 2003. Neuroendocrine tumors (carcinoid) of the thymic gland. Ann Thorac Surg 75:633. [DOI] [PubMed] [Google Scholar]
- 37. Cardillo G, Treggiari S, Paul MA, Carleo F, De Massimi AR, Remotti D, Graziano P, Martelli M. 2010. Primary neuroendocrine tumours of the thymus: a clinicopathologic and prognostic study in 19 patients. Eur J Cardiothorac Surg 37:814–818 [DOI] [PubMed] [Google Scholar]
- 38. Ozawa Y, Tomoyasu H, Takeshita A, Shishiba Y, Yamada S, Kovacs K, Matsushita H. 1996. Shift from CRH to ACTH production in a thymic carcinoid with Cushing's syndrome. Horm Res 45:264–268 [DOI] [PubMed] [Google Scholar]
- 39. Wick MR, Scott RE, Li CY, Carney JA. 1980. Carcinoid tumor of the thymus: a clinicopathologic report of seven cases with a review of the literature. Mayo Clin Proc 55:246–254 [PubMed] [Google Scholar]
- 40. Feelders RA, Hofland LJ, de Herder WW. 2010. Medical treatment of Cushing's syndrome: adrenal-blocking drugs and ketaconazole. Neuroendocrinology 92(Suppl 1):111–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.