Abstract
Context:
Increased adipose tissue lipolytic activity is considered an important factor in the pathogenesis of skeletal muscle insulin resistance associated with obesity.
Objective:
The objective of the study was to evaluate the relationship between the rate of release of free fatty acids (FFA) into plasma and skeletal muscle insulin sensitivity in human subjects.
Methods:
We determined the palmitate rate of appearance (Ra) per kilogram fat-free mass (an index of FFA availability to lean tissues) during basal conditions and during insulin infusion (to simulate postprandial insulin concentrations) and skeletal muscle insulin sensitivity, defined as the percent increase in the glucose rate of disappearance, in 110 nondiabetic women (body mass index 20.6–46.4 kg/m2) by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotope tracer methods.
Results:
Basal (rs = −0.379, P < 0.001) and insulin-suppressed (rs = −0.631, P < 0.001) palmitate Ra correlated negatively with skeletal muscle insulin sensitivity. However, the strength of the correlation was greater for palmitate Ra during insulin infusion than palmitate Ra during basal conditions (P = 0.0007) when lipolytic rates and FFA availability were reduced to less than 20% of basal values. The relative suppression of palmitate Ra correlated directly with the relative stimulation of glucose rate of disappearance during insulin infusion (rs = 0.530, P < 0.001).
Conclusion:
These data suggest that the correlation between FFA kinetics and muscle glucose metabolism is due to multiorgan insulin resistance rather than a direct effect of FFA itself on skeletal muscle insulin action and challenge the view that increased adipose tissue lipolytic rate is an important cause of insulin resistance.
It is widely believed that increased free fatty acid (FFA) release from adipose tissue is an important cause of insulin resistance in obese people (1). This notion is supported by data from a series of studies that demonstrate experimentally increasing plasma FFA concentrations causes insulin resistance in skeletal muscle (2–4), whereas experimentally decreasing plasma FFA concentrations improves skeletal muscle insulin sensitivity (5, 6). However, data from studies conducted in large numbers of subjects demonstrate that fasting plasma FFA concentration is a weak predictor (R2 ∼6%) of insulin-mediated glucose disposal (7). Circulating FFA in the basal state do not accurately reflect the day-long plasma FFA load on insulin-sensitive tissues (8). In response to a meal, insulin suppresses adipose tissue triglyceride lipolysis and the release of FFA into the circulation and stimulates skeletal muscle glucose uptake. We have found that insulin-resistant obese subjects have impaired insulin-mediated suppression of adipose tissue lipolysis compared with insulin-sensitive obese subjects, whereas basal rates of FFA release into plasma are the same in both groups (9). Therefore, it is possible that inadequate insulin-mediated suppression of lipolysis of adipose tissue triglycerides after meal ingestion is a more important determinant of skeletal muscle insulin action than basal rates of FFA release into plasma.
The purpose of the present study was to evaluate the relationship between FFA kinetics and insulin sensitivity in lean, overweight, and obese women to determine whether excessive adipose tissue lipolytic rate and release of FFA into the systemic circulation during basal and simulated postprandial (i.e. hyperinsulinemia) conditions are associated with skeletal muscle insulin resistance. Subjects who had diabetes were excluded from participating in this study to avoid the confounding influence of β-cell dysfunction and diabetes medications. Stable isotopically labeled tracer infusions and the hyperinsulinemic-euglycemic clamp procedure were used to assess FFA kinetics and skeletal muscle insulin action.
Materials and Methods
Subjects
A total of 110 middle-aged women with body mass index (BMI) ranging from 20.6 to 46.4 kg/m2 (median 32.8 kg/m2) participated in this study (Table 1). Many of them underwent a hyperinsulinemic-euglycemic clamp procedure as part of their participation in other studies (9–11). All subjects completed a comprehensive medical evaluation at screening, which included a 2-h oral glucose tolerance test (all subjects had 2 h plasma glucose concentrations < 200 mg/dl). Body fat mass and fat-free mass (FFM) were determined by using dual-energy x-ray absorptiometry (Delphi-W densitometer; Hologic, Waltham, MA). Potential participants who smoked cigarettes or had diabetes or other metabolic diseases were excluded. Subjects provided written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine.
Table 1.
Anthropometric and metabolic characteristics of the study subjects
| Median (Q1, Q3) | Range | |
|---|---|---|
| Age (yr) | 51 (40, 57) | 22–69 |
| BMI (kg/m2) | 32.8 (27.1, 38.4) | 20.6–46.4 |
| Body fat (%) | 42 (39, 45) | 25–57 |
| Basal glucose concentration (mg/dl) | 93 (89, 100) | 79–117 |
| Basal FFA concentration (μmol/liter) | 579 (470, 696) | 252–923 |
| FFA concentration during insulin infusion (μmol/liter) | 47 (32, 86) | 17–475 |
| Basal insulin concentration (mU/liter) | 12 (7, 21) | 2–42 |
| Insulin concentration during insulin infusion (mU/liter) | 91 (77, 111) | 41–176 |
| Glucose Rd during basal conditions (μmol/kg FFM per minute) | 15 (13, 16) | 10–23 |
| Glucose Rd during insulin infusion (μmol/kg FFM per minute) | 51 (33, 73) | 16–142 |
| Insulin-mediated stimulation of glucose Rd (%) | 254 (121, 348) | 19–893 |
| Palmitate Ra during basal conditions (μmol/kg FFM per minute) | 2.02 (1.70, 2.52) | 0.96–5.14 |
| Palmitate Ra during insulin infusion (μmol/kg FFM per minute) | 0.37 (0.25, 0.56) | 0.07–3.36 |
| Insulin-mediated suppression of palmitate Ra (%) | 80 (75, 87) | 35–97 |
Experimental protocol
Subjects were admitted to the Clinical Research Unit in the evening before the study and consumed a standard meal containing approximately 10 kcal/kg FFM (50% of total energy from carbohydrate, 30% from fat, and 20% from protein). The following morning, after subjects fasted for 12 h overnight, a hyperinsulinemic-euglycemic clamp procedure (3.5 h basal period and a 4 h insulin infusion) was performed. Stable isotopically labeled tracers ([6,6-2H2]glucose and [2,2-2H2]palmitate) were infused to evaluate glucose and fatty acid kinetics. After the 3.5-h basal period, insulin was infused at a rate of 50 mU/m2 body surface area per minute. Euglycemia was maintained at a blood glucose concentration of approximately 100 mg/dl (5.6 mm) by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained immediately before starting the tracer infusion and every 10 min during the final 30 min of the basal and insulin infusion periods to determine plasma glucose, FFA and insulin concentrations, and substrate kinetics, as described previously (11).
Statistical analysis
Data sets were examined for normality according to the Shapiro-Wilks criteria. With the exception of the basal plasma FFA concentration and plasma insulin concentration during insulin infusion, all other variables were not normally distributed. Therefore, Spearman's correlation coefficient (rs) was computed to examine the relationships between outcome variables, and data sets were ranked for presentation. Differences in the strength between different correlations were evaluated by using Steiger's Z-test for correlated correlations. Descriptive characteristics are presented as medians with quartiles. A P ≤ 0.05 was considered statistically significant.
Results
During basal conditions, there was a 5-fold range in palmitate rate of appearance (Ra) into the bloodstream and a 2-fold range in glucose rate of disappearance (Rd) from the bloodstream (Table 1). Median plasma insulin concentration increased from 12 (7, 21) mU/liter in the basal state to 91 (77, 111) mU/liter during the clamp procedure, which is within the physiological postprandial range (8, 12, 13). Insulin infusion caused a marked reduction in plasma FFA concentration and palmitate Ra and an increase in glucose Rd, but values varied greatly among subjects with a 9-fold range in the insulin-suppressed palmitate Ra and a 48-fold range in the insulin-stimulated increase in glucose Rd (Table 1). Values for BMI were positively associated with basal palmitate Ra (rs = 0.359) and palmitate Ra during insulin infusion (rs = 0.561), and negatively associated with insulin-mediated suppression of palmitate Ra (rs = −0.421) and insulin-mediated stimulation of glucose Rd (rs = −0.572) (all P < 0.001).
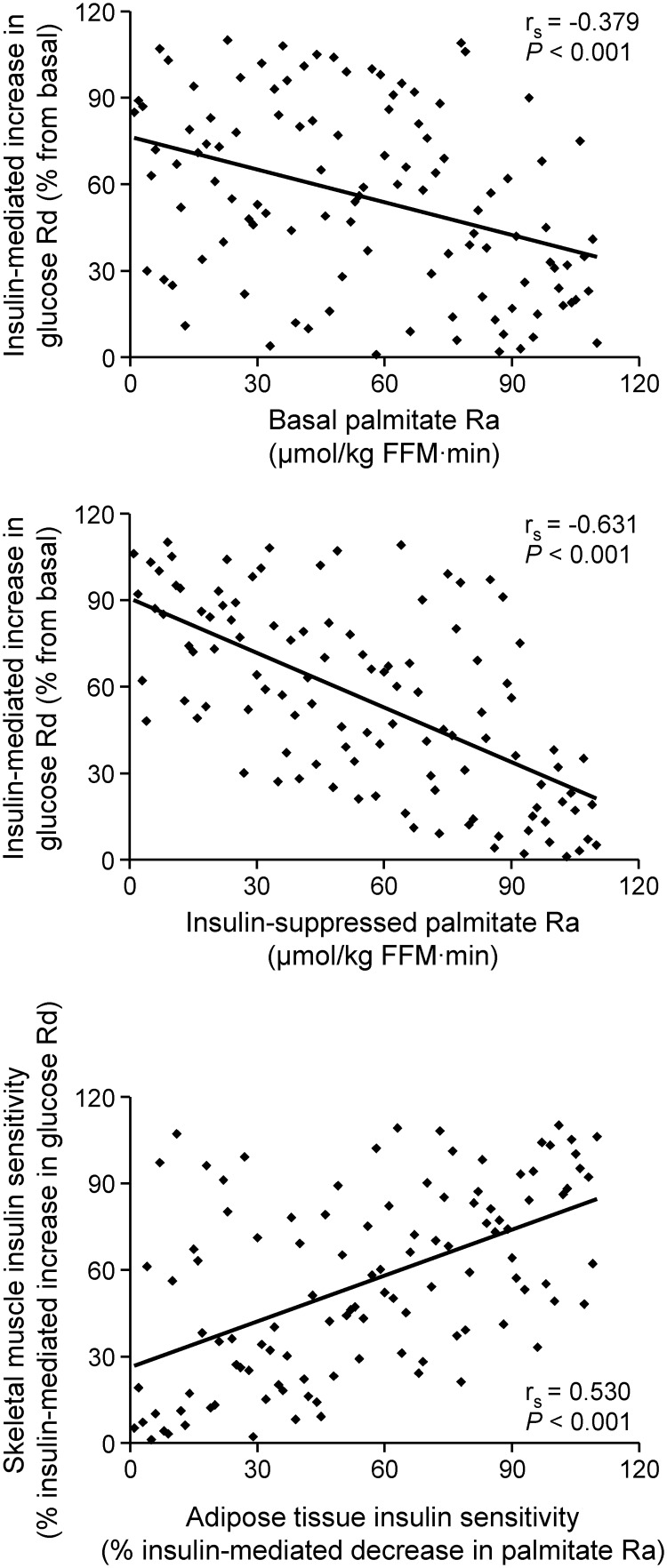
Values for palmitate Ra, expressed per kilogram of FFM, which provides an index of FFA availability to lean tissues, were negatively correlated with skeletal muscle insulin sensitivity (defined as the relative increase in glucose Rd during insulin infusion) during both basal conditions (rs = −0.379, P < 0.001, Fig. 1, top panel) and insulin infusion (rs = −0.631, P < 0.001, Fig. 1, middle panel). The strength of the correlation between palmitate Ra and skeletal muscle insulin sensitivity was greater for palmitate Ra during insulin infusion than palmitate Ra during basal conditions (P = 0.0007). The same relationships between palmitate Ra and glucose metabolism were obtained when the absolute values for insulin-mediated glucose Rd or the glucose infusion rate were used instead of the relative increase in glucose Rd during insulin infusion because these three parameters were highly intercorrelated (rs values > 0.92). In addition, similar relationships between fatty acid metabolism and skeletal muscle insulin sensitivity were obtained when either palmitate Ra expressed relative to the fat mass (rs = −0.112, P = 0.244 for basal palmitate Ra and rs = −0.557, P < 0.001 for insulin-suppressed palmitate Ra) or FFA concentration (rs = −0.102, P = 0.290 for basal plasma FFA concentration and rs = −0.706, P < 0.001 for insulin suppressed plasma FFA concentration) were used instead of palmitate Ra per kilogram of FFM. The relative suppression of palmitate Ra during insulin infusion (an index of adipose tissue insulin sensitivity) correlated directly with the relative stimulation of glucose Rd during insulin infusion (rs = 0.530, P < 0.001, Fig. 1, bottom panel).
Fig. 1.
Relationship between skeletal muscle insulin sensitivity, assessed as the relative increase in glucose rate of disappearance (Rd) during insulin infusion (% from basal), and palmitate rate of appearance (Ra) into the systemic circulation in the basal state (top) and during insulin infusion (middle), and adipose tissue insulin sensitivity (bottom), assessed as the relative decrease in palmitate Ra during insulin infusion (% from basal), in 110 non-diabetic women. Data are ranked for presentation.
Discussion
In this study, we evaluated whether increased adipose tissue lipolytic activity is associated with skeletal muscle insulin resistance. Accordingly, we measured FFA kinetics during both basal conditions and insulin infusion in a large number of nondiabetic women, who had a wide range in BMI, percent body fat, rates of FFA kinetics, and skeletal muscle insulin sensitivity, to enhance our ability to detect a link between adipose tissue lipolytic rate and insulin action. Our data demonstrate that palmitate Ra expressed per kilogram FFM, which provides an index of FFA availability to lean tissues, was negatively correlated with skeletal muscle insulin sensitivity during both basal conditions and during an infusion of insulin designed to simulate postprandial conditions. However, the overall rate of FFA release during both postabsorptive and simulated postprandial states accounted for less than half of the variability in skeletal muscle insulin sensitivity, indicating that other factors must be involved in the pathogenesis of insulin resistance. In addition, the association between FFA kinetics and insulin sensitivity was almost entirely due to FFA Ra during insulin infusion, when lipolytic rates and plasma FFA concentrations were markedly suppressed to less than 20% of basal values. These observations question whether increased adipose tissue lipolytic rate is an important cause of insulin resistance in obese people because the relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance was weakest when FFA availability was highest (basal state) and strongest when FFA availability was lowest (insulin infusion). The direct correlation between the suppression of palmitate Ra (adipose insulin sensitivity) and the increase in glucose Rd (skeletal muscle insulin sensitivity) during insulin infusion is likely due to simultaneous insulin resistance in multiple organs, rather than a direct effect of the adipose tissue FFA release on skeletal muscle insulin action.
The data from the present study challenge the view that excessive adipose tissue lipolytic activity is responsible for insulin resistance in obese people (1). This notion is consistent with a careful assessment of data from a series of studies that found no differences in: 1) FFA Ra expressed per kilogram of FFM measured over 24 h in insulin-sensitive lean and insulin-resistant abdominally obese men (8); 2) fasting and postprandial FFA Ra in insulin-resistant and insulin-sensitive overweight subjects (14); 3) basal FFA Ra in insulin-resistant and insulin-sensitive obese subjects (9); and 4) basal and insulin-stimulated glucose kinetics in upper-body obese and lower-body obese women, despite significant differences in FFA kinetics (15). However, fasting and postprandial plasma insulin concentrations were consistently greater in insulin-resistant than insulin-sensitive subjects (8, 9, 14), which could normalize lipolytic rates in insulin-resistant subjects because adipose tissue lipolytic activity is very sensitive to small increases in circulating insulin (16). Therefore, the composite of these data suggests that the hyperinsulinemia associated with insulin resistance is able to adequately suppress adipose tissue lipolytic rate and prevent excessive release of FFA into the bloodstream during both basal and postprandial conditions in nondiabetic, obese people.
Even though the rate of release of FFA from adipose tissue might not be very different in insulin-resistant and insulin-sensitive subjects, this does not mean that skeletal muscle fatty acid uptake is not increased in insulin-resistant people. We have previously found that gene expression and protein content of CD36, the major transport protein responsible for tissue FFA uptake (17), was lower in adipose tissue and higher in skeletal muscle in insulin-resistant than insulin-sensitive subjects (9). These findings are consistent with data from a recent study that found day-long plasma FFA availability to lean tissues was the same in insulin-sensitive lean and insulin-resistant obese subjects, but adipose tissue FFA uptake was impaired in the obese group (8). Together these observations suggest that skeletal muscle insulin resistance in obese people is associated with a redirection of circulating FFA away from adipose tissue and toward skeletal muscle, resulting in increased muscle FFA uptake (18) and generation of intramyocellular fatty acid metabolites (e.g. diacylglycerols, ceramides, and fatty acyl CoA/carnitines) that can impair insulin signaling (19, 20). Therefore, tissue FFA uptake and intracellular handling of FFA might be more important determinants of insulin action than the rate of release of FFA from adipose tissue.
Our study has several important limitations. We only studied nondiabetic women to avoid the potential influence of sex (21) and diabetes (16) on FFA kinetics. Therefore, we cannot exclude the possibility that our results might be different among men or in subjects with diabetes who have significantly impaired β-cell function and are unable to mount an adequate insulin response. Furthermore, we did not measure the rate of FFA release into plasma over 24 h. However, studying subjects during the basal state and during an infusion of insulin designed to simulate typical postprandial peak insulin concentrations allowed us to provide a robust evaluation of adipose tissue lipolytic activity and skeletal muscle insulin sensitivity. Finally, we cannot exclude the possibility that the relationship between FFA kinetics and insulin sensitivity might be different for hepatic insulin sensitivity, which could not be assessed reliably with our experimental design because the infusion of insulin resulted in complete or near-complete suppression (90–100%) of endogenous glucose production in half of our subjects.
The results from the present study demonstrate that the pathogenesis of skeletal muscle insulin resistance in obese people is complex. Our findings challenge the view that excessive rates of adipose tissue triglyceride lipolysis is an important cause of skeletal muscle insulin resistance associated with obesity in people who do not have diabetes. Nonetheless, these results do not preclude the possibility that fatty acids are important mediators of insulin resistance, and it is possible that redirection of plasma FFA trafficking away from adipose tissue and toward skeletal muscle and subsequent alterations in fatty acid metabolism within skeletal muscle itself lead to metabolic dysfunction.
Acknowledgments
We thank Freida Custodio, Jennifer Shew, and Dr. Adewole Okunade for technical assistance; the staff of the Clinical Research Unit for their help in performing the studies; and the study subjects for their participation.
This work was supported by National Institutes of Health Grants DK37948, DK56341 (Nutrition Obesity Research Center), RR024992 (Clinical and Translational Science Award), and RR00954 (Biomedical Mass Spectrometry Resource).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- FFA
- free fatty acid
- FFM
- fat-free mass
- Ra
- rate of appearance
- Rd
- rate of disappearance
- rs
- Spearman's correlation coefficient.
References
- 1. Boden G. 2011. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. 1983. Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. 1993. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roden M, Krssak M, Stingl H, Gruber S, Hofer A, Fürnsinn C, Moser E, Waldhäusl W. 1999. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 48:358–364 [DOI] [PubMed] [Google Scholar]
- 5. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. 1999. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841 [DOI] [PubMed] [Google Scholar]
- 6. Kleiber H, Munger R, Jallut D, Tappy L, Felley C, Golay A, Frascarolo P, Jéquier E, Felber JP. 1992. Interaction of lipid and carbohydrate metabolism after infusions of lipids or of lipid lowering agents: lack of a direct relationship between free fatty acid concentrations and glucose disposal. Diabetes Metab 18:84–90 [PubMed] [Google Scholar]
- 7. Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S, Coppack SW. 2000. Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. European Group for the Study of Insulin Resistance (EGIR). Eur J Clin Invest 30:45–52 [DOI] [PubMed] [Google Scholar]
- 8. McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KN, Karpe F. 2011. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. 2009. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106:15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. 2008. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 134:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. 2008. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134:1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk ML, Potts JL, Hockaday TD. 1992. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 41:264–272 [DOI] [PubMed] [Google Scholar]
- 13. Roust LR, Jensen MD. 1993. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42:1567–1573 [DOI] [PubMed] [Google Scholar]
- 14. Bickerton AS, Roberts R, Fielding BA, Tornqvist H, Blaak EE, Wagenmakers AJ, Gilbert M, Humphreys SM, Karpe F, Frayn KN. 2008. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia 51:1466–1474 [DOI] [PubMed] [Google Scholar]
- 15. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. 1989. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83:1168–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen MD, Caruso M, Heiling V, Miles JM. 1989. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38:1595–1601 [DOI] [PubMed] [Google Scholar]
- 17. Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. 2004. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18:1144–1146 [DOI] [PubMed] [Google Scholar]
- 18. Koonen DP, Glatz JF, Bonen A, Luiken JJ. 2005. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 1736:163–180 [DOI] [PubMed] [Google Scholar]
- 19. Eckel RH, Grundy SM, Zimmet PZ. 2005. The metabolic syndrome. Lancet 365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 20. Lewis GF, Carpentier A, Adeli K, Giacca A. 2002. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23:201–229 [DOI] [PubMed] [Google Scholar]
- 21. Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. 2009. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 17:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]