Abstract
Context:
We wanted to investigate vitamin D in low-risk prostate cancer.
Objectives:
The objective of the study was to determine whether vitamin D3 supplementation at 4000 IU/d for 1 yr is safe and would result in a decrease in serum levels of prostate-specific antigen (PSA) or in the rate of progression.
Design:
In this open-label clinical trial (Investigational New Drug 77,839), subjects were followed up until repeat biopsy.
Setting:
All subjects were enrolled through the Medical University of South Carolina and the Ralph H. Johnson Veterans Affairs Medical Center, both in Charleston, SC.
Patients and Other Participants:
All subjects had a diagnosis of low-risk prostate cancer. Fifty-two subjects were enrolled in the study, 48 completed 1 yr of supplementation, and 44 could be analyzed for both safety and efficacy objectives.
Intervention:
The intervention included vitamin D3 soft gels (4000 IU).
Main Outcome Measures:
Adverse events were monitored throughout the study. PSA serum levels were measured at entry and every 2 months for 1 yr. Biopsy procedures were performed before enrollment (for eligibility) and after 1 yr of supplementation.
Results:
No adverse events associated with vitamin D3 supplementation were observed. No significant changes in PSA levels were observed. However, 24 of 44 subjects (55%) showed a decrease in the number of positive cores or decrease in Gleason score; five subjects (11%) showed no change; 15 subjects (34%) showed an increase in the number of positive cores or Gleason score.
Conclusion:
Patients with low-risk prostate cancer under active surveillance may benefit from vitamin D3 supplementation at 4000 IU/d.
The hypothesis that vitamin D could provide anticancer benefits was recognized from epidemiological studies decades ago; Apperly (1) reported the association between latitude and cancer mortality based on sun exposure. After being forgotten for decades, the relationship between vitamin D and cancer was resurrected through basic science and further epidemiological studies. Abe et al. (2), while studying the metabolism of vitamin D in myeloid leukemia cells, made the important observation that 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] actually induced the differentiation of these neoplastic cells. It was later determined that this differentiation was due to the regulation of c-myc by 1,25(OH)2D3 (3). A few years later, it was hypothesized that inadequate vitamin D status, resulting from limited solar exposure at higher latitudes, was a risk factor for the development of various noncutaneous malignancies (4–6). Since that time, literally thousands of studies encompassing basic science, clinical trials, and epidemiology have been published with respect to vitamin D and cancer.
Prostate cancer is of great interest from the standpoint of vitamin D interaction because prostate cells express the vitamin D receptor (VDR), vitamin D-25-hydroxylase, 25-hydroxy-vitamin D-1-α-hydroxylase, and the 25-hydroxyvitamin D-24-hydroxylase (7–11). Recent observational studies investigating the association between vitamin D status, as defined by circulating 25-hydroxyvitamin D [25(OH)D], and prostate cancer have produced variable results ranging from protection to increased risk (12–15). Although one in six men will be diagnosed with prostate cancer during his lifetime, only one in 36 men will die of prostate cancer (American Cancer Society: Learn about Cancer; http://www.cancer.org/Cancer/ProstateCancer/DetailedGuide/prostate-cancer-key-statistics). Therefore, many indolent prostate cancers are monitored over time in an effort to determine which progress into clinically important, more aggressive cancers. Thus, it provides an excellent model in which to study the effects of enhancing vitamin D status and related changes in tumor progression over an extended period of time. Also, treatment with vitamin D3 is without any known potential side effects other than hypercalcemia and/or hypercalcuria, which rarely occur and are easily corrected.
Digital rectal examination and measurement of the serum level of prostate-specific antigen (PSA) remain the standard of care for prostate cancer screening, despite significant shortcomings (17). There is general agreement that the introduction of PSA testing led to a measurable decrease in U.S. death rates from prostate cancer (18), but two large randomized trials of PSA screening gave mixed results for improvements in overall survival (19, 20). Nonetheless, the latest analysis of the Surveillance, Epidemiology, and End Results program reveals a very favorable 10-yr outcome in the PSA era, after conservative management of early-stage, well-differentiated prostate cancer (21). Additional longitudinal studies have revealed that the risk of dying from prostate cancer after 20 yr was only 30% with a Gleason score of 6 (22). Therefore, a considerable number of patients diagnosed with low-grade, low-risk prostate cancer may not require definitive treatment (surgery, radiation, or a combination thereof), especially considering possible side effects such as urinary incontinence, bladder dysfunction, rectal irritation, and erectile dysfunction. Thus, the generally indolent nature of prostate cancer and the risk of side effects from surgery or radiation therapy have led to active surveillance as an option for early-stage, low-risk prostate cancer, in which definitive treatment is deferred to a later date or even indefinitely. However, active surveillance is essentially a monitoring regimen that does not specifically address the issue of how to treat low-risk disease.
It has been recently reported that low serum levels of 25(OH)D (<50 nmol/liter equivalent to <20 ng/ml) strongly correlate with death from prostate cancer (12), raising the possibility that vitamin D deficiency over time would favor the progression of subclinical prostate cancer to clinical disease (23). Additional studies have shown that LNCaP prostate cancer cells treated with 1,25(OH)2D3 undergo apoptotic cell death. Vitamin D-induced apoptosis correlates with down-regulation of Bcl-2 expression and appears to be caspase dependent by primarily triggering the mitochondrial pathway of cell death (24, 25). Furthermore, neoplastic progression in prostate tissue appears to be associated with loss of 25-hydroxyvitamin D3 1α-hydroxylase activity (26). However, the latest recommendations from the Institute of Medicine (IOM) concerning dietary reference intake for calcium and vitamin D (27) emphasize that the role of vitamin D in non-bone-related health issues such as cancer remains unresolved because of the conflicting nature of the available evidence. These doubts have been reaffirmed in a recent article (28). It appears that clinical studies involving robust and sustained, but nontoxic, vitamin D3 supplementation would shed some clarity on this important issue. Therefore, we sought to determine whether daily supplementation with 4000 IU vitamin D3 for 1 yr was safe and of clinical benefit in patients electing active surveillance for low-risk prostate cancer.
Subjects and Methods
Subjects
Eligible subjects had histologically confirmed diagnosis of adenocarcinoma of the prostate (Gleason score ≤6, PSA ≤10, clinical stage T1c or T2a) and had elected to be managed through active surveillance. For the purpose of eligibility, these additional criteria were verified: serum creatinine 2.0 mg/dl or less, serum phosphate (measured as phosphorus) greater than 2.3 and less than 4.8 mg/dl, and serum calcium greater than 8.5 and less than 10.5 mg/dl. Exclusion criteria were the following: any concurrent malignancy, except nonmelanoma skin cancer; history of sarcoidosis; vitamin D3 supplementation at 1000 IU/d or greater; history of hypercalcemia; or use of lithium as a medication. This clinical trial was approved by the Institutional Review Board of the Medical University of South Carolina (MUSC) and the Veterans Affairs Medical Center (www.ClinicalTrials.gov Identifier: NCT01045109).
Objectives
The objectives of this study were to determine whether vitamin D3 (4000 IU/d for 1 yr) would be safe and result in a measurable decrease of serum PSA levels in a significant number of enrolled subjects and be associated with a stabilization or improvement of their disease assessed through histological examination of repeat biopsy specimens (Gleason score and number of positive cores), obtained at the end of the study as part of the standard medical care for active surveillance.
Study medication
Food and Drug Administration Investigational New Drug no. 77,839 was granted for clinical studies designed to assess the effect of vitamin D3 (4000 IU/d for 1 yr) on subjects with early-stage prostate cancer. The vitamin D3 (cholecalciferol) was manufactured by J. R. Carlson Laboratories, Inc. (Arlington Heights, IL).
Methods
Serum levels of vitamin D3 (cholecalciferol) were determined by HPLC; 25(OH)D, 1,25-dihydroxyvitamin D [1,25(OH)2D], and PTH were determined by RIA, as previously described (29, 30). Serum levels of PSA were measured in the Clinical Laboratories of MUSC by the Siemens Advia Centaur Immunoassay System (Chemiluminescence Technology). Assessment of tissue biopsy specimens, before enrollment and after study completion, was part of the standard of care for each subject and was performed by pathologists who were blinded to the study.
Clinical trial study design
The open-label study enrolled 52 eligible subjects. Forty-eight subjects completed the study and were included in the safety analysis; these subjects had complete PSA laboratory results for inclusion in the evaluation of changes in PSA. Forty-four subjects had both baseline and repeat biopsy (as part of their standard of care) to compare the number of positive cores and Gleason score with baseline, after completing vitamin D3 supplementation. All subjects had study visits every 2 months for 1 yr to measure serum levels of 25(OH)D, PSA, phosphorus, and PTH, plus complete blood count, basic metabolic panel, and urinary calcium to creatinine ratio (to rule out any potential toxicities from vitamin D3 supplementation). In addition, circulating levels of vitamin D3 (cholecalciferol) and 1,25(OH)2D (calcitriol) were measured at baseline and exit in 19 subjects for whom extra serum samples were available.
Historical control subjects
From an institutional (MUSC) database of more than 700 patients diagnosed with prostate cancer within the last 5 yr, we identified 19 control subjects who were under active surveillance, underwent repeat biopsies, and met all eligibility criteria of the open-label clinical trial, except that they received no vitamin D3 supplementation. We selected only patients who had their follow-up biopsy at 10 months or later after their initial positive biopsy to ensure comparability with the timing of the follow-up visit of the patients in the open-label trial. All subjects who met eligibility criteria were included. From these control subjects, we abstracted information on their serum PSA levels and assessment of tissue biopsy specimens.
Statistical analysis
For PSA values, before comparison of means or paired differences by t test, a natural log transformation was performed to stabilize variance. As a result, hypothesis tests were based on differences in log(PSA+1). Paired t tests and signed rank tests were used to assess differences in serum levels of 25(OH)D, log PSA, PTH, cholecalciferol, and 1,25(OH)2D at enrollment and at the end of the study. Changes from baseline to follow-up in Gleason score and number of positive scores of biopsies were assessed using a sign test. Fisher's exact test was used to test for differences in proportions.
Random effects models, which included random effects per patient, were used to assess changes in the number of cores over time and to test that the resulting slope was different from zero. The log of number of cores+1 was used as the outcome to adhere to assumption of the regression (i.e. linearity of the trend and homoscedasticity). Due to differentials in times between baseline and follow-up biopsy, we had concerns regarding influence of follow-up biopsies that occurred relatively late (i.e. 48 months or longer after the baseline biopsy). Regression models were estimated including and excluding data points greater than 24, greater than 36, and greater than 48 months from baseline to determine how inferences were affected. The inferences were unchanged: the P value remained significant, and the magnitude of the slope changes were only slightly dependent on the inclusion or exclusion of the later biopsy data.
Results
Study population
Eligible subjects were enrolled through the Departments of Radiation Oncology and Urology at MUSC and through the Urology Clinic of the Ralph H. Johnson Veterans Affairs Medical Center. Fifty-two subjects were enrolled in this open-label study. Four subjects were removed from the study due to exclusionary criteria: a diagnosis of colorectal cancer, noncompliance, elevated serum PSA (>10 ng/ml), and voluntary withdrawal. Forty-eight subjects completed 1 yr of vitamin D3 supplementation. Forty-four subjects who completed supplementation and underwent 12-core biopsy at baseline and at the end of supplementation could be analyzed for both study objectives (Table 1).
Table 1.
Characteristics of supplemented subjects (n = 52)
| Variable | Baseline | Follow-up | P value |
|---|---|---|---|
| Age | |||
| Mean [range] | 65 [49, 78] | ||
| Race, n (%) | |||
| Caucasian | 37 (71%) | ||
| AA | 14 (27%) | ||
| Asian | 1 (2%) | ||
| 25(OH)D (ng/ml serum) | |||
| Mean (sd) [range] | 32.8 (13.3) [11.7, 75.0] | 66.2 (14.5) [35.7, 95.4] | <0.00001a |
| PTH (pg/ml serum) | |||
| Mean (sd) [range] | 45.5 (18.9) [7.6, 117.1] | 36.2 (15.3) [8.9, 71.4] | 0.00002a |
| Cholecalciferol (n = 19) (ng/ml serum) | |||
| Mean (sd) [range] | 6.23 (3.32) [2.2, 13.7] | 13.08 (3.73) [6.5, 20.6] | <0.00001 |
| 1,25(OH)2D (n = 19) (pg/ml serum) | |||
| Mean (sd) [range] | 37.8 (15.2) [17.3, 65.7] | 46.6 (19.6) [22.5, 107.5] | 0.02 |
| Months from baseline to follow-up biopsy | |||
| Mean (sd) [range] | 20.2 (9.8) [12, 63] | ||
| Gleason n(%) | |||
| 0 | 0 | 15 (34%) | 0.08b |
| 6 | 50 (100%) | 23 (52%) | |
| 7 | 0 | 4 (9%) | |
| 8 | 0 | 2 (5%) | |
| Missing | 2 | 8 | |
| Number of positive cores (of 12), n (%) | |||
| 0 | 0 (0%) | 15 (34%) | |
| 1 | 24 (48%) | 9 (20%) | 0.03b |
| 2 | 11 (22%) | 7 (16%) | |
| 3 | 7 (14%) | 5 (11%) | |
| 4 | 4 (8%) | 5 (11%) | |
| 5 | 3 (6%) | 3 (7%) | |
| 6 | 12 (%) | 0 (0%) |
AA, African-American.
P values for comparison of pre- and post-25(OH)D and PTH based on paired t test.
P values for comparison of pre- and post-Gleason score and number of positive cores based on the nonparametric sign test.
The 19 historical controls fit all eligibility criteria for the open-label trial and are described in Table 2. As can be seen from the age, race, and baseline distributions of Gleason scores and number of positive cores, the control population was similar to the open-label study population.
Table 2.
Characteristics of historical control subjects (n = 19)
| Variable | Baseline | Follow-up | P value |
|---|---|---|---|
| Age | |||
| Mean (sd) [range] | 66 (7) [50, 81] | ||
| Race, n (%) | |||
| Caucasian | 15 (79%) | ||
| AA | 4 (21%) | ||
| Asian | 0 (0%) | ||
| Months from baseline to follow-up biopsy | |||
| Mean (sd) [range] | 18.4 (8.0) [10, 42] | ||
| Gleason | |||
| 0 | 0 (0%) | 2 (11%) | 1.00a |
| 6 | 18 (95%) | 13 (68%) | |
| 7 | 1 (5%) | 4 (21%) | |
| Number of positive cores (of 12), n (%) | |||
| 0 | 0 (0%) | 2 (11%) | 0.12a |
| 1 | 11 (58%) | 4 (21%) | |
| 2 | 5 (26%) | 4 (21%) | |
| 3 | 2 (11%) | 4 (21%) | |
| 4 | 0 (0%) | 5 (26%) | |
| 5 | 1 (5%) | 0 (0%) |
AA, African-American.
P values for comparison of pre- and post-Gleason score and number of positive cores based on the nonparametric sign test.
Safety and efficacy
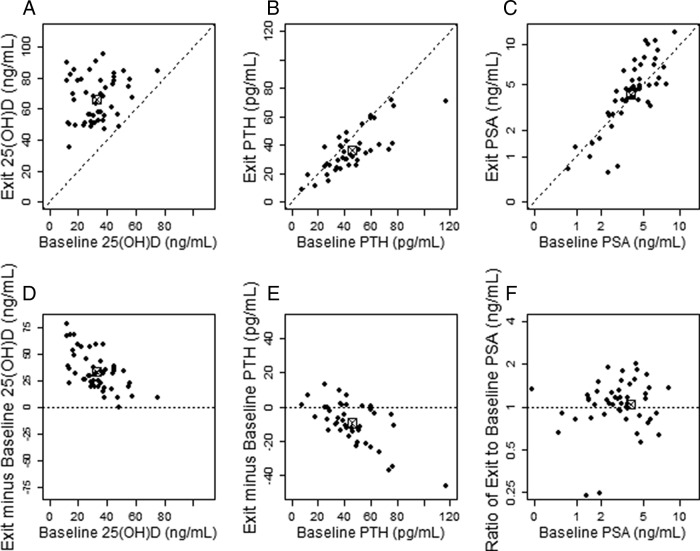
Supplementation with vitamin D3 at 4000 IU/d for 1 yr appeared to correct all cases of vitamin D deficiency or insufficiency, although the magnitude of the response varied among subjects. No adverse events linked to vitamin D3 supplementation at 4000 IU/d were observed. Table 1 and Fig. 1 show that exit values of serum 25(OH)D (expressed as mean ± sd in nanograms per milliliter) were significantly higher than entry values (66.2 ± 14.5 vs. 32.8 ± 13.3, respectively; P < 0.00001). As a result of supplementation, serum levels of PTH (expressed as mean ± sd in picograms per milliliter) were significantly lower at the end of the study (exit: 36.2 ± 15.3 vs. entry: 45.5 ± 18.9; P = 0.00002), confirming that secondary hyperparathyroidism can be a consequence of vitamin D insufficiency or deficiency. Figure 1, D and E, also shows the change in serum values of 25(OH)D and PTH (exit minus baseline value), respectively, as a function of baseline levels for each of 48 analyzable subjects enrolled in the study and suggests that subjects with lower baseline levels tend to have larger increases. However, serum PSA levels (expressed as median ± sd in nanograms per milliliter) remained relatively constant, and changes do not appear to be related to the baseline PSA level (entry: 4.1 ± 1.8 vs. exit: 4.5 ± 2.7; P = 0.27), as shown in Fig. 1, C and E. Furthermore, serum PSA levels did not seem to correlate with the repeat biopsy outcome (see below).
Fig. 1.
Entry vs. exit (after treatment) serum values of 25(OH)D, PTH, and PSA. Each black point represents an individual subject. A–C, Entry values are read off the horizontal x-axis (baseline); exit values are read off the vertical y-axis (exit). D–F, Change in serum values of 25(OH)D, PTH, and PSA, respectively. The marked empty square ([X]) in each panel represents the mean entry and posttreatment value (A–C) and the mean change in value for each parameter (D–F). Note the significant increase in 25(OH)D serum values (P < 0.00001), the significant decrease in PTH serum values (P = 0.00002), and the overall stability of PSA serum values after vitamin D3 supplementation (P = 0.27).
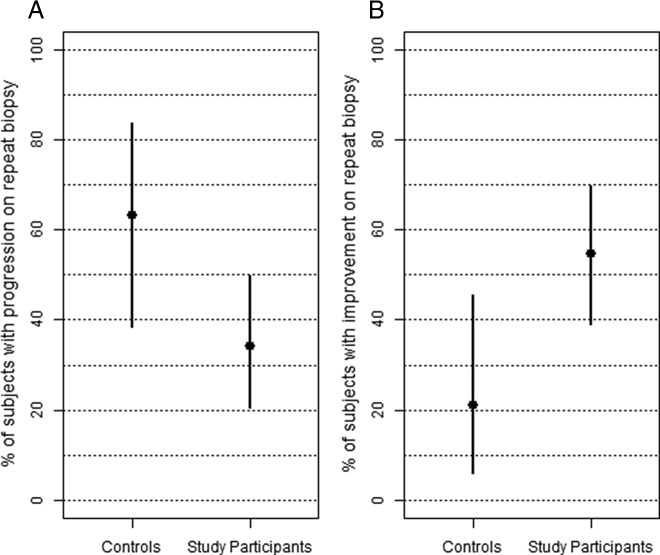
At baseline, the mean number of positive cores (±sd) for the 44 analyzable subjects was 2.1 ± 1.4; however, at repeat biopsy the corresponding mean value decreased to 1.7 ± 1.6. To determine whether individual subjects improved or progressed, we compared the number of positive cores and Gleason grade at baseline and repeat biopsy (Fig. 2). By these criteria, 34% of these subjects progressed (Fig. 2A), defined as an increase in the number of positive cores or in Gleason score; one of the subjects progressed due to an increase in Gleason score (from 6 to 8 in one core), despite a decrease in the number of positive cores (from five to two), and was classified as having had a progression. Five subjects (11%) showed no change. However, more than half of the subjects (55%) showed improvement (Fig. 2B), defined as a decrease in positive cores and no increase in Gleason score at repeat biopsy. Positive core data were also analyzed according to baseline levels of 25(OH)D, subdividing subjects into two groups using 20 ng/ml as a cutoff serum value (Fig. 2). These results suggest that subjects who are vitamin D deficient at baseline [25(OH)D <20 ng/ml serum] may benefit less than the other subjects from vitamin D3 supplementation, although this inference is premature because of the small number of subjects involved in these subsets analyses.
Fig. 2.
Comparison of repeat biopsy outcome in vitamin D3-supplemented subjects. Estimates (in percent) of progression (A) and improvement (B) for all analyzable subjects (n = 44, y-axis) and by entry values of serum 25(OH)D (<20 ng/ml vs. >20 ng/ml, x-axis) are shown. Bars are exact 95% confidence intervals (CI). Progression (CI in parentheses): 34% of all subjects (0.20, 0.50); 45% of vitamin D-deficient subjects (0.17, 0.77); and 30% of nondeficient subjects (0.16, 0.49). Improvement (CI in parentheses): 55% of all subjects (0.39, 0.70); 36% of vitamin D-deficient subjects (0.11, 0.69); and 61% of nondeficient subjects (0.42, 0.77).
We also compared the number of positive cores and Gleason grade at baseline and repeat biopsy in the supplemented group and the control group (Fig. 3). Using the same criteria, 63% of the subjects in the control group progressed (because of an increase in the number of positive cores or in Gleason score), compared with 34% in the supplementation group (P = 0.05), as seen in Fig. 3A. Furthermore, in the historical control group, four patients (21%) had improvements in biopsy results, three showed no differences (16%), and 12 (63%) progressed. Comparing the proportions who responded in each group (Fig. 3B), a significant difference was found (P = 0.025).
Fig. 3.
Comparison of repeat biopsy outcome between vitamin D3-supplemented subjects and historical controls. A, Supplemented subjects appeared to have a lower rate of progression (P = 0.0514) compared with baseline. B, Supplemented subjects appeared to have a significantly higher rate of improvement (P = 0.0257) compared with baseline. Bars are exact 95% confidence intervals (CI).
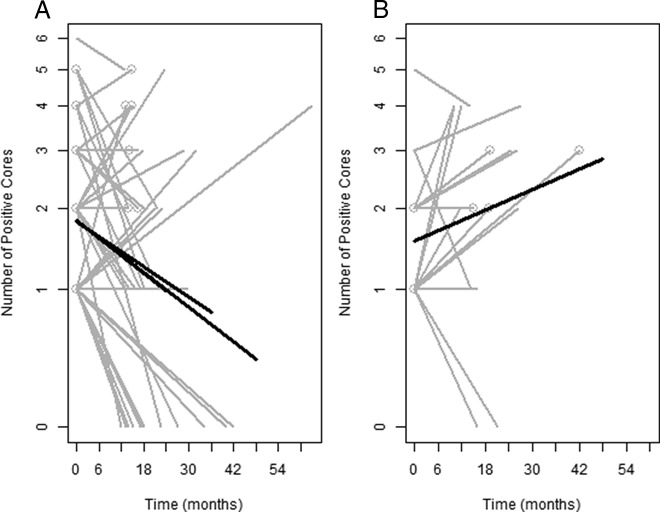
Figure 4, A and B, shows graphical displays of the positive cores over time in which baseline biopsy occurs at time 0. The baseline and the repeat biopsy for each individual are connected by gray lines. The fitted regression lines (in black) indicate that, over time, the number of positive cores tends to decrease in the supplementation group (Fig. 4A), whereas it tends to increase in the untreated control group (Fig. 4B). The slope of the regression line is not significantly different from 0 in the control group (P = 0.19), whereas it is significantly different in the supplementation group (P = 0.017). The slopes of the two fitted regression lines are significantly different from one another (P = 0.02). Data represented in Fig. 4A were analyzed using three different thresholds: follow-up through 24, 36, and 48 months, respectively (i.e. all data from time points beyond the threshold are not used in the model estimation). Interestingly, the slopes are identical with four decimal places when using the 24- and 48-month time thresholds (b = −0.0146), whereas the slope when using a threshold of 36 months is slightly flatter (−0.0125). All of these values are significant at the P < 0.01 level, evidence of a decline in the number of positive cores over time.
Fig. 4.
Trajectories of number of positive cores over time in subjects supplemented with vitamin D3 (A) and untreated historical control subjects (B). A and B, Gray lines represent individual patient data, and thick solid black lines are the fitted estimate including all data within 48 months of baseline. The gray circles at the ends of some of the gray lines refer to subjects with an increase of Gleason score at repeat biopsy or a decrease of Gleason score from baseline. A, The thick solid black lines are the fitted estimates including all data for 24-, 36-, and 48-month follow-up period, respectively. The slopes are identical between the 24- and 48-month time thresholds (b = −0.0146), whereas the slope of the 36-month threshold is slightly flatter (−0.0125). All corresponding P values are significant: 0.0090 (24 months); 0.0098 (36 months); 0.00093 (48 months). B, The slope is not significantly different from 0 (P = 0.17). However, the slopes in A and B (based on all follow-up data) are significantly different from each other (P = 0.002).
We compared serum levels of 25(OH)D (in nanograms per milliliter) at the end of the study between responders (66.8 ± 14.5) and nonresponders (65.5 ± 14.6), but the values were not significantly different (P = 0.77), suggesting that other factor(s), in addition to the level of vitamin D repletion, affect the response to supplementation.
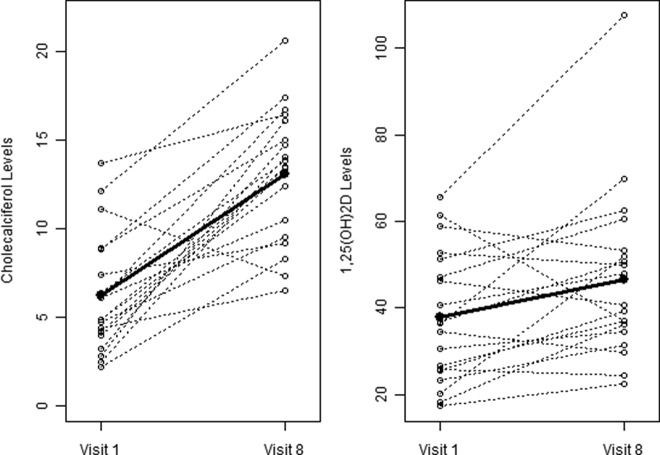
Circulating values of vitamin D3 (cholecalciferol) and 1,25(OH)2D (calcitriol) were measured in 19 subjects for whom extra serum samples were available, and we found significant differences between baseline and exit values of calcitriol (37.8 ± 15.2 vs. 46.6 ± 19.6 pg/ml, respectively; P = 0.023) but no obvious correlation with positive core data. However, there was a more significant increase from baseline in circulating levels of cholecalciferol (6.2 ± 3.3 vs. 13.1 ± 3.7 ng/ml, respectively; P < 0.0001), indicating that these levels are much more responsive to supplementation than calcitriol (Fig. 5).
Fig. 5.
Change in serum levels of cholecalciferol (left panel) and calcitriol (right panel) between baseline (visit 1) and exit (visit 8). Black lines and open circles represent individual values at baseline and exit; thick lines and closed circles show mean values at baseline and exit. Testing that there is a difference in visit 1 vs. visit 8 values, both measures show significance, although cholecalciferol levels (in nanograms per milliliter of serum) increased more dramatically than 1,25(OH)2D levels (in picograms per milliliter of serum) (calcitriol: P = 0.023 based on paired t test; P = 0.016, based on nonparametric signed rank test; cholecalciferol: P < 0.0001 based on paired t test; P = 0.0003 based on nonparametric signed rank test).
Discussion
Increasing numbers of patients diagnosed with low-risk prostate cancer are electing active surveillance due to the relative indolent nature of the disease as well as the risks of side effects from definitive treatment (surgery or radiation therapy). This approach includes regular monitoring of the disease through measurements of serum PSA levels, digital rectal examination, and repeat prostate biopsy in an attempt to identify those who are at risk for progressing to clinically relevant disease. To date, it is unclear what other parameters can help clinicians either identify those at risk for progression or provide treatment measures to prevent progression of disease.
The results of our clinical study suggest that serial measurements of PSA serum levels (every 2 months for 1 yr) are a relatively poor predictor of progression in patients with low-risk prostate cancer. However, the combination of active surveillance and vitamin D3 supplementation at 4000 IU/d resulted in a decreased number of positive cores at repeat biopsy in more than half of patients diagnosed with low-risk prostate cancer. These subjects (responders) are all eligible to remain on active surveillance and do not require definitive treatment (e.g. surgery or radiation therapy). Therefore, this regimen may decrease the chances of overtreatment for patients with low-risk prostate cancer who, based on the results of the repeat biopsy, respond to the combination and remain stable or improve. Conversely, vitamin D3 supplementation did not benefit 35–40% of subjects (nonresponders) for reasons yet to be investigated. Nevertheless, the combination of active surveillance and vitamin D3 supplementation may also help identify those patients (nonresponders) who are more likely to need definitive treatment.
The results of this clinical study indicate that changes in circulating levels vitamin D3 and 25(OH)D are quite substantial. In contrast, the much smaller differences between baseline and exit values of circulating 1,25(OH)2D suggest that renal production of 1,25(OH)2D may be less responsive to vitamin D3 supplementation and does not necessarily correlate with the tissue levels of calcitriol; furthermore, the prostate 25-hydroxyvitamin D-1-α-hydroxylase, unlike the renal enzyme, is not regulated by PTH and calcium (31). In turn, this suggests that vitamin D action on prostate tissue is through an autocrine/paracrine mechanism, rather than the classic renal endocrine pathway. The mechanism of action is likely to be mediated through the delivery of cholecalciferol and 25(OH)D to prostatic cells, with subsequent activation to 25(OH)D and 1,25(OH)2D by CYP2R1 and CYP27B1, respectively (9, 10). A similar mechanism has been described for the control of cathelicidin production by human monocytes and macrophages, for which circulating calcitriol levels are of little consequence (32). The increased circulating levels of cholecalciferol may be of considerable importance because its free concentration would be high enough to enter the cell at an accelerated rate to be hydroxylated first to 25(OH)D and subsequently to 1,25(OH)2D. It should also be noted that the rate of 25-hydroxylation of vitamin D3 decreases dramatically with higher initial circulating 25(OH)D levels. This has been previously reported in a recent study in which production of 25(OH)D decreases when circulating 25(OH)D levels approach 40 ng/ml, probably through feedback inhibition (30).
Comparison of the outcomes of repeat biopsy, between supplemented subjects and historical controls, suggests that supplementation with vitamin D3 at 4000 IU/d may benefit patients with early-stage, low-risk prostate cancer on active surveillance. However, we realize that there are many limitations to the use of historical controls for comparison purposes. The historical control cohort analyzed in this study was small and there may be additional differences in this group, leading to potential biases. Furthermore, we could not measure levels of circulating vitamin D in these control subjects. Therefore, it is essential to validate the effectiveness of vitamin D3 supplementation in active surveillance by conducting a larger-scale, randomized clinical trial.
This study was not intended to investigate any mechanism by which vitamin D may interfere with growth, viability, or survival of prostate cancer cells. However, it has been shown that calcitriol is capable of inducing apoptotic death of prostate cancer cells (24, 25). Also, high VDR expression in human prostate cancer is associated with a reduced risk of lethal disease (8). Furthermore, it has been recently reported that in mice a vitamin D3-deficient diet combined with low VDR expression results in a tissue environment favoring early procarcinogenic events that enhance prostate cancer risk (33). Therefore, our working hypothesis is that vitamin D3 supplementation at 4000 IU/d may benefit patients with low-risk prostate cancer by increasing the prostate tissue levels of 1,25(OH)2D3, which in turn will induce apoptosis of cancer cells. We also propose to use positive cores at repeat biopsy as a progression biomarker to assess the potential benefit of treatment options that specifically target early-stage, low-risk prostate cancer.
Prevention of cancer by vitamin D in humans has relied mostly on observational studies (34). It should be noted that these types of studies all compare the lowest with the highest levels found within a population that is not supplemented and do not have a comparable group with high enough 25(OH)D levels to inhibit cancer progression (35), as we have observed in this study. Using vitamin D to prevent cancer was recently declared ineffective based on three randomized controlled trials (28). Two of the three trials were probably not successful because of either too long intervals between supplementation doses (36) or providing an insufficient amount of vitamin D3 in the treatment arm, which essentially becomes another placebo group (37). Trivedi et al. (36) administered an oral dose of 100,000 IU vitamin D3 on a quarterly basis for 5 yr, which proved to be effective on skeletal homeostasis but was ineffective on cancer prevention. The Women's Health Initiative study, which involved thousands of subjects, was positive for colorectal cancer vs. circulating 25(OH)D levels based on their nested case-control data (37). However, the interventional arm of the study was negative because of insufficient supplementation: in this study, 400 IU/d of vitamin D3 for 7 yr as a treatment would have been unable to significantly raise systemic levels of 25(OH)D. In fact, the actual vitamin D intake in this large study was approximately 280 IU/d if their reported compliance rate is taken into account (37). Furthermore, this study did not perform any posttreatment 25(OH)D analyses on the subjects and implied that a 280 IU/d vitamin D supplement would be an effective dose, which is not likely (38). The third randomized controlled trial (39) was a vitamin D-based skeletal study; however, a secondary analysis of the data demonstrated a strong preventive effect on cancer. Our interventional trial is similar to that of Lappe et al. (39), in that both studies used a daily dose of vitamin D3 that would increase and maintain a stable circulating level of 25(OH)D during the study period, although the dosage used in our study (4000 IU/d) resulted in much higher systemic circulating levels of 25(OH)D. It is worth noting that in both the study by Lappe et al. (39) and our interventional trial, baseline 25(OH)D levels were 70–80 nmol and increased significantly after supplementation to produce the prevention/treatment effects on cancer. According to the recent IOM report, our mean basal circulating 25(OH)D level of 80 nmol far exceeded the recommended level of 50 nmol (27). Furthermore, the IOM report went as far as to say that circulating 25(OH)D levels exceeding 125 nmol could be a risk factor for developing cancer. Our study resulted in 25(OH)D levels reaching 175 nmol with no clear adverse events but with potential oncologic benefits. We therefore hypothesize that there are different vitamin D requirements or thresholds for different biological systems (e.g. skeletal vs. prostate).
It is important to point out the absence of any measurable toxicity of the vitamin D3 treatment used in this clinical study. Supplementation with 4000 IU/d for 1 yr elevated circulating 25(OH)D to a range recently recommended by The Endocrine Society practice guidelines (40). These guidelines, compared with those of the Food and Nutritional Board of the IOM (16), recommend a 2- to 3-fold increase in vitamin D intake, with a tolerable upper intake level of 10000 IU/d. The IOM report also concluded that circulating 25(OH)D levels were unrelated to various neoplasias, including prostate cancer, and in fact higher levels of circulating 25(OH)D could make the cancer worse. These conclusions of the IOM report are not supported by our current data. Our next objective is to conduct a definitive, randomized trial to validate the effectiveness of vitamin D3 supplementation in active surveillance, using an intervention strategy that is extremely cost effective and easy to implement.
Acknowledgments
The contents of this paper do not represent the views of the Department of Veterans Affairs or the United States Government. We thank Drs. Leander Cannick, Jason Zauls, Kyle Russo, and Lewis Cooper (Medical University of South Carolina Department of Radiation Oncology) for helping with the recruitment of eligible subjects.
This work was supported in part by Gateway for Cancer Research Grant G-06-068), the Health Services Research and Development Program of the Department of Veterans Affairs, Veterans Affairs Merit Award CX000163, the Biostatistics Shared Resource, Hollings Cancer Center's Support Grant P30 CA138313 (National Institutes of Health/National Center for Research Resources Grant UL1 RR029881), and South Carolina Clinical and Translational Research Institute Grant UL1 RR029882.
Disclosure Summary: B.W.H. is an academic consultant to the DiaSorin Corp. The other authors have nothing to disclose.
Footnotes
- IOM
- Institute of Medicine
- MUSC
- Medical University of South Carolina
- 25(OH)D
- 25-hydroxyvitamin D
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 1,25(OH)2D3
- 1,25 dihydroxyvitamin D3
- PSA
- prostate-specific antigen
- VDR
- vitamin D receptor.
References
- 1. Apperly FL. 1941. The relation of solar radiation to cancer mortality in North America. Cancer Res 1:191–195 [DOI] [PubMed] [Google Scholar]
- 2. Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. 1981. Differentiation of mouse myeloid leukemia cells induced by 1 alpha, 25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 78:4990–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reitsma PH, Rothberg PG, Astrin SM, Trial J, Bar-Shavit Z, Hall A, Teitelbaum SL, Kahn AJ. 1983. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature 306:492–494 [DOI] [PubMed] [Google Scholar]
- 4. Garland CF, Garland FC. 1980. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9:227–231 [DOI] [PubMed] [Google Scholar]
- 5. Garland FC, Garland CF, Gorham ED, Young JF. 1990. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19:614–622 [DOI] [PubMed] [Google Scholar]
- 6. Lefkowitz ES, Garland CF. 1994. Sunlight, vitamin D, and ovarian cancer mortality rates in U.S. women. Int J Epidemiol 23:1133–1136 [DOI] [PubMed] [Google Scholar]
- 7. Miller GJ, Stapleton GE, Ferrara JA, Lucia MS, Pfister S, Hedlund TE, Upadhya P. 1992. The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1α,25-dihydroxyvitamin D3. Cancer Res 52:515–520 [PubMed] [Google Scholar]
- 8. Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, Fiore C, Penney KL, Ma J, Kantoff PW, Stampfer MJ, Loda M, Mucci LA, Giovannucci E. 2011. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol 29:2378–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellfolk M, Norlin M, Gyllensten K, Wikvall K. 2009. Regulation of human vitamin D3 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol 75:1392–1399 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. 1998. Human prostate cells synthesize 1,25dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev 7:391–395 [PubMed] [Google Scholar]
- 11. Deeb KK, Luo W, Karpf AR, Omilian AR, Bshara W, Tian L, Tangrea MA, Morrison CD, Johnson CS, Trump DL. 2011. Differential vitamin D 24-hydroxylase/CYP24A1 gene promoter methylation in endothelium from benign and malignant human prostate. Epigenetics 6:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. 2009. Association between serum 25(OH)D and death from prostate cancer. Brit J Cancer 100:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang F, Kasperzyk JL, Shui I, Hendrickson W, Hollis BW, Fall K, Ma J, Gaziano JM, Stampfer MJ, Mucci LA, Giovannucci E. 2011. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS One 6:e18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yin L, Raum E, Haug U, Arndt V, Brenner H. 2009. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol 33:435–445 [DOI] [PubMed] [Google Scholar]
- 15. Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M. 2004. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer 108:104–108 [DOI] [PubMed] [Google Scholar]
- 16. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 report on dietary reference intake for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr 2004. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 350:2239–2246 [DOI] [PubMed] [Google Scholar]
- 18. Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, eds. 2009. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER [Google Scholar]
- 19. Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD; PLCO Project Team 2009. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A; ERSPC Investigators 2009. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360:1320–1328 [DOI] [PubMed] [Google Scholar]
- 21. Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, Barry MJ, Zietman A, O'Leary M, Walker-Corkery E, Yao SL. 2009. Outcomes of localized prostate cancer following conservative management. JAMA 302:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albertsen PC, Hanley JA, Fine J. 2005. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 293:2095–2101 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz GG. 2005. Vitamin D and the epidemiology of prostate cancer. Semin Dial 18:276–289 [DOI] [PubMed] [Google Scholar]
- 24. Blutt SE, McDonnell TJ, Polek TC, Weigel NL. 2000. Calcitriol-induced apoptosis in LNCaP cells is locked by overexpression of Bcl-2. Endocrinology 141:10–17 [DOI] [PubMed] [Google Scholar]
- 25. Guzey M, Kitada S, Reed JC. 2002. Apoptosis induction by 1α,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther 1:667–677 [PubMed] [Google Scholar]
- 26. Hsu JY, Feldman D, McNeal JE, Peehl DM. 2001. Reduced 1α-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25(OH)D3-induced growth inhibition. Cancer Res 61:2852–2856 [PubMed] [Google Scholar]
- 27. Institute of Medicine 2011. Dietary references intakes for calcium and vitamin D. Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- 28. Manson JE, Mayne ST, Clinton SK. 2011. Vitamin D and prevention of cancer: ready for prime time? N Engl J Med 364:1385–1387 [DOI] [PubMed] [Google Scholar]
- 29. Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. 1993. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- 30. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. 2011. Vitamin D supplementation during pregnancy: double blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Young MV, Schwartz GG, Wang L, Jamieson DP, Whitlatch LW, Flanagan JN, Lokeshwar BL, Holick MF, Chen TC. 2004. The prostate 25-hydroxyvitamin D-1α-hydroxylase is not influenced by parathyroid hormone and calcium: implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis 25:967–971 [DOI] [PubMed] [Google Scholar]
- 32. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzic SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 33. Kovalenko PL, Zhang Z, Yu JG, Li Y, Clinton SK, Fleet JC. 2011. Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res 4:1617–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giovannucci E. 2011. The epidemiology of vitamin D and cancer Risk. In: Feldman D, Pike JW, Adams JS, eds. Vitamin D. 3rd ed Academic Press, San Diego, CA; 1569–1590 [Google Scholar]
- 35. Krishnan AV, Feldman D. 2011. Vitamin D and prostate cancer. In Feldman D, Pike JW, Adams JS, eds. Vitamin D. 3rd ed Academic Press, San Diego, CA; 1675–1709 [Google Scholar]
- 36. Trivedi DP, Doll R, Khaw KT. 2003. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double-blind controlled trial. BMJ 326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE; Women's Health Initiative Investigators 2006. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354:684–696 [DOI] [PubMed] [Google Scholar]
- 38. Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. 2001. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr 55:1091–1097 [DOI] [PubMed] [Google Scholar]
- 39. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. 2007. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591 [DOI] [PubMed] [Google Scholar]
- 40. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]