Abstract
Context:
The intramyocellular deposition of lipid associates with metabolic dysregulation in adolescents and adults. Relatively little is known about the deposition of fat in muscle before the onset of puberty.
Objective:
Our objective was to describe the relationship between intramyocellular lipid (IMCL) accumulation and 1) visceral adiposity and 2) markers of insulin resistance and cardiovascular disease risk in a cohort of prepubertal and early pubertal children.
Study Design:
Data were collected as part of a retrospective cohort study, Exploring Perinatal Outcomes among Children (EPOCH). Multiple linear regression models were constructed for data analysis.
Results:
A total of 441 children participated in the study (226 prepubertal, 215 early pubertal). In prepubertal children, there was a significant relationship between IMCL and visceral fat (parameter estimate 0.019, P = 0.002) that remained after controlling for body mass index. Independent of overall adiposity, in all children, IMCL was associated with the triglyceride to high-density lipoprotein ratio (parameter estimate 0.1418, P = 0.002).
Conclusions:
This study demonstrates a concerning and related pattern of IMCL and visceral fat deposition in prepubertal children. Intramuscular fat deposition is linked to markers of insulin resistance and cardiovascular disease risk.
Obesity is widely considered to be the greatest public health challenge for the 21st century because of its role in causing disability and crippling healthcare costs (1, 2). Up to 90% of obese adolescents will continue to be obese 10 yr hence (3). This stubborn persistence of obesity in adulthood has generated interest in either primary prevention of obesity or interventions in overweight children before the onset of adolescence. Because up to 17% of obese adults [defined as body mass index (BMI) >30] appear to be metabolically healthy without insulin resistance or cardiovascular risks such as hyperlipidemia and hypertension, it is critical to identify those clinical characteristics early in life that predict adverse metabolic outcomes (4).
Conventional measures of adiposity, such as BMI, may lack specificity for metabolic disease because they mostly estimate sc fat and are less sensitive in estimating other, more harmful locations for fat storage. The deposition of fat in liver, muscle, and intraabdominal adipose depots has been suggested to better predict metabolic dysregulation, independent of overall adiposity (5, 6). In a cohort of obese, mainly postpubertal children, Caprio and colleagues demonstrated that visceral and intramyocellular lipid (IMCL) deposition discriminated strongly between insulin-sensitive and insulin-resistant subjects (7). Skeletal muscle glucose uptake is critical to the maintenance of insulin sensitivity. A direct causal link has been proposed between accrual of IMCL or its metabolites and interference with insulin-mediated glucose uptake via glucose transporter type 4 (8). Visceral adipocytes, when compared in vitro to sc adipocytes, have also been shown to be more proinflammatory and lipolytic (9, 10).
The transition from prepuberty to adulthood is associated with a stepwise increase in trunk fat (11). This transition in adiposity distribution is accompanied by an increase in insulin resistance, likely mediated via changes in GH, sex hormones, and SHBG (12, 13). The pubertal period, therefore, may represent a critical developmental window for the expansion of visceral fat. The demonstration of detrimental patterns of fat distribution in prepubertal children, even before the expected changes in fat distribution associated with adolescence, may help identify children at highest risk of later development of metabolic disease and further strengthen the case for early intervention and primary prevention.
Using a large cohort of mostly prepubertal or early pubertal children, we sought to explore 1) the relationship between IMCL deposition and patterns of fat distribution and 2) the relationship between IMCL deposition and markers of insulin resistance [triglyceride (TG) to high-density lipoprotein cholesterol (HDL-c) ratio (14)] and cardiovascular risk (adiponectin, blood pressure, and lipid levels).
Subjects and Methods
Participants
This report includes data from a retrospective cohort study conducted in Colorado: Exploring Perinatal Outcomes among Children (EPOCH). Participants were healthy 6- to 13-yr old children whose mothers were members of the Kaiser Permanente of Colorado health plan and who attended an in-person fasting study visit. All participants provided informed consent, and youths provided written assent. The study was approved by the Colorado Multiple Institutional Review Board.
Measures of adiposity and fat distribution
All measurements were performed by a trained technician. Children were weighed and measured in light clothing and without shoes. Weight was measured to the nearest 0.1 kg using a portable electronic SECA scale (Hanover, MD), and height was measured to the nearest 0.1 cm, using a portable SECA stadiometer. BMI was calculated as kilograms per square meter. Age- and sex-specific BMI z-scores were calculated according to Centers for Disease Control standard growth charts (15). Waist circumference was measured to the nearest 1 mm at the midpoint between the lower ribs and the pelvic bone with a fiberglass non-spring-loaded tape measure. Skinfolds were measured in triplicate using Holtain calipers (average for subscapular, 20 mm below the tip of the scapula; for triceps, halfway between the acromion process and the olecranon process).
Magnetic resonance imaging of the abdominal region was used to quantify visceral adipose tissue (VAT) and sc adipose tissue (SAT) with a 3T HDx imager (General Electric, Waukesha WI) by a trained technician. Each study participant was placed supine, and a series of T1-weighted coronal images were taken to locate the L4/L5 plane. One axial, 10-mm, T1-weighted image at the L4/L5 disc space was analyzed to determine SAT and VAT content. IMCL and extramyocellular lipid (EMCL) were measured via proton (1H) magnetic resonance spectroscopy. Each subject was positioned to image the midcalf area, using a T1-weighted image as a localizer, and homogenous muscle regions were selected for measurement. This technique exploits the small (about 2 parts per million) frequency shift between IMCL and EMCL resonances observed when muscle fibers are roughly aligned in the direction of the magnetic field in a magnetic resonance scanner, as is the case with soleus and tibialis anterior muscles. Spectra were analyzed with LCModel software as previously described (16, 17). IMCL and EMCL concentrations, obtained by reference to the unsuppressed water peak, are reported in arbitrary concentration units.
Markers of insulin resistance and cardiovascular risk
Fasting blood samples were obtained, and TG and HDL-c were measured using the Olympus (Center Valley PA) AU400 advanced chemistry analyzer system. Adiponectin was measured by RIA (Millipore, Billerica MA). The TG to HDL-c ratio was calculated as a marker of insulin resistance (18).
Other measurements
Race/ethnicity was self-reported using 2000 U.S. Census-based questions and categorized as Hispanic (any race), non-Hispanic white, and non-Hispanic African-American. Pubertal development was self-assessed using a diagrammatic representation of Tanner staging adapted from Marshall and Tanner (19) Sexual maturation self-assessment was recently shown to be in excellent agreement with physician-assessed Tanner stage (20). Youth were categorized as prepubertal (Tanner <2) and early pubertal (Tanner 2–4). Maternal level of education and total household income were self-reported at the study visit. Children's total energy intake (kilocalories per day) was assessed using the Block Kid's Food Questionnaire (21). Self-reported key activities, both sedentary and nonsedentary, performed during the previous 3 d was queried using a 3-d Physical Activity Recall (3DPAR) questionnaire (22). Each 30-min block of activity was assigned a metabolic equivalent variable to accommodate the energy expenditure. Results were reported as the average number of 30-min blocks of moderate-to-vigorous activity per day.
Statistical analysis
Multiple linear regression analysis was used to examine the association between IMCL and other adiposity and fat distribution parameters including BMI, SAT, VAT, waist circumference, and subscapular and triceps skinfolds, controlling for potential confounders, separately among prepubertal and early pubertal children. Model 1 (base model) was adjusted for demographic factors (age, sex, and race/ethnicity) and current lifestyle factors (child's self-reported physical activity and total daily energy intake). Model 2 additionally adjusted for current childhood BMI to explore whether current childhood body size mediates the association between IMCL levels and other measures of adiposity.
A second set of multiple linear regression analyses were conducted to explore the relationship between IMCL and the TG to HDL-c ratio, as marker of insulin resistance, as well as other cardiovascular risk factors, including adiponectin, systolic and diastolic blood pressure, and TG and HLD-c levels. These models included all children (prepubertal and early pubertal) and used a base model (adjusted for age, sex, race/ethnicity, and an interaction term for age and Tanner stage) followed by additional adjustment for BMI, SAT, and VAT in separate models (23). Each marker of insulin resistance and cardiovascular risk was assessed independently in these models. Linear and nonlinear relationships between IMCL and insulin resistance/cardiovascular disease risk factors were explored by testing first-, second-, and third-order polynomial functions in our base model. A linear model was determined to be most appropriate for all parameters based on examination of P values for the significance of polynomial functions and the fraction of variance explained by each model. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) at an α-level for significance of P < 0.05.
Results
Table 1 depicts clinical characteristics of the 441 children who participated in the EPOCH study, stratified according to Tanner Stage classification into prepubertal and early pubertal groups. The cohort was representative of the larger cohort described in the National Health and Nutrition Examination Survey (NHANES) in terms of prevalence of obesity (prepubertal, 13.7%; early pubertal, 15.4%) (24). There were no differences between prepubertal and early pubertal children in terms of gender, race/ethnicity, habitual physical activity level, or total daily caloric intake. There was a slightly higher percent intake of calories from fat in the early pubertal vs. prepubertal group (36.2 vs. 35.2%, P = 0.03). All anthropometric and radiological measures of adiposity were significantly higher in the early pubertal compared with prepubertal group. In addition, there were higher levels of resting systolic and diastolic blood pressure in the early pubertal group. Also, biochemical differences between early pubertal and prepubertal groups included lower insulin sensitivity as estimated by the higher TG to HDL-c ratio.
Table 1.
EPOCH study participant characteristics
| Prepubertal (n = 226) | Early pubertal (n = 215) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yr) | 9.9 ± 1.16 | 11.4 ± 1.06 | <0.0001 |
| Males [n (%)] | 108 (47.8) | 107 (49.8) | 0.27 |
| Race/ethnicity [n (%)] | 0.13 | ||
| Non-Hispanic White | 104 (46.0) | 105 (48.8) | |
| Hispanic | 106 (46.9) | 85 (39.5) | |
| African-American | 16 (7.1) | 25 (11.6) | |
| Physical activity (block/d)a | 4.4 (2.8) | 4.3 (2.9) | 0.6 |
| Total daily calories (kcal/d) | 1772.3 ± 567.0 | 1864.5 ± 570.2 | 0.9 |
| Percent calories from fat (%) | 35.2 ± 4.8 | 36.2 ± 4.9 | 0.03 |
| Adiposity and fat patterning | |||
| BMI (kg/m2) | 17.96 ± 4.28 | 20.11 ± 4.64 | <0.0001 |
| BMI z-score | 0.03 ± 1.26 | 0.47 ± 1.13 | 0.0001 |
| Normal BMI [n (%)] | 171 (75.66) | 144 (66.98) | 0.07 |
| Overweight [n (%)] | 24 (10.62) | 38 (17.67) | |
| Obese [n (%)] | 31 (13.72) | 33 (15.35) | |
| VAT (cm2) | 20.6 ± 17.6 | 24.2 ± 14.4 | 0.02 |
| SAT (cm2) | 100.9 ± 98.1 | 142.8 ± 115.9 | <0.0001 |
| Waist circumference (cm) | 62.59 ± 11.16 | 68.91 ± 12.28 | <0.0001 |
| Waist/hip | 0.87 ± 0.05 | 0.85 ± 0.06 | 0.03 |
| Triceps skinfold (cm) | 15.41 ± 7.20 | 17.38 ± 7.34 | 0.005 |
| Subscapular skinfold (cm) | 11.73 ± 8.35 | 14.16 ± 8.62 | 0.003 |
| IMCL (institutional units) | 2.23 ± 1.20 | 2.50 ± 1.30 | 0.03 |
| Markers of insulin resistance | |||
| TG to HDL-c ratio | 1.80 ± 1.22 | 2.23 ± 1.43 | 0.0008 |
| Markers of cardiovascular risk | |||
| Adiponectin | 12.27 ± 5.92 | 11.36 ± 5.1 | 0.09 |
| TG (mg/dl) | 83.4 ± 78.2 | 97.2 ± 90.8 | 0.001 |
| HDL-c (mg/dl) | 50.78 ± 10.74 | 47.80 ± 11.77 | 0.006 |
| Diastolic BP (mm HG) | 68.7 ± 7.9 | 70.9 ± 8.1 | 0.003 |
| Systolic BP (mm HG) | 101.4 ± 10.4 | 104.4 ± 9.7 | 0.002 |
Data are presented as means ± sd or n (%). BP, Blood pressure.
Percentage score reflecting the extent to which the number of 30-min blocks of moderate-to-vigorous physical activity reported over 3 d meets recommended levels.
Relationship between IMCL and adiposity estimates
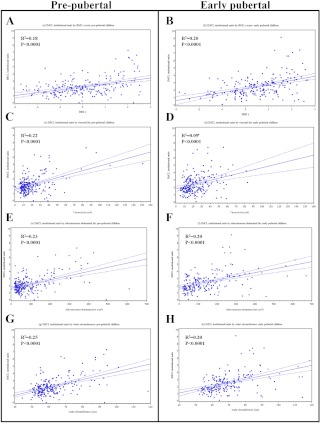
The unadjusted associations of IMCL concentration (institutional units) with adiposity measures in pre- and early-pubertal children are shown in Fig. 1, A–H. Table 2 displays the same relationship in our base (model 1) and fully adjusted (model 2) analyses. Higher levels of each adiposity measure were significantly associated with higher IMCL levels after adjustment for age, sex, race/ethnicity, current childhood physical activity, and current total daily calories in both prepubertal and early pubertal children (model 1). Model 2 shows that, among prepubertal children, even after controlling for BMI, a 10-cm2 increase in VAT was associated with 0.19 U higher IMCL (P = 0.002), and a 10-cm increase in waist circumference resulted in 0.44 U higher IMCL levels (P = 0.08). Among early pubertal children, the associations between VAT/waist circumference and IMCL were very similar but did not reach statistical significance. In this group, SAT was the only adiposity parameter significantly associated with IMCL (P = 0.047), after adjustment for BMI.
Fig. 1.
Unadjusted association of IMCL institutional units to adiposity parameters in pre- and early-pubertal children. A, BMI z prepubertal; B, BMI z early pubertal; C, visceral fat (square centimeters) prepubertal; D, visceral fat (square centimeters) early pubertal; E, sc abdominal fat prepubertal (square centimeters); F, sc abdominal fat early pubertal (square centimeters); G, waist circumference (centimeters) prepubertal; H, waist circumference (centimeters) early pubertal.
Table 2.
The relationship between IMCL and markers of fat distribution in pre- and early-pubertal children
| Prepubertal |
Early pubertal |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 adjusted for BMI |
Model 1 |
Model 2 adjusted for BMI |
|||||||||
| β (se) | P | R2 | β (se) | P | R2 | P | R2 | β (se) | P | R2 | ||
| BMI (kg/m2) | 0.137 (0.017) | 0.0001 | 0.28 | 0.112 (0.02) | <0.0001 | 0.23 | ||||||
| VAT (cm2) | 0.032 (0.004) | 0.0001 | 0.28 | 0.019 (0.006) | 0.002 | 0.31 | 0.027 (0.006) | <0.0001 | 0.19 | 0.011 (0.008) | 0.1 | 0.24 |
| SAT (cm2) | 0.0059 (0.0007) | 0.0001 | 0.27 | 0.002 (0.002) | 0.2 | 0.28 | 0.0045 (0.0008) | <0.0001 | 0.24 | 0.004 (0.002) | 0.05 | 0.24 |
| WC (cm) | 0.057 (0.007) | 0.0001 | 0.30 | 0.044 (0.017) | 0.008 | 0.30 | 0.043 (0.008) | <0.0001 | 0.23 | 0.024 (0.02) | 0.3 | 0.24 |
| Waist/hip | 6.598 (1.508) | <0.0001 | 0.14 | 1.790 (1.566) | 0.2 | 0.28 | 3.797 (1.576) | 0.02 | 0.14 | −0.344 (1.72) | 0.8 | 0.23 |
| Subscapular skinfold (cm) | 0.065 (0.009) | 0.0001 | 0.26 | 0.026 (0.016) | 0.1 | 0.29 | 0.049 (0.011) | <0.0001 | 0.20 | 0.004 (0.02) | 0.8 | 0.23 |
| Triceps skinfold (cm) | 0.068 (0.010) | 0.0001 | 0.22 | 0.004 (0.019) | 0.8 | 0.28 | 0.063 (0.012) | <0.0001 | 0.22 | 0.033 (0.019) | 0.08 | 0.24 |
The β relates to the change in IMCL institutional units for every 1-U increase in the adiposity measure. R2 indicates the degree to which IMCL levels were predicted by the adiposity marker included in the model. Model 1 is adjusted for age, sex, race/ethnicity, current child physical activity, and current total daily calories Model 2 is the base model plus BMI. WC, Waist circumference.
IMCL and markers of insulin resistance/cardiovascular risk
For these analyses, IMCL was the outcome variable, and various clinical and biochemical measures served as explanatory variables (Table 3). The β-estimate represents the change in IMCL for every one unit increase in the marker of insulin resistance/cardiovascular risk. Our initial analyses divided prepubertal and early pubertal children, but we found no differences between these two groups in the relationships between IMCL and markers of insulin resistance or cardiovascular risk; therefore, the two groups were combined in the final analyses.
Table 3.
The relationship between IMCL, and markers of insulin resistance/cardiovascular risk in children aged 6–13 from multiple linear regression models, adjusted for adiposity parameters
| Model 1 |
Model 2 adjusted for BMI |
Model 3 adjusted for SAT |
Model 4 adjusted for VAT |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (se) | P | R2 | β (se) | P | R2 | β (se) | P | R2 | β (se) | P | R2 | |
| TG to HDL-c | 0.2475 (0.045) | <0.0001 | 0.15 | 0.1418 (0.04) | 0.002 | 0.26 | 0.1351 (0.04) | 0.003 | 0.26 | 0.1159 (0.047) | 0.01 | 0.23 |
| Adiponectin | −0.027 (0.01) | 0.02 | 0.10 | −0.011 (0.01) | 0.30 | 0.24 | −0.011 (0.01) | 0.30 | 0.25 | −0.014 (0.01) | 0.2 | 0.23 |
| Systolic BP (mm HG) | 0.025 (0.006) | <0.0001 | 0.12 | 0.007 (0.006) | 0.29 | 0.24 | 0.008 (0.006) | 0.18 | 0.25 | 0.012 (0.006) | 0.04 | 0.23 |
| Diastolic BP (mm HG) | 0.020 (0.008) | 0.01 | 0.10 | 0.001 (0.007) | 0.86 | 0.24 | 0.0005 (0.007) | 0.95 | 0.24 | 0.007 (0.007) | 0.4 | 0.22 |
| Triglyceride (mg/dl) | 0.008 (0.001) | <0.0001 | 0.17 | 0.005 (0.001) | <0.0001 | 0.27 | 0.005 (0.001) | 0.0001 | 0.27 | 0.005 (0.001) | <0.0001 | 0.25 |
| HDL-c (mg/dl) | −0.0096 (0.006) | 0.09 | 0.09 | −0.00003 (0.005) | 1.0 | 0.24 | 0.0015 (0.005) | 0.78 | 0.24 | 0.004 (0.005) | 0.5 | 0.22 |
The β relates to the change in IMCL institutional units for every 1-U increase in the CVD risk factor. R2 indicates the degree to which IMCL levels were predicted by the marker of insulin resistance/cardiovascular risk included in the model. Model 1 is adjusted for age, sex, race/ethnicity, Tanner, Tanner × age interaction, current child physical activity, and current total daily calories, Model 2 is model 1 plus BMI. Model 3 is model 1 plus SAT. Model 4 is model 1 plus VAT. BP, Blood pressure.
In model 1, there were significant positive associations between IMCL and blood pressure and TG levels, with negative associations between IMCL and TG to HDL-c ratio, adiponectin, and HDL-c. In models 2 and 3, adjustment for either BMI or SAT reduced the relationship between IMCL and blood pressure, adiponectin, and HDL-c, but the relationship with serum TG and TG to HDL-c remained significant. Similarly, in model 4, adjustment for VAT did not impact the significant relationship between IMCL and serum TG as well as TG to HDL-c ratio.
Discussion
To our knowledge, this is the largest study to examine the relationship between IMCL, VAT, and markers of insulin resistance and cardiovascular risk in prepubertal and early pubertal children. Both cross-sectional and longitudinal data have demonstrated large reductions in insulin sensitivity and changes in fat deposition with the onset of puberty (25, 26). The importance of studying prepubertal children lies in the ability to detect early abnormalities in fat partitioning at a stage where intervention is more likely to be successful. The inclusion of a large number of prepubertal children in our study allowed us to demonstrate a significant relationship between IMCL and VAT in prepubertal children that exists independent of overall adiposity. IMCL was also strongly and independently associated with serum TG levels and TG to HLD-c ratio in all children.
Our data demonstrate that abnormal patterning of fat deposition, specifically storage of fat in muscle and intraabdominal locations, already exists in prepubertal children. These data are consistent with recently reported findings from the Mechanisms of the Metabolic Syndrome in Prepubertal Youth study, which showed higher IMCL levels in obese prepubertal children compared with lean controls (27, 28). In that study, the relationship between IMCL and obesity disappeared after adjustment for total body fat, whereas in our sample of 226 prepubertal children, the correlation between ectopic fat (IMCL) and VAT was still present after adjustment for BMI.
Adjustment for BMI attenuated to nonsignificance the association between IMCL and VAT among our early pubertal youth, although the β-estimate was similar to the one seen in the prepubertal group. We hypothesize several reasons for the change in this relationship with puberty. First, this may be due, in part, to the smaller number of subjects in this group. Second, there was greater variability in IMCL seen in our early pubertal youth, perhaps due to the diversity in their pubertal stage. Third, the known increase in truncal fat with pubertal stage may attenuate the relationship between IMCL and visceral fat. The association between VAT and IMCL accumulation has been previously shown in pubertal adolescents (29) and in a high-fat rodent model (30), but the underlying mechanisms remain unclear.
Visceral adipocytes may drive higher levels of lipids through enhanced lipolytic activity (31). However, the overall contribution of VAT to fatty acid flux must be limited because VAT comprises only 10% of total fat volume in children. Saturated fatty acids may play a role in fat oxidation in muscle (32), but evidence implicating dietary fatty acid profile and VAT accumulation is lacking. Altered expression of lipid-regulating genes such as SREBP-1 in the setting of insulin resistance may also contribute to ectopic lipid deposition (33). Recent data linking the PNLAP3 gene to hepatic fat deposition demonstrates that genetic profiles provide risk for ectopic fat deposition (34). Although a specific gene has not been identified that is linked to IMCL deposition, Shulman and colleagues (35) showed higher IMCL deposition and impairment in skeletal muscle mitochondrial oxidation in the healthy, normal-weight, but insulin-resistant offspring of subjects with type 2 diabetes mellitus, suggesting a genetic predisposition for development of exaggerated IMCL. The differences in VAT accumulation seen across racial and ethnic groups (36, 37) also support a role for genetic background risk, but genetic factors controlling VAT accumulation are likely separate from those risk loci contributing to overall obesity risk (38).
We found associations between markers of cardiovascular risk and IMCL deposition, but most appeared to be mediated by SAT deposition because they lost significance after adjustment for either BMI or sc fat. However, the relationships between IMCL and TG to HDL-c ratio (as estimate of insulin resistance) and serum TG levels were independent of all other measures of adiposity. The TG to HDL-c ratio has been shown in children and adults to correlate with insulin resistance and an atherogenic lipid profile (14, 39–41). Higher TG levels were associated with increased aortic intimal-medial thickness, a marker of early atherosclerosis, in the Muscatine Offspring cohort (42). We also demonstrated in our cohort that waist circumference correlated with IMCL deposition, independent of BMI (P = 0.008). These data are consistent with findings from the Metabolic Syndrome in Prepubertal Youth study, which showed a significant correlation of waist circumference with IMCL and hepatic lipid (28). The relationship between waist circumference and IMCL may be mediated by VAT deposition, because waist circumference has been demonstrated to correlate well with VAT (R2 = 0.64) in a cohort of 407 children that had a large number of prepubertal subjects (43). In large epidemiological studies, waist circumference has been shown to better correlate with insulin sensitivity and components of the metabolic syndrome than BMI (44). Waist circumference norms have been developed and could be implemented in primary care settings to identify those children at highest risk of later disease (45).
Our study included a large number of children representing a broad range of ages, pubertal status, race/ethnicity, and adiposity. Our measures of adiposity were robust for ectopic fat deposition (IMCL) and depot-specific information (VAT vs. SAT). A weakness of our study was its lack of direct measures of insulin sensitivity and hepatic fat deposition, although the correlation between hepatic fat and IMCL has been shown previously (28).
In conclusion, our study shows the concerning presence of ectopic fat deposition in early pubertal and even prepubertal youth and a relationship between VAT and IMCL in children that may be independent of overall adiposity. Waist circumference also appears to correlate well with ectopic fat deposition. The genetic, epigenetic, and dietary factors that contribute to this worrisome pattern of fat deposition in childhood are incompletely understood. In our study, higher IMCL deposition was associated with insulin resistance as estimated by the TG to HDL-c ratio and elevations in serum TG, a marker of cardiovascular disease risk, independent of adiposity. We believe that these findings contribute to a growing body of data that emphasize the need for intervention and/or primary prevention of metabolic disease in early childhood.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant R01 DK06800107 to D.D. and General Clinical Research Centers NIH Program M01 RR00069.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- BMI
- Body mass index
- EMCL
- extramyocellular lipid
- EPOCH
- Exploring Perinatal Outcomes among Children
- HDL-c
- high-density lipoprotein cholesterol
- IMCL
- intramyocellular lipid
- SAT
- sc adipose tissue
- TG
- triglyceride
- VAT
- visceral adipose tissue.
References
- 1. Reuser M, Bonneux LG, Willekens FJ. 2009. Smoking kills, obesity disables: a multistate approach of the US Health and Retirement Survey. Obesity (Silver Spring) 17:783–789 [DOI] [PubMed] [Google Scholar]
- 2. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. 2011. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378:815–825 [DOI] [PubMed] [Google Scholar]
- 3. Gordon-Larsen P, The NS, Adair LS. 2010. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring) 18:1801–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. 2008. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 5. Després JP, Lemieux I. 2006. Abdominal obesity and metabolic syndrome. Nature 444:881–887 [DOI] [PubMed] [Google Scholar]
- 6. Lara-Castro C, Garvey WT. 2008. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am 37:841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S. 2005. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 90:3731–3737 [DOI] [PubMed] [Google Scholar]
- 8. Samuel VT, Petersen KF, Shulman GI. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. 2010. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 18:879–883 [DOI] [PubMed] [Google Scholar]
- 10. Thörne A, Löfgren P, Hoffstedt J. 2010. Increased visceral adipocyte lipolysis: a pathogenic role in nonalcoholic fatty liver disease? J Clin Endocrinol Metab 95:E209–E213 [DOI] [PubMed] [Google Scholar]
- 11. Taylor RW, Grant AM, Williams SM, Goulding A. 2010. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 18:1410–1416 [DOI] [PubMed] [Google Scholar]
- 12. Moran A, Jacobs DR, Jr, Steinberger J, Cohen P, Hong CP, Prineas R, Sinaiko AR. 2002. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 87:4817–4820 [DOI] [PubMed] [Google Scholar]
- 13. Sørensen K, Aksglaede L, Munch-Andersen T, Aachmann-Andersen NJ, Petersen JH, Hilsted L, Helge JW, Juul A. 2009. Sex hormone-binding globulin levels predict insulin sensitivity, disposition index, and cardiovascular risk during puberty. Diabetes Care 32:909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abbasi F, Reaven GM. 2011. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 60:1673–1676 [DOI] [PubMed] [Google Scholar]
- 15. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC growth charts: United States. Adv Data 314:1–27 [PubMed] [Google Scholar]
- 16. Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. 2009. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab 94:3687–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen C, Jacob S, Schick F, Häring HU, Stumvoll M. 2003. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791 [DOI] [PubMed] [Google Scholar]
- 18. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. 2003. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 139:802–809 [DOI] [PubMed] [Google Scholar]
- 19. Marshall WA, Tanner JM. 1968. Growth and physiological development during adolescence. Annu Rev Med 19:283–300 [DOI] [PubMed] [Google Scholar]
- 20. Lamb MM, Beers L, Reed-Gillette D, McDowell MA. 2011. Feasibility of an audio computer-assisted self-interview method to self-assess sexual maturation. J Adolesc Health 48:325–330 [DOI] [PubMed] [Google Scholar]
- 21. Cullen KW, Watson K, Azkievi I. 2008. Relative reliability and validity of the Block kids questionnaire among youth aged 10 to 17 years. J Amer Diet Assoc 108:862–866 [DOI] [PubMed] [Google Scholar]
- 22. Pate RR, Ross R, Dowda M, Trost SG. 2003. Validation of a three-day physical activity recall instrument in female youth. Pediatr Exerc Sci 15:257–265 [Google Scholar]
- 23. Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, McDuffie R, Bischoff K, Hamman RF, Norris JM, Dabelea D. 2011. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 54:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. 2010. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 303:242–249 [DOI] [PubMed] [Google Scholar]
- 25. Hannon TS, Janosky J, Arslanian SA. 2006. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 60:759–763 [DOI] [PubMed] [Google Scholar]
- 26. Pilia S, Casini MR, Foschini ML, Minerba L, Musiu MC, Marras V, Civolani P, Loche S. 2009. The effect of puberty on insulin resistance in obese children. J Endocrinol Invest 32:401–405 [DOI] [PubMed] [Google Scholar]
- 27. Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, Chalew S, Gordon S, Smith SR, Newcomer BR, Goran M, Sothern M. 2012. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity (Silver Spring) 20:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larson-Meyer DE, Newcomer BR, Ravussin E, Volaufova J, Bennett B, Chalew S, Cefalu WT, Sothern M. 2011. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia 54:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. 2002. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027 [DOI] [PubMed] [Google Scholar]
- 30. Korach-André M, Gao J, Gounarides JS, Deacon R, Islam A, Laurent D. 2005. Relationship between visceral adiposity and intramyocellular lipid content in two rat models of insulin resistance. Am J Physiol Endocrinol Metab 288:E106–E116 [DOI] [PubMed] [Google Scholar]
- 31. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. 2004. Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. 2008. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283:11107–11116 [DOI] [PubMed] [Google Scholar]
- 33. Nadeau KJ, Leitner JW, Gurerich I, Draznin B. 2004. Insulin regulation of sterol regulatory element-binding protein-1 expression in L-6 muscle cells and 3T3 L1 adipocytes. J Biol Chem 279:34380–34387 [DOI] [PubMed] [Google Scholar]
- 34. Goran MI, Walker R, Le KA, Mahurkar S, Vikman S, Davis JN, Spruijt-Metz D, Weigensberg MJ, Allayee H. 2010. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes 59:3127–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. 2010. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr 91:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liska D, Dufour S, Zern TL, Taksali S, Calí AM, Dziura J, Shulman GI, Pierpont BM, Caprio S. 2007. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One 2:e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haupt A, Thamer C, Heni M, Machicao F, Machann J, Schick F, Stefan N, Fritsche A, Haring HU, Staiger H. 2010. Novel obesity risk loci do not determine distribution of body fat depots: a whole-body MRI/MRS study. Obesity (Silver Spring) 18:1212–1217 [DOI] [PubMed] [Google Scholar]
- 39. Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, Pierpont B, Weiss R. 2011. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 34:1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiss R, Otvos JD, Sinnreich R, Miserez AR, Kark JD. 2011. The triglyceride to high-density lipoprotein-cholesterol ratio in adolescence and subsequent weight gain predict nuclear magnetic resonance-measured lipoprotein subclasses in adulthood. J Pediatr 158:44–50 [DOI] [PubMed] [Google Scholar]
- 41. Quijada Z, Paoli M, Zerpa Y, Camacho N, Cichetti R, Villarroel V, Arata-Bellabarba G, Lanes R. 2008. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes 9:464–471 [DOI] [PubMed] [Google Scholar]
- 42. Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M. 2004. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 110:2678–2686 [DOI] [PubMed] [Google Scholar]
- 43. Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, Peters DM, Barbeau P, De Simone M, Pietrobelli A. 2006. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes (Lond) 30:23–30 [DOI] [PubMed] [Google Scholar]
- 44. l'Allemand-Jander D. 2010. Clinical diagnosis of metabolic and cardiovascular risks in overweight children: early development of chronic diseases in the obese child. Int J Obes (Lond) 34(Suppl 2):S32–S36 [DOI] [PubMed] [Google Scholar]
- 45. Cook S, Auinger P, Huang TT. 2009. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr 155:S6 e15–e26 [DOI] [PMC free article] [PubMed] [Google Scholar]