Abstract
Context:
Vitamin D deficiency and obesity are associated with increased tissue renin-angiotensin system (RAS) activity.
Objective:
The objective of the study was to evaluate whether vitamin D3 therapy in obesity reduces tissue-RAS activity, as indicated by an increase in tissue sensitivity to angiotensin II (AngII).
Participants:
Participants included obese subjects with hypertension and 25-hydroxyvitamin D less than 25 ng/ml.
Design:
Subjects were studied before and after 1 month of vitamin D3 15,000 IU/d, while in dietary sodium balance, and off all interfering medications. Fourteen subjects successfully completed all study procedures.
Setting:
The study was conducted at a clinical research center.
Outcome Measures:
At each study visit, tissue sensitivity to AngII was assessed by measuring renal plasma flow (RPF), mean arterial pressure (MAP), and adrenal secretion of aldosterone during an infusion of AngII. Subjects were then given captopril, and a second AngII infusion to evaluate the effect of captopril on tissue-RAS activity.
Results:
Vitamin D3 therapy increased 25-hydroxyvitamin D (18 to 52 ng/ml) and basal RPF (+5%) and lowered supine MAP (−3%) (all P < 0.01). There was a greater decline in RPF and higher stimulation of aldosterone with AngII infusion after vitamin D3 therapy (both P < 0.05). As anticipated, captopril increased the renal-vascular, MAP, and adrenal sensitivity to AngII, but this effect was much smaller after vitamin D3 therapy, indicating that vitamin D3 therapy corrected the tissue sensitivity to AngII akin to captopril.
Conclusions:
Vitamin D3 therapy in obese hypertensives modified RPF, MAP, and tissue sensitivity to AngII similar to converting enzyme inhibition. Whether chronic vitamin D3 therapy abrogates the development of diseases associated with excess RAS activity warrants investigation.
Obesity is associated with the development of hypertension (HTN), chronic kidney disease (CKD), and death (1, 2); one of the leading mechanisms for these obesity-related complications is increased activity of the local tissue renin-angiotensin system (RAS) (3–6). With increasing adiposity, excess activity of the RAS in vascular, cardiac, adipose, and other tissues may contribute to systemic inflammation and end-organ dysfunction (3–7).
Vitamin D has been implicated as a negative regulator of renin expression and RAS activity; translational studies have shown that the 1,25 dihydroxyvitamin D3 [1,25(OH)2D]-vitamin D receptor (VDR) complex lowers renin expression (8). We, and others, have previously observed that 25-hydroxyvitamin D [25(OH)D] concentrations are inversely associated with circulating plasma renin activity (PRA) (9, 10), renin concentration (11), and angiotensin II (AngII) in humans (12). Furthermore, we observed a blunted tissue sensitivity to AngII in individuals with lower 25(OH)D levels, when compared with those with higher 25(OH)D, that was particularly evident among obese individuals (12, 13); these findings suggested higher tissue-RAS activity in obese individuals with low 25(OH)D concentrations. Because 25(OH)D concentrations are known to be lower in obesity (14, 15), this insufficiency may contribute to excess tissue-RAS activity and the development of HTN and CKD; however, prospective interventional studies examining the mechanistic influence of vitamin D metabolites on the RAS in human obesity have not been reported.
We hypothesized that high-dose vitamin D3 therapy in obese individuals with low vitamin D status could lower tissue-RAS activity and thus increase the tissue sensitivity to AngII. To test this, we prospectively enrolled obese subjects with HTN, normal kidney function, and low 25(OH)D levels and evaluated their tissue sensitivity to AngII before, and after, 1 month of vitamin D3 15,000 IU/d therapy. These studies were conducted with the control of posture, dietary electrolyte intake, and time of day and after stopping all the antihypertensive and interfering medications for up to 3 months to enhance the accuracy and interpretability of our findings.
Materials and Methods
Study participants
Participants were recruited from the greater Boston metropolitan area. A study physician at the Brigham and Women's Hospital assessed eligibility criteria during a formal screening evaluation. Subjects were enrolled if they had body mass index 30 kg/m2 or greater, 25(OH)D less than 25 ng/ml, mild to moderate hypertension (defined as an untreated blood pressure of 140/90 to 159/99 mm Hg or the use of any antihypertensive drugs for a diagnosis of hypertension), age younger than 65 yr, normal renal function (estimated glomerular filtration rate >60 ml/min using the CKD-Epidemiology Collaboration equation and serum creatinine <1.5 mg/dl), and a normal resting electrocardiogram. Subjects were ineligible for enrolment if they had any history of coronary artery disease, heart failure, cerebrovascular disease, liver disease, history of parathyroid or granulomatous or primary bone disorders, hyper- or hypocalcemia, illness requiring overnight hospitalization in the past 6 months, alcohol intake greater than 12 oz/wk, use of tobacco or any recreational drugs, use of oral or transdermal contraceptives, or use of antipsychotic or benzodiazepene or bisphosphonate drugs or were pregnant or breast-feeding. The Human Subjects Committees/Institutional Review Board at the Brigham and Women's Hospital approved all study protocols, and informed written consent was obtained from each subject.
Drug washout protocol
Enrolled subjects underwent a medication washout before study. Consistent with our prior protocols designed to reliably measure the RAS with minimal drug interference (9), angiotensin-converting enzyme inhibitors (ACEi) and angiotensin and mineralocorticoid receptor antagonists were stopped for 3 months; β-blockers and diuretics were stopped for 1 month; and calcium channel blockers were stopped for 2 wk before the study. Thereafter, all antihypertensive medications were withheld for the duration of the study. Additionally, subjects were asked to discontinue use of nonsteroidal antiinflammatory agents, decongestants, and cold medicines at least 2 wk before study visits. During the medication washout periods, subjects were trained to use a home sphygmomanometer and report daily blood pressure readings to study staff; if blood pressure readings consistently exceeded 159/99 mm Hg for 5 d, amlodipine was prescribed to target a blood pressure less than 159/99 mm Hg but was stopped at least 2 wk before any study visit. Subjects were withdrawn from the study and resumed on their antihypertensive regimen if their blood pressure could not be maintained below 159/99 mm Hg (with or without amlodipine).
Study diet protocol
To ensure reliable measurements of the circulating and tissue-RAS, uniform dietary electrolyte conditions were prescribed for 1 wk before all study visits. Study diets included more than 200 mmol of sodium daily, 100 mmol of potassium daily, and 20 mmol of calcium daily. The liberal sodium intake in this diet was designed to ensure homogenous suppression of the circulating RAS that was similar to the typical Western diet (16, 17).
Inpatient clinical research center study visits
After completing an antihypertensive medication washout and 1 wk of study diet, subjects were admitted overnight to the clinical research center for the first overnight study visit (see below) and then discharged with vitamin D3 therapy for 1 month (see below), and returned while on the same study diet for a second overnight study visit.
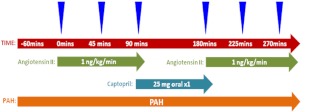
On admission to the clinical research center, subjects completed a 24-h urine collection to evaluate compliance with the study diet; a urinary sodium clearance of at least 150 mmol per 24 h was required to be considered in compliance. Subjects were kept in a strict supine position overnight while fasting and commenced study procedures at 0700 h the following morning. A weight-based infusion of paraaminohippurate (PAH) was administered and continued throughout the study visit to maintain a steady-state circulating concentration of PAH to evaluate renal plasma flow (RPF) at various time points (Fig. 1); at each time point, PAH was measured in serial triplicate for optimal precision (18). After the initial 60 min of PAH infusion, continuous iv AngII (1 ng/kg · min) was initiated to evaluate the tissue sensitivity to AngII, with blood sampling at baseline and 45 and 90 min; the tissue response to exogenous AngII infusion has previously been shown to be inversely proportional to local tissue-RAS activity (19–21). During AngII infusion, blood pressure was assessed every 2 min for safety using a Dinamap automated device (Critikon, Tampa, FL). The AngII infusion was terminated if at any time any of the following occurred: 1) mean arterial pressure (MAP) rose more than 30 mm Hg from baseline, 2) blood pressure exceeded 200/110 mm Hg, or 3) subjects developed any symptoms concerning for an acute cardiovascular insult. MAP measurements were recorded as five consecutive readings at baseline and 45 and 90 min of each AngII infusion to maximize precision (Fig. 1). The AngII infusion was terminated after 90 min, and subjects received a single oral dose of captopril 25 mg and were maintained in continued supine position. Ninety minutes after the administration of captopril, a second infusion of AngII (1 ng/kg · min) was initiated (with blood sampling at 45 and 90 min) to evaluate the tissue sensitivity to AngII after treatment with an ACEi (20). In the reported results, Figs. 2A, 3A, and 4A depict the tissue sensitivities to AngII before and after the vitamin D3 intervention, whereas Figs. 2B, 3B, and 4B depict the change in tissue sensitivities to AngII induced by vitamin D3 in comparison with the change induced by captopril.
Fig. 1.
Study schema. After completing the drug washout and study diet, subjects were admitted to the clinical research center, maintained in a supine position overnight, and commenced study procedures at 0700 h in the morning. Subjects received a continuous PAH infusion throughout the study day, an infusion of AngII (1 ng/kg · min) for 90 min, followed by a single dose of captopril 25 mg and then 90 min later received a second infusion of AngII (1 ng/kg · min). Hemodynamic and blood measurements were taken at baseline and 45 and 90 min of each AngII infusion (arrowheads).
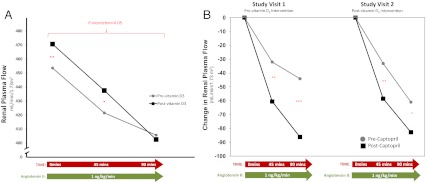
Fig. 2.
The renal-vascular sensitivity to AngII. Baseline RPF and the change in RPF with AngII infusion (A) and the change in RPF with angiotensin II before and after captopril (B) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
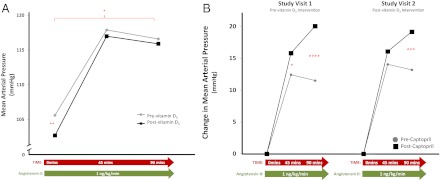
Fig. 3.
The systemic vascular sensitivity to AngII. Baseline MAP and the change in MAP with AngII infusion (A) and the change in MAP with angiotensin II before and after captopril (B) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
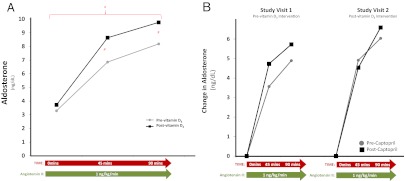
Fig. 4.
The adrenal sensitivity to AngII. Baseline serum aldosterone and the change in aldosterone with AngII infusion (A) and the change in aldosterone with angiotensin II before and after captopril (B) are shown. #, P < 0.10; *, P < 0.05.
Vitamin D3 therapy protocol
After completing the first study visit, subjects were given vitamin D3 15,000 IU/d for 1 month (450,000 IU total) and scheduled to return for their second study visit after completing the same study diet for 1 wk. Our institutional Investigational Drug Service supplied commercially manufactured vitamin D3 capsules after verifying certificates of analyses on purity and dose; three 5000-IU capsules were prescribed for each day. Similar dose regimens have been shown to safely raise 25(OH)D in obesity without adverse effects (22–25). No study participants developed signs or symptoms of hypercalcemia or hypercalciuria.
Laboratory measurements
PTH (Beckman Coulter, Fullerton, CA), 25(OH)D (Diasorin, Inc., Stillwater, MN), 1,25(OH)2D (Diasorin), serum calcium, and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP; Roche, Indianapolis, IN) were measured at baseline in the morning of both study visits after overnight supine rest in dietary sodium balance. Measures of the circulating RAS, including PRA (Diasorin) and serum aldosterone (Siemens, Los Angeles, CA) were obtained as single measurements at baseline and before and during AngII infusions (baseline, 45 and 90 min). PAH was measured in serial triplicate at baseline, and during AngII infusions (baseline, 45 and 90 min), by the same technician using an autoanalyzer technique (12).
Statistical analyses
Demographic and laboratory data before and after vitamin D3 therapy were compared using paired t tests; all tabular data are presented as means ± the sem. A repeated-measures regression analysis model (PROC MIXED) was used to examine the effect of vitamin D3 therapy on RPF (three measures of PAH per time point per subject), aldosterone (one measure per time point per subject), and MAP (five measures per time point per subject) before and during AngII infusions (baseline, 45 and 90 min) at each study visit (before and after vitamin D3 therapy). This model accounted for variation in all raw measurements at each time point and the lack of independence between intraindividual observations. The point estimates for RPF, aldosterone, and MAP are reported at baseline and 45 and 90 min of each AngII infusion, and an interaction analysis was performed to evaluate whether vitamin D3 intervention modified the influence of AngII infusion on each outcome. Although our study design and primary analysis were focused on the paired comparison of tissue responses to AngII before and after vitamin D3 intervention, we performed exploratory secondary analyses to assess the correlation of changes in 25(OH)D [Δ25(OH)D] and PTH (ΔPTH) with tissue responses to AngII using spearman correlation coefficients. Data analyses were performed using SAS (version 9.1) statistical software (SAS Institute, Cary, NC).
Results
Study participants
A total of 55 subjects were screened for participation, of which 26 met all eligibility criteria. Nine of these participants were disempaneled during the medication washout phase of the study due to blood pressure elevations or noncompliance with routine communications with research staff coordinators. Fourteen of the remaining 17 subjects who completed the full study protocol demonstrated 24-h urinary sodium excretions of 150 mmol or greater at both study visits, suggesting compliance with the study diet and achievement of the target liberal sodium balance and were thus included in the final analysis. The mean age of this study population was 50 ± 2.5 yr (range 28–64), 64% were female, and 30% were Caucasian, whereas 70% were Black. Other basic demographic and laboratory characteristics of this study population from the screening visit (before medication washout and study initiation) are depicted in Table 1; participants were morbidly obese, had evidence of impaired glycemic control, had mean 25(OH)D concentrations less than 20 ng/ml, and were generally taking at least one antihypertensive medication.
Table 1.
Initial screen population characteristics
| Baseline characteristics | |
|---|---|
| Weight (kg) | 103.1 ± 3.39 |
| Height (m) | 1.70 ± 0.02 |
| Body mass index (kg/m2) | 36.0 ± 1.27 |
| Systolic blood pressure (mm Hg) | 128.95 ± 2.60 |
| Diastolic blood pressure (mm Hg) | 81.81 ± 2.18 |
| MAP (mm Hg) | 97.51 ± 2.06 |
| Antihypertensive therapy | |
| None (%) | 14.3 |
| One-drug regimen (%) | 71.4 |
| Two-drug regimen (%) | 14.3 |
| 25(OH)D (ng/ml) | 16.55 ± 1.93 |
| PTH (pg/ml) | 47.86 ± 6.69 |
| Hemoglobin A1c (%) | 6.03 ± 0.08 |
| Thyroid-stimulating hormonea | 1.84 ± 0.20 |
| Serum creatinine (mg/dl) | 0.93 ± 0.04 |
| Low-density lipoprotein (mg/dl) | 131.77 ± 6.69 |
| Serum calcium (mg/dl)b | 9.37 ± 0.11 |
Characteristics of the study population were obtained at the first screening visit (prior to medication washout). Data are presented as means ± sem.
Normal range 0.5–5.0 mIU/liter.
Normal range 8.7–10.2 mg/dl.
Baseline changes with vitamin D3 intervention
The baseline demographics and lab data obtained at each study visit are presented in Table 2. There was an expected rise in 25(OH)D and 1,25(OH)2D after 1 month of vitamin D3 therapy and a reciprocal decline in PTH; no hypercalcemia or hypercalciuria was noted. The mean sodium balance at both study visits was greater than 150 mmol per 24 h, confirming compliance with the liberal sodium study diet and resulting in an expected physiological suppression of circulating PRA and aldosterone. Not surprisingly, urine albumin excretion did not change during this short study. In contrast to prior studies, high-dose vitamin D3 therapy did not significantly influence levels of the natriuretic peptide NT-pro-BNP (26), despite being measured after overnight supine rest, while fasting, in controlled sodium balance, and without interfering medications.
Table 2.
Population characteristics during study interventions
| Characteristics | Study visit 1 (before vitamin D3 intervention) | Study visit 2 (after vitamin D3 intervention) |
|---|---|---|
| n | 14 | |
| Demographic variables | ||
| Weight (kg) | 102.9 ± 3.3 | 103.4 ± 3.3 |
| Body mass index (kg/m2) | 36.3 ± 1.2 | 36.3 ± 1.3 |
| Serum creatinine (mg/dl) | 0.90 ± 0.1 | 0.92 ± 0.1 |
| Calcium regulatory variables | ||
| 25(OH)D (ng/ml) | 18.2 ± 1.8 | 51.9 ± 3.1a |
| 1,25(OH)2D (pg/ml) | 38.1 ± 3.3 | 57.7 ± 4.5a |
| PTH (pg/ml) | 46.4 ± 4.3 | 37.1 ± 3.1b |
| Serum calcium (mg/dl)c | 8.9 ± 0.1 | 9.1 ± 0.8d |
| 24-h urine variables | ||
| Urine creatinine (mg) | 1599.5 ± 103.4 | 1692.6 ± 131.8 |
| Urine sodium (mmol) | 343.5 ± 37.1 | 339.0 ± 34.8 |
| Urine potassium (mmol) | 95.0 ± 8.3 | 91.5 ± 8.0 |
| Urine calcium (mg/kg) | 1.9 ± 0.3 | 2.2 ± 0.2 |
| Urine albumin (mg) | 8.2 ± 1.4 | 11.0 ± 2.7 |
| Urinary calcium (mg) to creatinine (mg) ratio | 0.12 ± 0.0 | 0.14 ± 0.0 |
| Sodium regulatory variables | ||
| NT-pro-BNP (pg/ml) | 92.4 ± 38.9 | 56.4 ± 16.1 |
| PRA (ng/ml · h) | 0.21 ± 0.0 | 0.26 ± 0.1 |
| Aldosterone (ng/dl) | 3.3 ± 0.2 | 3.7 ± 0.4 |
| 24-h aldosterone excretion rate (μg/d) | 6.5 ± 1.4 | 5.8 ± 1.8 |
Baseline study visit demographics and laboratory data were obtained after medication washout and completion of the study diet. Data are presented as means ± sem.
P < 0.0001
P < 0.01.
Normal range is 8.7–10.2 mg/dl.
P < 0.05.
The impact of vitamin D3 therapy on the renal-vascular sensitivity to AngII
RPF and the change in RPF during AngII infusion were evaluated as measures of renal-vascular tissue-RAS activity (20). Therapy with vitamin D3 significantly raised baseline RPF by 5% and significantly modified the influence of a continuous AngII infusion on RPF (P interaction <0.05) (Fig. 2A); the renal-vascular sensitivity to AngII was increased after vitamin D3 intervention, consistent with a lowering of renal-vascular tissue-RAS activity. The administration of captopril increased RPF from baseline to the same degree at both study visits (503.5 ± 13.7 vs. 509.8 ± 14.0, respectively, for study visits 1 and 2; P = NS). As anticipated, the renal-vascular sensitivity to AngII after captopril was improved at both visits, but this improvement was much smaller following vitamin D3 therapy (Fig. 2B), indicating that the vitamin D3 therapy corrected the difference in the renal-vascular sensitivity to AngII induced by an ACEi.
The impact of vitamin D3 therapy on the systemic vascular sensitivity to AngII
MAP and the change in MAP with AngII infusion were evaluated as surrogates for systemic vascular tissue-RAS activity. Basal supine MAP was significantly lowered by 3% (P < 0.01) after vitamin D3 therapy (Fig. 3A). Although the mean AngII-stimulated MAP was lower after intervention with vitamin D3, there was no significant interaction to suggest that vitamin D3 therapy modified the MAP response to AngII. Captopril administration lowered MAP to the same degree at both study visits (96.9 ± 2.1 vs. 95.1 ± 2.0 mm Hg, respectively, for study visits 1 and 2; P = NS) and improved the MAP sensitivity to AngII (Fig. 3B); however, the improvement in MAP sensitivity to AngII induced by captopril was significantly diminished following vitamin D3 therapy (P < 0.01), suggesting that vitamin D3 intervention increased the vascular sensitivity to AngII akin to the action of an ACEi (Fig. 3B).
The impact of vitamin D3 therapy on the adrenal sensitivity to AngII
Baseline serum aldosterone and urinary aldosterone excretion were unchanged after vitamin D3, reflecting suppression of the circulating RAS by liberal sodium intake prescribed in the study diet (Table 2). With infusion of AngII, there was a greater increase in serum aldosterone after vitamin D3 therapy; although point estimates at 45 and 90 min were not statistically different, integrated mean aldosterone levels across the AngII infusion were significantly higher after vitamin D3 therapy (P < 0.05) (Fig. 4A). The administration of captopril decreased serum aldosterone levels to similar degrees at both study visits (2.88 ± 0.23 vs. 3.09 ± 0.29 ng/dl, respectively, for study visits 1 and 2; P = NS). There was a trend suggesting that the administration of captopril increased the adrenal sensitivity to AngII before the vitamin D3 intervention but not after the vitamin D3 intervention, indicating that vitamin D3 therapy corrected the adrenal sensitivity to AngII in a similar manner to an ACEi (27); however, these differences were not statistically significant (Fig. 4B).
Correlating changes in 25(OH)D and PTH with tissue sensitivities to AngII
We explored the relationship between tissue responses to AngII with Δ25(OH)D and ΔPTH in a secondary correlation analysis. In support of our aforementioned findings, a larger decline in RPF with AngII infusion was correlated with greater increases in 25(OH)D and greater declines in PTH (Δ25(OH)D: rs = −0.30; ΔPTH: rs = 0.33). Similarly, the MAP and aldosterone responses to AngII were positively correlated with Δ25(OH)D (rs = 0.27 and rs = 0.24, respectively) and inversely correlated with ΔPTH (rs = −0.61 and rs = −0.10, respectively). However, as anticipated due to our small sample size and study design, these correlation coefficients did not reach statistical significance.
Discussion
Herein we report the results of an intervention study that evaluated the impact of high-dose vitamin D3 therapy on the tissue sensitivity to AngII. Because tissue responsiveness to AngII is inversely proportional to end-organ tissue-RAS activity (19, 20, 27), our study design assessed whether vitamin D3 therapy could physiologically modulate tissue-RAS activity in a population of obese individuals with hypertension, two conditions that are predisposed to high tissue-RAS activity (5, 7). We observed that high-dose vitamin D3 therapy increased 25(OH)D and 1,25(OH)2D and decreased PTH, and also raised basal RPF and decreased supine MAP. Furthermore, vitamin D3 therapy heightened the tissue sensitivity to AngII, and marginalized the typical tissue sensitization to AngII induced by an ACEi. These collective findings are supportive of the hypothesis that vitamin D3 therapy reduces unfavorable tissue-RAS activity; vitamin D3 corrects the tissue sensitivity to AngII in a manner similar to that previously induced by an ACEi (20, 27).
The implications of our physiological findings are best understood in the context of prior experimentations in hypertensives. Shoback et al. (19) originally reported that a subset of hypertensive individuals demonstrate a blunted renal-vascular and adrenal sensitivity to AngII. The mechanism for this blunted response was speculated to be high local tissue-AngII concentrations and tissue-RAS activity, resulting in down-regulated or desensitized AngII receptors. Indeed, subsequent physiology experiments in hypertension confirmed that treatment with an ACEi corrected this abnormal renal-vascular and adrenal responsiveness to AngII (20, 27); lowering the local tissue-RAS with an ACEi increased the tissue sensitivity to AngII. Although these prior experiments provided convincing data to support the tissue responsiveness to AngII as an inverse measure of local tissue-RAS activity in humans, this methodology involves indirect measurements that cannot conclusively confirm the biology of tissue-RAS activity. Nonetheless, these agonist-antagonist physiology experimentations have provided considerable insight into tissue-RAS activity in humans over several decades; our previous cross-sectional observations demonstrated that lower 25(OH)D concentrations were associated with a blunted vascular sensitivity to AngII, particularly with higher adiposity (12, 13). These findings suggested that vitamin D deficiency may contribute to abnormally high tissue-RAS, akin to that observed in the subset of nonresponsive hypertension by Shoback et al. (19) and led us to hypothesize that vitamin D3 therapy could potentially correct the sensitivity to AngII similar to an ACEi. In support of this hypothesis, herein we observed that vitamin D3 therapy lowered basal MAP and raised RPF, improved tissue sensitivities to AngII, and reduced the influence of an acute dose of captopril on the tissue sensitivity to AngII. Of clinical significance, because obesity and elevated blood pressure predict a higher risk for kidney and vascular diseases and treatment with ACEi has been shown to diminish this risk (28), our findings may support the maintenance of higher 25(OH)D status in obese hypertensives as a method to lower tissue-RAS activity and abrogate the development of vascular and metabolic diseases associated with these conditions. It is worth noting that to attain desired increases in 25(OH)D in our obese population within the study period, we used vitamin D3 doses that were considerably higher than the current guidelines would recommend (29); our observations alone are insufficient to support changes in vitamin D3 prescribing practices. Future corroborative studies are needed to evaluate the benefits and risks of high-dose vitamin D3 therapy on extraskeletal outcomes.
Although some large clinical studies have observed vascular, metabolic, and mortality benefits with vitamin D3 therapy (30–35), others with differing population demographics and supplementation doses have not (36–38). Our current results provide insight into the interpretation of these discordant and conflicting findings in the literature: the beneficial effects of vitamin D3 therapy on cardiovascular health outcomes may be maximally seen in younger individuals with low 25(OH)D levels, who are obese and hypertensive and who receive larger doses of vitamin D3. Furthermore, if the mechanism by which vitamin D3 therapy imparts health benefits is via modest reductions in local tissue-RAS activity, then the influence on clinical outcomes (such as cardiovascular events) may require prolonged therapy and follow-up and may be obscured when pharmacological RAS antagonists are concomitantly used.
Our findings are consistent with and extend those of prior animal and human studies that have implicated vitamin D metabolites as negative regulators of the RAS (8). Prior investigators (39, 40) have reported multiple experiments supporting the 1,25(OH)2d-VDR complex as a negative regulator of renin expression and RAS activity in mice. In mouse models of diabetes that displayed enhanced tissue-RAS activity and the development of nephropathy, therapy with VDR agonists attenuated proteinuria and renal injury (41–43). Human cross-sectional studies have translated these findings by showing associations between lower 25(OH)D levels and higher tissue RAS activity (12, 13), the prevalence and incidence of hypertension (30, 32), worsening renal function (44), and even death (35). Although we observed a notable reduction in basal supine MAP with vitamin D3 therapy, other prospective human studies to date have yielded mixed results on the impact of vitamin D therapy on blood pressure (30). In contrast, it has been consistently observed that VDR agonism in human CKD significantly lowers proteinuria (8, 45), even though these studies were not designed to evaluate whether the beneficial mechanism of vitamin D therapy was via reductions in the renal-vascular RAS. In this regard, our current observations may implicate a lowering of the renal-vascular RAS as a key mechanism for proteinuria reductions in CKD seen with VDR agonism (45) and may further suggest that future interventional study designs should consider the RAS as a key mechanistic mediator when evaluating the influence of vitamin D on clinical outcomes in humans.
Our findings must be interpreted in the context of the strengths and limitations of our study design. A major study strength was the assessment of circulating and tissue RAS measures after withdrawing all interfering medications, under controlled posture, dietary electrolyte balance, and by using agonists (AngII) and antagonists (captopril) to illicit functional responses. Although consistent evidence suggests the tissue responses to AngII are an inverse surrogate for tissue-RAS activity (12, 20, 27), this method of measuring tissue-RAS activity is limited by its indirect and inconclusive nature. The liberal dietary sodium intake in our study diet suppressed circulating RAS components and thus prevented detection of basal changes in these components with vitamin D3 therapy; however, it ensured reliable comparisons of tissue-RAS responses. Although emerging data associate circulating aldosterone levels with cardiovascular risk (46), our study diet and protocol were not designed to shed further light on this important topic. Our study conclusions are limited by the fact that our overall study population was small, was not compared with a placebo intervention, and did not assess clinical outcomes; future studies with a larger sample size and a blinded placebo control design [such as the ongoing MODERATE (Modifiable Effectors of Renin System Activation: Treatment Evaluation) study (NCT01320722)] may expand on our initial mechanistic observations. Our observations that 1 month of high-dose vitamin D3 therapy did not influence NT-pro-BNP or proteinuria need confirmation in a larger population. We observed a modest reduction in basal supine MAP after vitamin D3 therapy, but prior prospective studies did not see any influence of vitamin D3 supplementation on blood pressure in obesity (37). The influence of vitamin D and PTH on blood pressure remains inconclusive and fraught with mixed results (30); ongoing studies that use superior methods to assess blood pressure may provide more conclusive results [i.e. the DAYLIGHT study (NCT01240512) and the Vitamin D and OmegA-3 Trial (NCT01169259)]. We tested the hypothesis that raising 25(OH)D and 1,25(OH)2D concentrations with vitamin D3 therapy would reduce tissue-RAS activity; however, our intervention also modulated PTH and calcium levels as expected. Although our study design was based on the hypothesis that 1,25(OH)2D is a direct mediator of the RAS, it was not designed to assess whether changes in PTH and calcium, which have both been implicated with RAS modulation (47, 48), could have also contributed to our observed findings. Our exploratory secondary correlation analyses demonstrated supportive trends that did not reach statistical significance because of the insufficient sample size and the likely nonlinear relationships between vitamin D3, 25(OH)D, PTH, and tissue-RAS activity.
In summary, we observed that vitamin D3 therapy for obese hypertensives with low 25(OH)D concentrations raised basal RPF, lowered supine MAP, and modulated the vascular and adrenal sensitivities to AngII akin to the actions of a pharmacological ACE inhibitor. These findings are consistent with prior animal and human data implicating 1,25(OH)2D as a negative mediator of the RAS. Whether the chronic maintenance of higher 25(OH)D status in obesity and HTN reduces unfavorable clinical outcomes associated with high tissue-RAS activity warrants further investigation.
Acknowledgments
We thank our funding sources: F32 HL104776-02 and K23 HL111771-01 (to A.V.), American Medical Association Foundation Seed Grant (to A.V.), K23 HL08236-03 (to J.S.W.), U54LM008748 from the National Library of Medicine and UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources and M01-RR02635, Brigham & Women's Hospital, General Clinical Research Center, from the National Center for Research Resources, and the Specialized Center of Research (SCOR) in Molecular Genetics of Hypertension P50HL055000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Library of Medicine, the National Institutes of Health, or the National Center for Research Resources.
This work was supported by Grants F32 HL104776-02 and K23 HL111771-01 (to A.V.), K23 HL08236-03 (to J.S.W.), and U54LM008748 from the National Library of Medicine; an American Medical Association Foundation Seed Grant (to A.V.); Grant UL1 RR025758 (to the Harvard Clinical and Translational Science Center) from the National Center for Research Resources; Grant M01-RR02635 (to Brigham and Women's Hospital, General Clinical Research Center) from the National Center for Research Resources; and the Specialized Center of Research in Molecular Genetics of Hypertension Grant P50HL055000.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACEi
- Angiotensin-converting enzyme inhibitor
- AngII
- angiotensin II
- CKD
- chronic kidney disease
- HTN
- hypertension
- MAP
- mean arterial pressure
- NT-pro-BNP
- N-terminal pro-B-type natriuretic peptide
- 1,25(OH)2D
- 1,25 dihydroxyvitamin D3
- 25(OH)D
- 25-hydroxyvitamin D
- Δ25(OH)D
- change in 25(OH)D
- PAH
- paraaminohippurate
- PRA
- plasma renin activity
- ΔPTH
- change in PTH
- RAS
- renin-angiotensin system
- RPF
- renal plasma flow
- VDR
- vitamin D receptor.
References
- 1. Hossain P, Kawar B, El Nahas M. 2007. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 356:213–215 [DOI] [PubMed] [Google Scholar]
- 2. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. 2010. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engeli S, Negrel R, Sharma AM. 2000. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 35:1270–1277 [DOI] [PubMed] [Google Scholar]
- 4. Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM. 2003. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35:807–825 [DOI] [PubMed] [Google Scholar]
- 5. Faloia E, Gatti C, Camilloni MA, Mariniello B, Sardu C, Garrapa GG, Mantero F, Giacchetti G. 2002. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J Endocrinol Invest 25:309–314 [DOI] [PubMed] [Google Scholar]
- 6. Yvan-Charvet L, Quignard-Boulangé A. 2011. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int 79:162–168 [DOI] [PubMed] [Google Scholar]
- 7. Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A, Ohashi N, Urushihara M, Kobori H, Morimoto N, Kawazoe T, Naitoh M, Okada M, Sakaue H, Suzuki S, Nakao K. 2010. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens 23:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaidya A, Williams JS. 2012. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 61:450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaidya A, Forman JP, Seely EW, Williams JS. 2011. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst 12:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaidya A, Sun B, Forman JP, Hopkins PN, Brown NJ, Kolatkar NS, Williams GH, Williams JS. 2011. The Vitamin D receptor gene polymorphism Fok1 is associated with plasma renin activity in Caucasians. Clin Endocrinol (Oxf) 74:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. 2010. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system. The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin Chim Acta 411:1354–1360 [DOI] [PubMed] [Google Scholar]
- 12. Forman JP, Williams JS, Fisher ND. 2010. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 55:1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaidya A, Forman JP, Williams JS. 2011. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens 25:672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beydoun MA, Boueiz A, Shroff MR, Beydoun HA, Wang Y, Zonderman AB. 2010. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in United States adults. J Clin Endocrinol Metab 95:3814–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. 2005. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123 [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention 1996. The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) reference manuals and reports [CD-ROM]. Bethesda, MD: National Center for Health Statistics [Google Scholar]
- 17. Dickinson BD, Havas S. 2007. Reducing the population burden of cardiovascular disease by reducing sodium intake: a report of the Council on Science and Public Health. Arch Intern Med 167:1460–1468 [DOI] [PubMed] [Google Scholar]
- 18. Fisher ND, Allan D, Kifor I, Gaboury CL, Williams GH, Moore TJ, Hollenberg NK. 1994. Responses to converting enzyme and renin inhibition. Role of angiotensin II in humans. Hypertension 23:44–51 [DOI] [PubMed] [Google Scholar]
- 19. Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. 1983. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest 72:2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. 1985. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest 75:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chamarthi B, Williams JS, Williams GH. 2010. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens 28:1020–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amir E, Simmons CE, Freedman OC, Dranitsaris G, Cole DE, Vieth R, Ooi WS, Clemons M. 2010. A phase 2 trial exploring the effects of high-dose (10,000 IU/day) vitamin D(3) in breast cancer patients with bone metastases. Cancer 116:284–291 [DOI] [PubMed] [Google Scholar]
- 23. Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. 2009. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med 122:1056–1060 [DOI] [PubMed] [Google Scholar]
- 24. Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. 2008. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int 19:1621–1628 [DOI] [PubMed] [Google Scholar]
- 25. Wu F, Staykova T, Horne A, Clearwater J, Ames R, Mason B, Orr-Walker B, Gamble G, Scott M, Reid I. 2003. Efficacy of an oral, 10-day course of high-dose calciferol in correcting vitamin D deficiency. N Z Med J 116:U536. [PubMed] [Google Scholar]
- 26. Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. 2010. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 53:2112–2119 [DOI] [PubMed] [Google Scholar]
- 27. Taylor T, Moore TJ, Hollenberg NK, Williams GH. 1984. Converting-enzyme inhibition corrects the altered adrenal response to angiotensin II in essential hypertension. Hypertension 6:92–99 [DOI] [PubMed] [Google Scholar]
- 28. Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Remuzzi G, Zoccali C. 2011. ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol 22:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaidya A, Forman JP. 2010. Vitamin D and hypertension: current evidence and future directions. Hypertension 56:774–779 [DOI] [PubMed] [Google Scholar]
- 31. Forman JP, Curhan GC, Taylor EN. 2008. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 52:828–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. 2007. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49:1063–1069 [DOI] [PubMed] [Google Scholar]
- 33. Griffin FC, Gadegbeku CA, Sowers MR. 2011. Vitamin D and subsequent systolic hypertension among women. Am J Hypertens 24:316–321 [DOI] [PubMed] [Google Scholar]
- 34. Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. 2011. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the cardiovascular health study. Hypertension 58:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. 2012. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol 109:359–363 [DOI] [PubMed] [Google Scholar]
- 36. Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, Beresford SA, Connelly SA, Curb JD, Grimm RH, Jr, Kotchen TA, Kuller LH, Wassertheil-Smoller S, Thomson CA, Torner JC. 2008. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension 52:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jorde R, Sneve M, Torjesen P, Figenschau Y. 2010. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med 267:462–472 [DOI] [PubMed] [Google Scholar]
- 38. Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. 2012. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD Trial). J Clin Endocrinol Metab 97:614–622 [DOI] [PubMed] [Google Scholar]
- 39. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 2002. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. 2008. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 74:170–179 [DOI] [PubMed] [Google Scholar]
- 41. Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC. 2010. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int 77:1000–1009 [DOI] [PubMed] [Google Scholar]
- 42. Ohara I, Tanimoto M, Gohda T, Yamazaki T, Hagiwara S, Murakoshi M, Aoki T, Toyoda H, Ishikawa Y, Funabiki K, Horikoshi S, Tomino Y. 2011. Effect of combination therapy with angiotensin receptor blocker and 1,25-dihydroxyvitamin D3 in type 2 diabetic nephropathy in KK-Ay/Ta mice. Nephron Exp Nephrol 117:e124–e132 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. 2008. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA 105:15896–15901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. 2011. Serum 25-hydroxyvitamin d and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 6:2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. 2010. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376:1543–1551 [DOI] [PubMed] [Google Scholar]
- 46. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. 2010. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J 31:1237–1247 [DOI] [PubMed] [Google Scholar]
- 47. Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, Meinitzer A, März W, Pieber TR, Tomaschitz A. 2012. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab 97:E75–E79 [DOI] [PubMed] [Google Scholar]
- 48. Atchison DK, Harding P, Beierwaltes WH. 2011. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension 58:604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]