Abstract
Context:
Previous studies have identified an obese phenotype without the burden of adiposity-associated cardiometabolic risk factors, although the health effects remain unclear.
Objective:
We examined the association between metabolically healthy obesity and risk of cardiovascular disease (CVD) and all-cause mortality.
Design and Setting:
This was an observational study with prospective linkage to mortality records in community-dwelling adults from the general population in Scotland and England.
Participants:
A total of 22,203 men and women [aged 54.1 (SD 12.7 yr), 45.2% men] without known history of CVD at baseline.
Interventions:
Based on blood pressure, high-density lipoprotein-cholesterol, diabetes diagnosis, waist circumference, and low-grade inflammation (C-reactive protein ≥ 3 mg/liter), participants were classified as metabolically healthy (0 or 1 metabolic abnormality) or unhealthy (two or more metabolic abnormalities). Obesity was defined as a body mass index of 30 kg/m2 or greater.
Main Outcome Measure:
Study members were followed up, on average, more than 7.0 ± 3.0 yr for cause-specific mortality. Cox proportional hazards models were used to examine the association of metabolic health/obesity categories with mortality.
Results:
There were 604 CVD and 1868 all-cause deaths, respectively. Compared with the metabolically healthy nonobese participants, their obese counterparts were not at elevated risk of CVD [hazard ratio (HR) 1.26, 95% confidence interval (CI) 0.74–2.13], although both nonobese (HR 1.59, 95% CI 1.30–1.94) and obese (HR 1.64, 95% CI 1.17–2.30) participants with two or more metabolic abnormalities were at elevated risk. Metabolically unhealthy obese participants were at elevated risk of all-cause mortality compared with their metabolically healthy obese counterparts (HR 1.72, 95% CI 1.23–2.41).
Conclusion:
Metabolically healthy obese participants were not at increased risk of CVD and all-cause mortality over 7 yr.
Obesity is typically accompanied by unfavorable metabolic profiles, such as impaired glucose metabolism, adverse lipid profiles, systemic inflammation, and elevated blood pressure, but it is increasingly recognized that this may not always be the case. The term, metabolically healthy obesity, has been used to describe an obese phenotype that does not have the burden of any metabolic disorder (1). There is convincing evidence to show adverse effects of obesity on health (2, 3), although in a recent study, the addition of information on measures such as body mass index (BMI), waist circumference, or waist to hip ratio to a cardiovascular disease risk prediction model containing conventional risk factors did not improve risk discrimination (4). Several epidemiological studies have shown that metabolically healthy obese participants are not at increased risk of developing cardiovascular disease (CVD) over 3–13 yr of follow-up compared with healthy nonobese (5–10) and are at lower risk compared with metabolically unhealthy obese participants (11). However, there are inconsistencies in the data, and studies with an extended follow up period (>15 yr) showed that obese participants without metabolic syndrome at baseline were still at increased risk of major CVD events compared with healthy nonobese (12, 13). In addition, metabolically healthy obese women had greater burden of subclinical atherosclerosis than nonobese women, although lower burden compared with metabolically unhealthy obese women (14). Taken together, it is difficult to draw firm conclusions from the present literature. The aim of this study was to explore the association between metabolically healthy obesity and risk of mortality in a large nationally representative data set of men and women initially free of CVD.
Materials and Methods
Study design and participants
Participants were recruited into the Health Survey for England (HSE) and Scottish Health Survey (SHS), both representative, general population-based study sampling individuals living in households (15). HSE/SHS samples are selected using multistage stratified probability design to give a representative sample of the target population. Stratification is based on geographical entities and not on individual characteristics: postcode sectors selected at the first stage and household addresses selected at the second stage. The overall response rate (interviewer home visit) ranged between 60 and 90% for different survey years, with 33–41% of all eligible participants seeing a nurse during a subsequent home visit. Participants for the present analysis were merged together from a range of different survey years (from HSE 1998, 1999, 2003, and 2004 and SHS 1998 and 2003) and were linked prospectively to National Health Service mortality data; thus, the analyses were based on a prospective cohort design. Study participants gave full written informed consent and ethical approval was obtained from the London Research Ethics Council.
Demographic and clinical variables
During the first household visit, interviewers collected information using computer-assisted personal interviewing modules. Various self-reported information was collected, including smoking (current/ex-smoker/never) and participation in moderate to vigorous leisure time physical activity including walking and cycling for any purpose (number of sessions per week lasting at least 30 min). The physical activity questionnaire has been validated using objective accelerometry recordings (16). Socioeconomic status was assessed using the Registrar General Classification; I/II professional/intermediate, III skilled nonmanual/skilled manual, IV/V part skilled/unskilled. Trained nurses collected information about physician-diagnosed CVD (stroke, angina, heart attack), hypertension, and diabetes mellitus (types 1 and 2); collected nonfasting blood samples; measured resting blood pressure (BP); and collected information on prescribed medication. Participants' body weight was measured using Tanita electronic scales (Tokyo, Japan) without shoes in light clothing, and height was measured using a stadiometer with the Frankfort plane in the horizontal position. BMI was calculated as the following: [weight (kilograms)/height (meters) squared]. Waist circumference was taken twice to the nearest millimeter midway between the iliac crest and lower rib using a measuring tape. An average from the first two measurements was used, although if the first and second reading differed by more than 3 cm, a third reading was taken. Blood samples were analyzed for C-reactive protein (CRP) and high-density lipoprotein (HDL) cholesterol. The analysis of CRP levels from serum was performed using the N Latex high-sensitivity CRP monoimmunoassay on the Behring Nephelometer II analyzer (Deerfield, IL; coefficient of variation < 6%). HDL cholesterol was measured using cholesterol oxidase assays on an Olympus 640 analyzer (Tokyo, Japan). Systolic and diastolic BP was measured with an Omron HEM-907 blood pressure monitor (Omron, Japan) three times in the sitting position after 5-min rest between each reading. The initial reading was discarded and an average of the second and third BP recordings was used for the present analyses.
Mortality follow-up
Classification of the primary (underlying) cause of death was based on information collected from the death certificate together with any additional information provided subsequently by the certifying doctor (e.g. secondary death cause). Diagnoses for the primary cause of death were recorded using the International Classification of Diseases, Ninth and 10th revisions. Cardiovascular disease codes were 390–459 for International Classification of Diseases, ninth revision, and I01-I99 for International Classification of Diseases, 10th revision.
Statistical analyses
Nonobese participants were defined as those with a BMI of 18–29.9 kg/m2 and obesity as a BMI of 30 kg/m2 or greater. Metabolic risk was based on an adaptation of previous criteria (17, 18) according to availability of data, and defined as two or more metabolic risk factors, including large waist (waist > 102 cm in men and > 88 cm in women), hypertension risk (clinic BP > 130/85 mm Hg or hypertension diagnosis or use of antihypertensive medication), doctor-diagnosed diabetes, low-grade inflammation (CRP ≥ 3 mg/liter), adverse HDL cholesterol (<1.03 mmol/liter in men and <1.30 mmol/liter in women). Participants were then categorized into four groups consisting of metabolically healthy nonobese; metabolically unhealthy nonobese; metabolically healthy obese; and metabolically unhealthy obese. We used χ2 and ANOVA with Scheffé post hoc tests to examine differences in baseline characteristics with respect to these categories. Having first ascertained that the proportional hazards assumption had not been violated, CVD or all-cause death as the outcome of interest, we used Cox proportional hazards models to compute hazard ratios (HR) with accompanying 95% confidence intervals (CI) for the association with metabolic health/obesity categories. The proportional hazards assumption was examined by comparing the cumulative hazard plots grouped on the various exposure variables, although no appreciable violations were noted. Months were the time scale, and for participants with no record of an event, the data were censored at February 28, 2008 (HSE), and December 31, 2008 (SHS). Each model was adjusted for age and sex, and we also ran additional models that included further adjustment for physical activity (none; one to two; or three or more sessions per week), smoking (never; previous; or current), socioeconomic group (I/II, professional and intermediate; III, skilled nonmanual and skilled manual; IV/V, part skilled/unskilled). In addition, we adjusted the models for BMI to determine whether the associations were independent of morbid obesity. We conducted a second set of analyses to examine mortality risk in relation to metabolic health and waist measurements, using a similar approach described above, although metabolic risk was defined as one or more risk factors, including hypertension risk, diabetes, inflammation, and adverse an HDL profile. All analyses were conducted using SPSS version 14 (SPSS Inc., Chicago, IL) and a significance level of P < 0.05 was used.
Results
The initial study sample consisted of 25,608 adults who had consented to a blood draw during the nurse visit, although 11% (n = 2,753) did not consent to mortality follow-up and were therefore removed from any analysis. Nonconsenting adults were younger than those consenting (50.1 vs. 54.4 yr, P < 0.001). After the exclusion of 135 underweight (BMI < 18 kg/m2) participants and 517 with CVD at baseline, the final analytic sample consisted of 22,203 participants [aged 54.1 (sd 12.7 yr), 45.2% men].
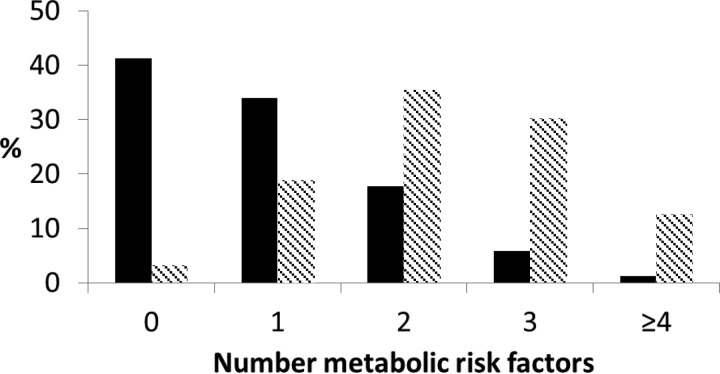
Twenty-four percent of the sample were defined as obese, and within the obese sample, 22% were categorized as metabolically healthy (Table 1). On average, obese participants displayed more metabolic risk factors than nonobese (2.3 vs. 0.9, P < 0.001), which is displayed in Fig. 1. Metabolically healthy obese participants were comparable with their healthy nonobese counterparts on a number of variables, including age, social status, physical activity, HDL cholesterol, CRP, and a low prevalence of diabetes and hypertension. The metabolically healthy obese participants were less likely to be smokers but displayed higher waist circumference compared with nonobese. However, the metabolically healthy obese participants displayed lower waist and BMI compared with their unhealthy obese counterparts. Participants at metabolic risk tended to be older than the metabolically healthy.
Table 1.
Characteristics of the study population at baseline (n = 22,203)
| Variable | Metabolically healthy nonobese (n = 12,716) | Metabolically unhealthy nonobese (n = 4,201) | Metabolically healthy obese (n = 1,160) | Metabolically unhealthy obese (n = 4,128) |
|---|---|---|---|---|
| Age (yr) | 51.9 ± 12.4 | 59.3 ± 12.5 | 51.3 ± 11.5a | 56.3 ± 12.3 |
| Men (%) | 45.4 | 47.2 | 52.6 | 40.4 |
| Highest socioeconomic group (I/II) (%) | 42.5 | 36.9 | 38.9a | 32.8 |
| Current smokers (%) | 25.6 | 28.7 | 16.7b | 19.7 |
| Physical activity (% at least three times per week MVPA) | 35.1 | 21.0 | 28.9a | 19.3 |
| HDL cholesterol (mmol/liter) | 1.6 ± 0.4 | 1.3 ± 0.4 | 1.5 ± 0.3b | 1.3 ± 0.4 |
| BMI (kg/m2) | 24.8 ± 2.6 | 26.7 ± 2.3 | 32.2 ± 2.7b | 34.2 ± 3.8 |
| Waist (cm) | 84.5 ± 9.6 | 93.1 ± 9.5 | 99.5 ± 10.1b | 106.1 ± 10.4 |
| Diabetes (%) | 0.5 | 7.0 | 0.3a | 6.5 |
| Systolic BP (mm Hg) | 129.2 ± 17.5 | 142.8 ± 17.5 | 131.9 ± 15.5b | 141.6 ± 20.6 |
| Hypertension risk (%) | 18.6 | 71.2 | 10.9b | 64.8 |
| CRP (mg/liter) | 2.0 ± 4.5 | 6.2 ± 8.6 | 2.1 ± 3.1a | 6.1 ± 7.0 |
| Inflammation (CRP ≥ 3 mg/liter) (%) | 12.5 | 65.5 | 8.8a | 67.5 |
Values are means ± sd unless otherwise stated. Obesity is defined as a BMI 30 kg/m2 or greater; metabolically unhealthy is defined as two or more metabolic risk factors, including large waist (>102 cm in men and > 88 cm in women), hypertension risk (clinical BP > 130/85 mm Hg or hypertension diagnosis or use of antihypertensive medication), doctor's diagnosed diabetes, low-grade inflammation (CRP ≥ 3 mg/liter), adverse lipid profile (HDL cholesterol < 1.03 mmol/liter in men and < 1.30 mmol/liter in women). MVPA, Moderate to vigorous physical activity.
Significantly different from metabolic at-risk groups.
Significantly different (P < 0.001) from all other groups.
Fig. 1.
The distribution of metabolic risk factors in nonobese (black bars) and obese (hatched bars) participants.
There were 604 and 1868 CVD and all-cause deaths, respectively. In mutually adjusted models, the metabolic risk factors that were associated with future CVD included hypertension (HR 1.55, 95% CI 1.30–1.84), diabetes (HR 1.77, 95% CI 1.33–2.35), low-grade inflammation as indexed by CRP of 3 mg/liter or greater (HR 1.67, 95% CI 1.42–1.97), and adverse HDL cholesterol profile (HR 1.31, 95% CI 1.09–1.58). Compared with the metabolically healthy nonobese subjects, their metabolically healthy obese counterparts were not at elevated risk of CVD (fully adjusted HR 1.26, 95% CI 0.74–2.13), although both the nonobese (HR 1.59, 95% CI 1.30–1.94) and obese (HR 1.64, 95% CI 1.17–2.30) participants with two or more metabolic abnormalities were at elevated risk (Table 2). Similar results were seen for all-cause mortality (Table 3). In analyses stratified by sex, obese men with metabolic risk factors were at the highest risk of CVD, whereas in women only the nonobese with metabolic risk factors were at elevated risk after adjustment for covariates. Nevertheless, in both sexes the metabolically healthy obese were not at elevated risk of CVD or all-cause mortality. Because it was feasible that some participants in the reference group were overweight (BMI 25–30 kg/m2) and had one metabolic risk factor, we performed a sensitivity analysis in which the reference group was redefined as participants with a BMI less than 25 kg/m2 and zero metabolic risk factors (n = 4034). However, the results remained largely unchanged; compared with the lean participants without any metabolic risk factors, the metabolically healthy obese were not at risk of CVD (fully adjusted HR 1.41, 95% CI 0.82–2.42), in contrast to their metabolically unhealthy obese counterparts (fully adjusted HR 1.82, 95% CI 1.30–2.53).
Table 2.
The association between metabolic health, obesity, and CVD mortality
| Cases/n | Age- and sex-adjusted HR (95% CI) | Fully adjusted HR (95% CI)a | |
|---|---|---|---|
| Whole sample | |||
| Metabolically healthy nonobese | 225/12716 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 216/4201 | 1.66 (1.37–2.00) | 1.59 (1.30–1.94) |
| Metabolically healthy obese | 18/1160 | 1.02 (0.63–1.65) | 1.26 (0.74–2.13) |
| Metabolically unhealthy obese | 145/4128 | 1.58 (1.28–1.95) | 1.64 (1.17–2.30) |
| P trend | <0.001 | <0.001 | |
| Men | |||
| Metabolically healthy nonobese | 132/5771 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 110/1983 | 1.45 (1.12–1.87) | 1.36 (1.04–1.78) |
| Metabolically healthy obese | 11/610 | 1.09 (0.59–2.02) | 1.45 (0.73–2.89) |
| Metabolically unhealthy obese | 79/1669 | 1.69 (1.28–2.24) | 1.84 (1.17–2.90) |
| P trend | 0.001 | 0.039 | |
| Women | |||
| Metabolically healthy nonobese | 93/6945 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 106/2218 | 1.95 (1.47–2.59) | 1.90 (1.41–2.57) |
| Metabolically healthy obese | 7/550 | 0.95 (0.44–2.05) | 1.04 (0.45–2.38) |
| Metabolically unhealthy obese | 66/2459 | 1.47 (1.07–2.02) | 1.47 (0.88–2.46) |
| P trend | <0.001 | <0.001 |
Sample contains participants without a history of CVD at baseline (n = 22,203).
Contains adjustment for age, sex, smoking, physical activity, socioeconomic group, and BMI.
Table 3.
The association between metabolic health, obesity, and all-cause mortality
| Cases/n | Age- and sex-adjusted HR (95% CI) | Fully adjusted HR (95% CI)a | |
|---|---|---|---|
| Whole sample | |||
| Metabolically healthy nonobese | 777/12716 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 656/4201 | 1.56 (1.40–1.73) | 1.59 (1.42–1.77) |
| Metabolically healthy obese | 38/1160 | 0.60 (0.43–0.83) | 0.91 (0.64–1.29) |
| Metabolically unhealthy obese | 397/4128 | 1.25 (1.11–1.41) | 1.79 (1.47–2.17) |
| P trend | <0.001 | <0.001 | |
| Men | |||
| Metabolically healthy nonobese | 417/5771 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 334/1983 | 1.46 (1.26–1.69) | 1.46 (1.25–1.69) |
| Metabolically healthy obese | 23/610 | 0.69 (0.45–1.05) | 1.09 (0.68–1.75) |
| Metabolically unhealthy obese | 203/1669 | 1.41 (1.20–1.67) | 2.09 (1.60–2.73) |
| P trend | <0.001 | <0.001 | |
| Women | |||
| Metabolically healthy nonobese | 360/6945 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy nonobese | 322/2218 | 1.69 (1.45–1.97) | 1.71 (1.45–2.01) |
| Metabolically healthy obese | 15/550 | 0.51 (0.30–0.86) | 0.73 (0.42–1.27) |
| Metabolically unhealthy obese | 194/2459 | 1.12 (0.94–1.33) | 1.56 (1.17–2.08) |
| P trend | <0.001 | <0.001 |
Sample contains participants without a history of CVD at baseline (n = 22,203).
Contains adjustment for age, sex, smoking, physical activity, socioeconomic group, and BMI.
We conducted a second set of analyses using waist circumference instead of BMI (Table 4). Thirty-four percent of the sample had large waist (>102 cm in men and > 88 cm in women), although 20.3% of these participants were categorized as metabolically healthy. The results largely replicated those for BMI, showing that participants with metabolic risk factors were at higher CVD risk compared with the metabolically healthy, regardless of waist circumference. In addition, we examined the joint effects of elevated waist and BMI. When we used both waist and BMI to define obesity, 20.7% of the sample had both a large waist circumference (>102 cm men and > 88 cm women) and BMI greater than 30 kg/m2. Within this obese sample, 15.2% (n = 699) were classified as metabolically healthy. Again, the results largely replicated those for general obesity, showing that participants with metabolic risk factors were at higher CVD risk compared with the metabolically healthy, regardless of obesity status (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Table 4.
The association between metabolic health (defined by blood pressure, HDL cholesterol, diabetes, CRP), waist, and mortalitya
| Cases/n | Age- and sex-adjusted HR (95% CI) | Fully adjusted HR (95% CI)b | |
|---|---|---|---|
| CVD death | |||
| Metabolically healthy, normal waist | 83/7129 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy, normal waist | 280/7535 | 1.78 (1.39–2.28) | 1.61 (1.26–2.08) |
| Metabolically healthy, large waist | 18/1529 | 0.81 (0.49–1.35) | 0.82 (0.49–1.40) |
| Metabolically unhealthy, large waist | 224/6019 | 1.78 (1.38–2.30) | 1.57 (1.15–2.15) |
| P trend | <0.001 | <0.001 | |
| All-cause death | |||
| Metabolically healthy, normal waist | 302/7129 | 1.00 (referent) | 1.00 |
| Metabolically unhealthy, normal waist | 850/7535 | 1.59 (1.39–1.81) | 1.51 (1.32–1.73) |
| Metabolically healthy, large waist | 66/1529 | 0.84 (0.64–1.09) | 1.03 (0.78–1.37) |
| Metabolically unhealthy, large waist | 651/6019 | 1.51 (1.31–1.73) | 1.84 (1.54–2.18) |
| P trend | <0.001 | <0.001 |
Sample contains participants without history of CVD at baseline (n = 22,203).
Large waist defined as greater than 102 cm in men and greater than 88 cm in women.
Contains adjustment for age, sex, smoking, physical activity, socioeconomic group, and BMI.
Discussion
The aim of this study was to examine the association between metabolically healthy obesity and risk of CVD in a large nationally representative sample of men and women initially free of CVD. A metabolically healthy phenotype was observed in 24% of the obese sample from the present study, which is largely comparable with other studies, although the prevalence has ranged from 10 to 30% (1, 17), depending on the definition used (11). The main findings from this study show that metabolically healthy obese participants were not at increased risk of CVD or all-cause mortality compared with the metabolically healthy nonobese reference group. In fact, this finding persisted, even when we used a more conservative approach that characterized the reference group as lean (BMI < 25 kg/m2) without any metabolic risk factors. These results are consistent with some (5–11) but not all previous work (12, 13). One of the main reasons for the inconsistencies might be the length of follow-up; studies with shorter follow-up (<15 yr), including ours, have generally shown that the metabolically healthy obese are not at future risk, which contradicts other studies using longer follow-up periods. However, it might be argued that obese participants who are initially metabolically healthy at baseline could go on to develop metabolic risk factors over a longer follow-up period, thus explaining their increased risk. Nevertheless, recent data (14) that showed metabolically healthy obese women had greater burden of subclinical atherosclerosis suggest that these individuals might be at increased risk but likely develop overt disease more slowly than their at-risk counterparts. In contrast to these findings, the present results suggest that in women, only the nonobese with metabolic risk factors were at elevated CVD risk after adjustment for covariates including BMI. Thus, taken together, our findings suggest that metabolic risk factors are more important predictors of CVD than overall adiposity. This is consistent with recent data from a collaborative analysis of 58 prospective studies (4).
We found that the metabolically healthy obese participants (as defined from BMI) had intermediate levels of waist circumference compared with metabolically unhealthy obese and nonobese, which is consistent with other data showing more favorable fat distribution in the healthy obese, characterized by lower visceral fat and greater thigh sc fat (19). Other data also indicate that participants with elevated body fat are at increased risk of developing cardiometabolic disease despite having a normal BMI (20). However, redefining the categories based on combined waist and BMI measures did not alter the differences in risk seen between the groups. In addition, the metabolically healthy obese participants had, on average, lower BMI than the at-risk obese, although adjustment for BMI did not alter the results. This might suggest that it is the presence of other cardiometabolic risk factors that is important in determining CVD risk, although another possibility is that we may not have measured the most relevant indices of adiposity such as visceral and ectopic fat. Other mechanisms might involve adipose tissue morphology and function. Indeed, independently of total body fat mass, increased visceral fat accumulation and adipose tissue dysfunction are associated with insulin-resistant obesity (21). Genetics may also play an important role (22).
The nonobese participants with metabolic risk factors were also at elevated risk of CVD. One of the striking features of this group was the high prevalence of hypertension and systemic inflammation, which was comparable with the levels seen in the metabolically unhealthy obese group. Interestingly, metabolically healthy obese participants had remarkably normal levels of CRP, which is consistent with some (19) but not other data (23). In addition, both metabolically unhealthy nonobese and obese participants displayed lower physical activity levels compared with the metabolically healthy, which is consistent with previous reports (17, 24). Because physical activity is known to have antiinflammatory effects (25), this might have contributed to the lower levels of CRP seen in the more active metabolically healthy obese participants. Clinically, it is important to identify the nonobese at-risk individuals because early intervention with exercise and diet may help prevent these participants from developing obesity and diabetes (26) and delay the onset of overt disease.
Strengths and limitations
The present study adds to the extant literature in several ways; this study included a large and well-characterized sample of the general population and used a plethora of contextual variables for statistical adjustments as well as the availability of objectively measured BMI and waist circumference. There are presently no unified criteria for the definition of metabolically healthy obesity. In the present study, metabolic risk was based on an adaptation of previous criteria (17, 18) according to availability of data, although measures of fasting glucose and triglycerides were not available. Thus, we cannot rule out the possibility of undetected impaired glucose tolerance, which is an important component of metabolic dysfunction. Thus, the present study might have overestimated the prevalence of metabolically healthy obesity, although this would have potentially increased the chances of finding a false-positive association with incident CVD in this group. We were unable to assess metabolic risk factors during the follow-up period; thus, it is possible that some of the healthy participants at baseline did go on to develop metabolic risk factors and overt disease. Nevertheless, we carefully removed those participants with a history of CVD at baseline to avoid possible reverse causation effects.
In summary, metabolically healthy obese participants were not at increased risk of CVD and all-cause mortality compared with healthy nonobese individuals. Thus, stratification of obese individuals based on their metabolic phenotype may be important to identify those who are to be prioritized for early pharmacological treatment in addition to lifestyle intervention.
Acknowledgments
The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and not necessarily of the funding bodies. M.H. and E.S. had full access to the data and take responsibility for the integrity of the data and accuracy of the data analyses. Both authors contributed to the concept and design of study, drafting, and critical revision of the manuscript.
The Health Survey for England is part of a program of surveys commissioned by the U.K. National Health Service Information Centre for health and social care and carried out since 1994 by the Joint Health Surveys Unit of the National Centre for Social Research (NatCen) and the Department of Epidemiology and Public Health at the University College London Medical School. The Scottish Health Survey is funded by the Scottish Executive. M.H. is supported by the British Heart Foundation (Grant RE/10/005/28296), and E.S. is a National Institute of Health Research Career Development Fellow.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2283
- BMI
- Body mass index
- BP
- blood pressure
- CI
- confidence interval
- CRP
- C-reactive protein
- CVD
- cardiovascular disease
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- HSE
- Health Survey for England
- SHS
- Scottish Health Survey.
References
- 1. Blüher M. 2010. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 21:38–43 [DOI] [PubMed] [Google Scholar]
- 2. Kopelman PG. 2000. Obesity as a medical problem. Nature 404:635–643 [DOI] [PubMed] [Google Scholar]
- 3. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. 2009. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emerging Risk Factors Collaboration, Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. 2011. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377:1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katzmarzyk PT, Janssen I, Ross R, Church TS, Blair SN. 2006. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care 29:404–409 [DOI] [PubMed] [Google Scholar]
- 6. Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. 2004. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation 109:706–713 [DOI] [PubMed] [Google Scholar]
- 7. Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. 2007. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol 100:1654–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. 2006. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 9. St-Pierre AC, Cantin B, Mauriège P, Bergeron J, Dagenais GR, Després JP, Lamarche B. 2005. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ 172:1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, Perseghin G. 2011. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. 2012. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 20:651–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnlöv J, Ingelsson E, Sundström J, Lind L. 2010. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 121:230–236 [DOI] [PubMed] [Google Scholar]
- 13. Flint AJ, Hu FB, Glynn RJ, Caspard H, Manson JE, Willett WC, Rimm EB. 2010. Excess weight and the risk of incident coronary heart disease among men and women. Obesity 18:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Wildman RP. 2011. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: the Study of Women's Health Across the Nation (SWAN). Atherosclerosis 217:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Scottish Government Statistics 2007. Scottish Health Survey Publications. http://www.scotland.gov.uk/Topics/Statistics/Browse/Health/scottish-health survey/Publications (accessed November 2007)
- 16. Joint Health Surveys Unit 2007. Health Survey for England Physical Activity Validation Study: substantive report. Leeds, UK: Health and Social Care Information Centre [Google Scholar]
- 17. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. 2008. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the U.S. population (NHANES 1999–2004). Arch Intern Med 168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239 [DOI] [PubMed] [Google Scholar]
- 19. Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, Satterfield S, Goodpaster BH, Ferrucci L, Harris TB. 2010. Health ABC Study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 18:2354–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shea JL, King MT, Yi Y, Gulliver W, Sun G. 5 January 2011. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis 10.1016/j.numecd.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 21. Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. 2010. Insulin sensitive obesity. Am J Physiol Endocrinol Metab 299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 22. Kilpeläinen TO, Zillikens MC, Stanèákova A, Finucane FM, Ried JS, Langenberg C, Zhang W, Beckmann JS, Luan J, Vandenput L, Styrkarsdottir U, Zhou Y, Smith AV, Zhao JH, Amin N, Vedantam S, Shin SY, Haritunians T, Fu M, Feitosa MF, Kumari M, Halldorsson BV, Tikkanen E, Mangino M, Hayward C, et al. 2011. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet 43:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wildman RP, Kaplan R, Manson JE, Rajkovic A, Connelly SA, Mackey RH, Tinker LF, Curb JD, Eaton CB, Wassertheil-Smoller S. 2011. Body size phenotypes and inflammation in the Women's Health Initiative Observational Study. Obesity 19:1482–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, Poehlman ET. 2004. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab 89:5013–5020 [DOI] [PubMed] [Google Scholar]
- 25. Hamer M. 2007. The relative influences of fitness and fatness on inflammatory factors. Prev Med 44:3–11 [DOI] [PubMed] [Google Scholar]
- 26. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J. 2006. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368:1673–1679 [DOI] [PubMed] [Google Scholar]