Abstract
Context:
Roux-en-Y gastric bypass (RYGB) is one of the most effective long-term therapies for the treatment of severe obesity. Recent evidence indicates that RYGB effects weight loss through multiple physiological mechanisms, including changes in energy expenditure, food intake, food preference, and reward pathways.
Objective:
Because central melanocortin signaling plays an important role in the regulation of energy homeostasis, we investigated whether genetic disruption of the melanocortin-4 receptor (MC4R) in rodents and humans affects weight loss after RYGB.
Methods and Results:
Here we report that MC4R−/− mice lost substantially less weight after surgery than wild-type animals, indicating that MC4R signaling is necessary for the weight loss effects of RYGB in this model. Mice heterozygous for MC4R remain fully responsive to gastric bypass. To determine whether mutations affect surgically induced weight loss in humans, we sequenced the MC4R gene in 972 patients undergoing RYGB. Patients heterozygous for MC4R mutations exhibited the same magnitude and distribution of postoperative weight loss as patients without such mutations, suggesting that although two normal copies of the MC4R gene are necessary for normal weight regulation, a single normal copy of the MC4R gene is sufficient to mediate the weight loss effects of RYGB.
Conclusions:
MC4R is the first gene identified that is required for the sustained effects of bariatric surgery. The need for MC4R signaling for the weight loss effects of RYGB in mice underscores the physiological mechanisms of action of this procedure and demonstrates that RYGB both influences and is dependent on the normal pathways that regulate energy balance.
It is estimated that 34% of adults in the United States are overweight and an additional 32% have obesity (1). More than 5% have severe obesity [body mass index (BMI) ≥ 40 kg/m2], and this subgroup is the fastest growing segment of the population (2). Obesity leads to many metabolic, inflammatory, degenerative, cognitive, and neoplastic disorders, together accounting for nearly 10% of all health care expenditures in the United States, with a cost of more than $168 billion annually (3).
Behavioral and pharmacological interventions for the treatment of obesity have had limited long-term success (4–7). In contrast, gastrointestinal weight loss surgery, particularly Roux-en-Y gastric bypass (RYGB), results in substantial and durable weight loss (8). Patients lose an average of 70% of their excess body weight and maintain approximately 80% of this weight loss over decades (9). With this weight loss comes an increase in life expectancy (9, 10) and a decrease in comorbidities, including type 2 diabetes, hypertension, hyperlipidemia, obstructive sleep apnea, and gastroesophageal reflux disease (8, 11). Increasing evidence indicates that the improvement in glucose homeostasis results from both weight loss-dependent and -independent mechanisms (10). Moreover, RYGB is associated with changes in the expression of several peripheral and central neuroendocrine peptides that regulate energy balance, including ghrelin, glucagon-like polypeptide-1, peptide YY, neuropeptide Y, and α-MSH (12). Diabetes improves within days after RYGB, before any significant weight loss has occurred, and patients maintain remission of diabetes over time, despite later increases in food intake. In addition, unlike diet-induced weight loss, RYGB is associated with decreased appetitive drive and significantly altered food preferences (13, 14). In rodents, RYGB is also associated with increased total energy expenditure, due primarily to an increase in diet-induced thermogenesis (DIT), which accounts for up to half of the resulting weight loss (10). We have recently demonstrated that this increase in DIT is associated with sympathetic activation of brown adipose tissue (15).
Long-term energy balance is controlled by complex interrelated systems that coordinately regulate food intake, energy expenditure, and metabolic efficiency (16). Essential for weight homeostasis is the integration of orexigenic and anorexigenic signals by leptin- and melanocortin-dependent pathways within the central nervous system (17). Central to this regulatory system is the melanocortin-4 receptor (MC4R), a G protein-coupled receptor expressed primarily in the central and peripheral nervous system (18–20). Genetic disruption of melanocortin signaling, including mutations in proopiomelanocortin and MC4R, has been associated with obesity in both humans and rodent models (21, 22). MC4R null (MC4R−/−) mice exhibit increased food intake (23) and decreased basal metabolic rate (24). MC4R−/− mice also have a profound disruption in diet-induced thermogenesis in response to fat intake (25), and MC4R has been shown to be critical for brown adipose tissue-mediated thermogenesis in response to both feeding and cold stimuli (26). Mutations that result in loss of a single functional copy of the MC4R gene are the most common mutations associated with obesity in human patients, present in up to 6% of individuals with severe early-onset obesity (27); individuals with two defective copies of the MC4R gene are extremely rare (27, 28). Given its central role in the regulation of food intake and thermogenesis, we hypothesized that genetic alterations in MC4R would interfere with the effectiveness of RYGB.
Materials and Methods
Animals and dietary manipulation
All experiments using the mouse RYGB procedure that includes a proximal gastric pouch (RYGB-H) were performed in compliance with and were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital. RYGB-H experiments used three different strains of male mice, including wild-type (WT) mice with a C57BL/6 background (Jackson Laboratories, Bar Harbor, ME), WT mice with a 129 background (Charles River Laboratories, Wilmington, MA), and MC4R knockout mice on both a 129 background and a C57BL/6J-129 mixed background (a generous gift from B. Lowell, Beth Israel Deaconess Medical Center, Boston, MA). MC4R−/− status was confirmed via PCR using an S1000 thermal cycler (Bio-Rad, Hercules, CA). WT C57BL/6J diet-induced obese mice were fed a high-fat diet (D12492 diet; Research Diets, New Brunswick, NJ), whereas C57BL/6J and C57BL/6J-129 animals were fed normal chow (Prolab Isopro RMH 3000, LabDiet; PMI Nutrition International Inc., Brentwood, MO) throughout the study, except during the 7–14 d after surgery, when all animals were maintained on a defined postoperative protocol that progressed from water only to liquid diet to solid diet (10). Animals were individually housed and maintained in a 12-h light, 12-h dark cycle under controlled temperature and humidity.
All experiments with mouse RYGB that completely bypasses the stomach (RYGB-C) were performed in compliance with and were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Male age- and littermate-matched melanocortin-4 receptor knockout (MC4R−/−), heterozygous (MC4R+/−), and WT mice derived from the original colony (29) on a C57BL/6J genetic background were obtained from breeding colonies maintained at Vanderbilt University (Nashville, TN). All animals had continuous ad libitum access to water and a normal chow diet (Purina rodent diet 5001, LabDiet; PMI Nutrition International) in a 12-h light, 12-h dark cycle under controlled temperature and humidity. Mice were offered an additional choice of a high-fat diet (HFD; D12492; 60% Kcal from fat; Research Diets) before surgery and again beginning 4 wk after surgery.
Surgical procedures: rodent studies
RYGB-H closely approximates a model in rats that has been described in detail elsewhere (10). In the RYGB-H procedure, the stomach was divided into a gastric pouch and distal stomach using a vascular clip (Ethicon Endo-Surgery, Cincinnati, OH). For each of the Roux and biliopancreatic limbs, the length of the intestine was 6 cm, chosen to approximate the 12–15% intestinal bypass in a human RYGB. The Roux limb was then secured to the gastric fundus by a gastrojejunal anastamosis. Sham operations consisted of a laparotomy and repair. At 12 wk of age, animals weighing at least 40 g were randomized to RYGB-H or sham operation. Before surgery, animals were fasted overnight for 12 h with access to water only. During all procedures, animals were maintained on inhaled anesthesia using a scavenged mask circuit of isoflurane (10).
RYGB-C surgical procedures were performed as described by Yin et al. (30) as a modified RYGB procedure. At 12 wk of age, mice were randomized to RYGB-C or sham operation. Briefly, the surgery included a bypass of the entire stomach and duodenum with the jejunum anastomosed to the esophagus through a side-to-side anastomosis. Sham operations consisted of isolation of the esophagus, stomach, and jejunum without any section or anastomosis. All mice received daily ketoprofen (10 mg/kg) for 2 d after surgery and were provided with approximately 2 ml of fresh Ensure (Abbott Laboratories, Abbott Park, IL)daily in a Petri dish for 1 wk after the operation. Beginning 1 wk after surgery, RYGB mice received weekly iron dextran injections (10 mg/kg; Durvet, Inc., Blue Springs, MO) to prevent anemia. All mice were monitored for severe anemia and eliminated from the study if the hematocrit was less than 20%. Morbidity-free survival rates were approximately 60% for both RYGB-H and RYGB-C and did not differ significantly among genetic groups.
Statistical analysis: rodent studies
At 8 wk after surgery, body composition was determined by whole-body composition measured with an mq10NMR (Bruker Optics Inc., Billerica, MA). Statistical analyses were performed using GraphPad PRISM (GraphPad Software, La Jolla, CA). Repeated-measures ANOVA and Mann-Whitney U tests were used for data analysis, as appropriate. Data are reported as mean ± sem. A P < 0.05 was considered statistically significant.
Human study population
Participants were recruited from the population of patients undergoing RYGB at a single academic center that is part of a larger hospital network in the Boston metropolitan area. From February 2000 until April 2007, we obtained consent to collect tissue samples removed at the time of surgery from 1018 of the patients undergoing RYGB at this center (97%). Operations included open and laparoscopic RYGB performed by one of two surgeons using the same operative techniques; the surgical methods have been described previously (31). For the open procedure, the stomach was partitioned but not divided, and for the laparoscopic procedure, the proximal gastric pouch was divided from the distal stomach. Otherwise, the techniques were the same, with an approximately 30-ml pouch, a 100- to 120-cm Roux limb fashioned in a retrocolic, retrogastric configuration, and a pancreaticobiliary limb extending approximately 75 cm beyond the ligament of Treitz. Intraoperative tissue samples were collected from all consenting patients, and genomic DNA was extracted from the liver samples. This study was approved by the Institutional Review Board of Massachusetts General Hospital.
Genetic sequencing: human study
As described previously (32), the coding region of the MC4R gene was amplified by PCR using BioTaq (Bioline, London, UK). Nested primers were constructed and genetic sequencing performed using Big Dye terminator chemistry (PerkinElmer, Foster City, CA) and electrophoresed on an ABI 377 automated DNA sequencer. Sequences were assembled and analyzed using Sequencer software (Gene Codes, Ann Arbor, MI). MC4R was successfully genotyped in 970 patients. Mutations in MC4R are considered pathogenic when they cosegregate with obesity in families and can be shown to result in a loss of function in in vitro studies (33).
End point and covariate assessment: human study
Demographic, clinical, and medical information was extracted by review of electronic medical records from all sites within the hospital network. Chart-derived weights were validated via telephone interviews in a subset of patients (n = 306); there was a 94% correlation between these two sources for the lowest weight after surgery (nadir weight). We calculated the percent excess body weight lost at 1 yr (range 10–14 months), 2 yr (22–26 months), and 3 yr (34–38 months) and nadir weight by subtracting the patient's end point weight from his or her preoperative weight and dividing this difference by the difference between the patient's initial weight and his or her calculated weight at a BMI of 25 kg/m2. Percent weight change was calculated as the patient's weight at each end point divided by his or her initial weight.
Statistical analyses: human study
Patients were sorted into groups by the presence or absence of MC4R mutations. Groups included the following: 1) no mutations, 2) nonpathogenic mutations (variants that do not impair receptor signaling in in vitro studies), and 3) pathogenic mutations that have been shown to impair receptor signaling in vitro (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). We compared baseline age, BMI, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, glucose, insulin, and glycosylated hemoglobin (HbA1c) before and after surgery in patients with and without MC4Rp mutations using Wilcoxon rank sum tests. We compared sex, type 2 diabetes status, and antidiabetes medications using χ2 tests. We used multiple linear regression models adjusted for race, age, sex, preoperative BMI, and diabetes status to examine weight loss at 1 and 2 yr, and at weight nadir after surgery. In addition to analyzing weight loss at specific time points, we analyzed weight loss trajectories over time (months) using mixed-effects models adjusted for age, sex, preoperative BMI, and type 2 diabetes status and additionally allowing for the slope of the curve to vary at 6, 12, 18, and 24 months (knots at these time points). Patients were excluded from all statistical analyses if they were on weight-lowering medications after surgery (1.7%) or if they had cancer or other severe illness (2.4%), leaving a cohort of 928 patients at baseline. Postoperative BMI at least 10 months after RYGB could not be determined in an additional 8% of patients, leading to a final sample of 847 patients (83.3% of the total cohort). There was no difference in rates of loss to follow-up across the different genetic groups (data not shown). Results were unchanged when percent weight change was used instead of percent excess body weight loss (Supplemental Table 2). Analyses were conducted using SAS statistical software (SAS Institute, Cary, NC).
Results
RYGB induces sustained weight loss in WT mice
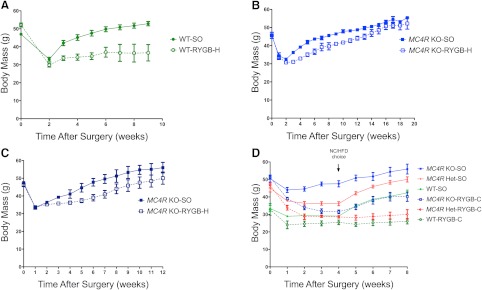
We have developed two mouse models of RYGB that mimic the anatomy of the human procedure (RYGB-H, Supplemental Fig. 1, A and B; RYGB-C, Supplemental Fig. 1C). Both RYGB-H and RYGC-C cause a bypass of the gastric endocrine mucosa and reroute the duodenum and jejunum in a manner comparable with the human procedure. Like the human procedure, the RYGB-H includes a small gastric pouch derived from the aglandular forestomach, whereas the RYGB-C excludes the entire stomach from the nutrient flow. To determine whether RYGB induces weight loss in mice, wild-type diet-induced obese C57BL/6J mice weighing at least 40 g were randomized to either sham operation (WT-SO) or RYGB-H (WT-RYGB-H). As we have seen in rats (10), both RYGB-H and sham operation induced short-term weight loss from the catabolic effects of surgery and the postoperative dietary protocol. Three weeks after surgery, however, WT-SO mice regained nearly all of the lost weight, and they continued to gain weight normally thereafter (Fig. 1A). In contrast, WT-RYGB-H mice maintained the weight loss, weighing 38% less than the WT-SO group by postoperative wk 9 (P = 0.018).
Fig. 1.
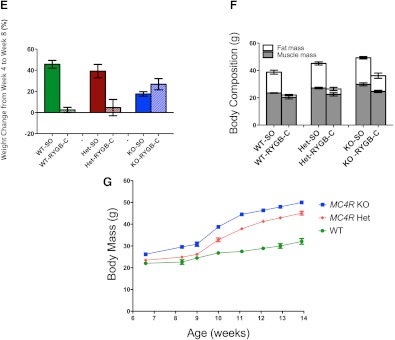
Body weight change over time in WT, MC4R+/− heterozygous (Het), and MC4R−/− null (KO) mice before and after gastric bypass surgery. A, Body weight (grams) of WT mice on a C57BL/6J background after RYGB-H (dashed green line and open green circles; n = 5) or SO (solid green line and circles; n = 5). B, MC4R−/− mice on a 129 background after RYGB-H (dashed blue line and open blue circles; n = 3) or SO (solid blue line and circles; n = 3). C, MC4R−/− mice on a 129/C57BL6 mixed background after RYGB (dashed blue line and open blue circles; n = 3) or SO (solid blue line and circles; n = 3). D, WT (green), Het (red), and MC4R−/− (blue) mice on a C57BL/6J background after RYGB-C (dashed lines and open circles; n = 3, n = 4, and n = 7, respectively) or SO (solid lines and circles; n = 3, n = 6, and n = 3, respectively). E, Change in body weight (grams) from postoperative wk 4 to postoperative wk 8 in RYGB-C and SO mice. F, Body weight (grams) 8 wk after surgery in WT (left), Het (center), and MC4R−/− (right) mice on a C57BL/6J background. Dark gray bars indicate muscle mass. White bars indicate fat mass. G, Body weight (grams) growth curves of untouched WT, Het, and MC4R−/− mice. Error bars, sem.
RYGB fails to induce sustained weight loss in MC4R−/− mice
To determine whether homozygous disruption of MC4R affects weight loss after RYGB in mice, we performed RYGB-H or SO (KO-RYGB-H and KO-SO, respectively) in MC4R−/− mice on 129 and mixed 129-C57BL/6J backgrounds. In contrast to WT mice, which exhibit durable weight loss after RYGB, KO-RYGB-H mice on both backgrounds steadily regained weight in a pattern similar to that of KO-SO animals (Fig. 1, B and C). At postoperative wk 52, WT-RYGB-H mice weighed 35.4% less than WT-SO animals (P < 0.001; Supplemental Fig. 2), but there was no statistically significant weight difference between the KO-RYGB-H and KO-SO groups. KO-RYGB-H mice weighed an average of 9.1% less than KO-SO animals (P > 0.05), indicating that interruption of MC4R signaling hinders at least 74% of the weight loss normally observed after RYGB in this strain.
To determine whether these observations were due to the genetic background of the mice or particular to the surgical technique, we replicated these studies in WT and MC4R−/− mice on a C57BL/6J background using a RYGB procedure that completely excludes the stomach from nutrient flow [RYGB-C (30); Supplemental Fig. 1C]. In WT mice, both procedures yielded similar outcomes. WT-RYGB-C and WT-SO mice lost weight immediately after surgery, which persisted for the first 4 wk after surgery while animals were recovering on a normal chow diet (Fig. 1D and Supplemental Fig. 3). When exposed to a HFD, WT-SO mice rapidly gained weight, whereas WT-RYGB-C mice maintained their lower body weight (Fig. 1, D and E), similar to what we observed after RYGB-H (Fig. 1A). In contrast, both KO-SO and KO-RYGB-C mice gained weight after the introduction of the HFD. Decreased body fat accounted for nearly all of the weight loss after RYGB in WT mice, and the loss of body fat after RYGB-C was severely blunted in KO mice (Fig. 1F). Thus, it was the loss of MC4R signaling rather than variations in genetic background or surgical technique that accounted for the blunted weight and body fat loss after RYGB in the MC4R−/− mice.
RYGB induces sustained weight loss in MC4R+/− mice
Because MC4R-related human obesity is primarily due to haploinsufficiency rather than to homozygous mutations in the MC4R gene, we examined the effects of RYGB-C in mice heterozygous for MC4R. As seen previously (29), MC4R+/− mice develop obesity when exposed to a HFD, with an obesity phenotype only slightly less severe than that seen in MC4R−/− mice (Fig. 1G). Unlike MC4R−/− animals, however, MC4R+/− mice responded to RYGB-C similarly to WT mice, maintaining their weight loss, even when challenged with a HFD (Fig. 1, D and E). These MC4R heterozygous animals, despite having a strong predisposition to obesity, are fully responsive to the effects of RYGB.
Human patients heterozygous for mutations in MC4R respond normally to RYGB
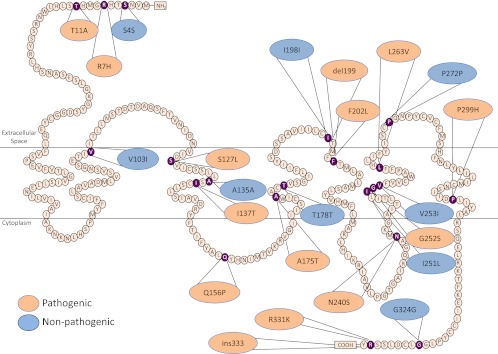
To determine whether mutations in MC4R affect weight loss after RYGB in human patients, we sequenced the MC4R gene in 972 patients undergoing RYGB. Of these 972 patients, 62 (6.4%) had at least one heterozygous mutation in MC4R (Fig. 2). Mutations in MC4R can be considered to be pathogenic when they cosegregate with obesity in families and have been shown to result in loss of function in vitro (33). There are several common variants in MC4R that are considered to be nonpathogenic because they are found at a comparable frequency in lean and obese cohorts, do not cosegregate with obesity in families, and do not impair signaling through MC4R in vitro. In our cohort, we identified 15 patients with pathogenic and 44 with nonpathogenic mutations (Supplemental Table 1). The baseline BMI and metabolic profile of patients heterozygous for pathogenic and nonpathogenic mutations in MC4R were similar to the group without MC4R mutations (Table 1).
Fig. 2.
Structure of the MC4R protein and location of mutations present in a cohort of 970 men and women undergoing RYGB. Positions of the mutations identified in this cohort are indicated in purple. Mutations shown to be pathogenic are shaded orange; nonpathogenic mutations are shaded blue.
Table 1.
Baseline characteristics by type of MC4R mutation among 928 men and women who underwent RYGB
| Variable | Type of MC4R mutation |
||
|---|---|---|---|
| None | Single-copy pathogenic | Single-copy nonpathogenic | |
| Number of participants | 869 | 15 | 44 |
| Age (yr) | 44.5 | 45.9 | 46.2 |
| Sex (% female) | 75.0 | 66.7 | 72.7 |
| BMI (kg/m2) | 50.7 | 50.1 | 49.4 |
| LDL cholesterol (mg/dl) | 109.7 | 115.5 | 100.8 |
| HDL cholesterol (mg/dl) | 48.2 | 50.1 | 48.3 |
| Diabetes (%) | 42.5 | 40.0 | 47.8 |
| Use of diabetes medication(s) (%) | 13.5 | 13.3 | 20.5 |
| Glucose (mg/dl) | 123.9 | 118.2 | 129.4 |
| Insulin (μIU/ml) | 22.9 | 18.8 | 24.8 |
| HbA1c | 6.4 | 6.0 | 6.7 |
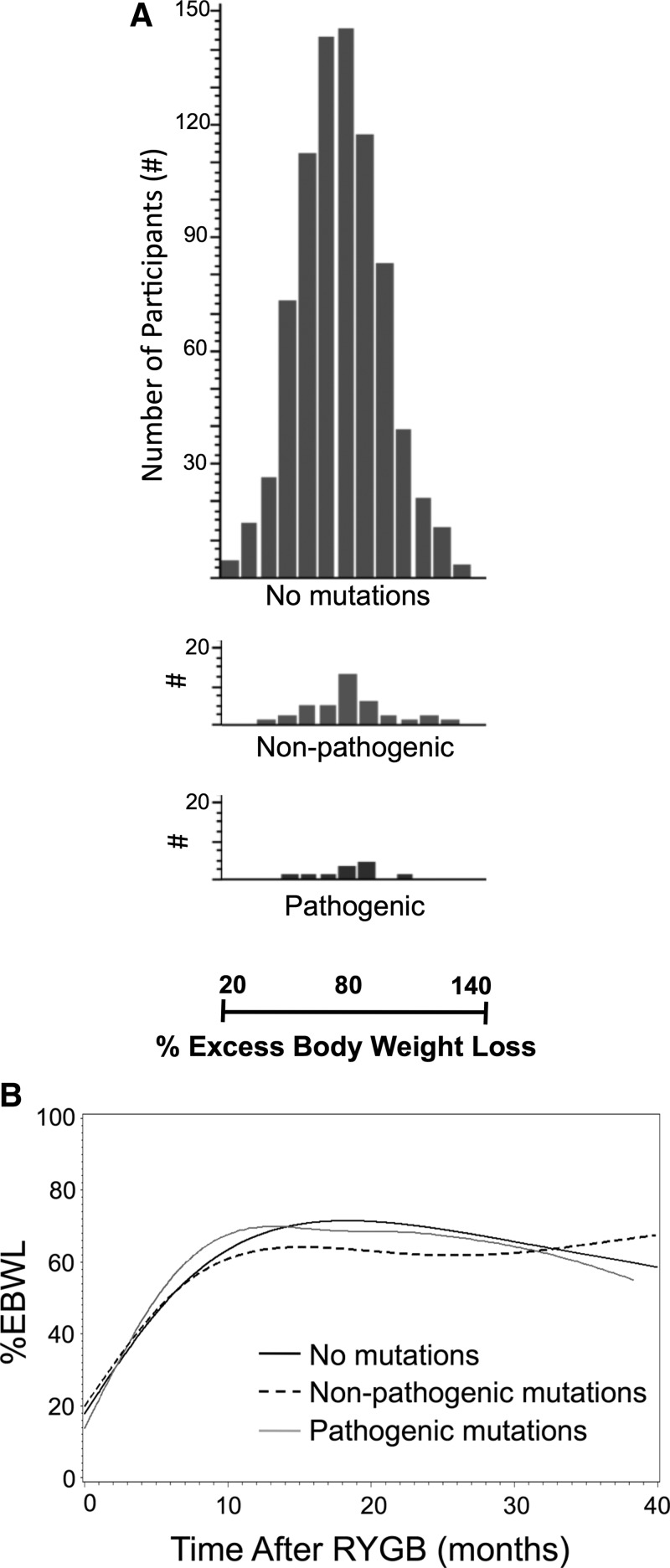
After RYGB, patients with either pathogenic or nonpathogenic MC4R mutations lost similar amounts of weight as patients without MC4R mutations. Both the mean and distribution of weight loss were similar among groups (Fig. 3A). Patients without mutations lost an average of 79.6% of their excess body weight, patients with pathogenic mutations lost an average of 87.2% (P = 0.41 vs. patients without MC4R mutations), and patients with nonpathogenic mutations lost 78.2% (P = 0.52 vs. patients without MC4R mutations). The patterns of weight loss over time were also similar in all three groups (Fig. 3B and Supplemental Table 2). We observed no differences in weight loss or other metabolic parameters in the most common mutations, V103I and I251L (Supplemental Table 3), both of which are nonpathogenic mutations. In addition, we observed no significant differences among these groups in their metabolic profiles after RYGB (Table 2).
Fig. 3.
Weight loss after RYGB among 847 men and women who underwent RYGB by type of heterozygous MC4R mutation. A, Distribution of percent excess weight loss after RYGB in patients with no mutations (upper panel), heterozygous nonpathogenic mutations (middle panel), or heterozygous pathogenic mutations (bottom panel). B, Weight loss curves over time in patients with no mutations (solid black line), nonpathogenic mutations (dashed black line), or pathogenic mutations (solid gray line) %EBWL, Percent excess body weight loss.
Table 2.
Metabolic characteristics by type of MC4R mutation among 847 men and women 1 yr after RYGB
| Variable | Type of MC4R mutation |
||||
|---|---|---|---|---|---|
| None | Single-copy pathogenic | P vs. none | Single-copy nonpathogenic | P vs. none | |
| LDL cholesterol (mg/dl) (sd) | 86.9 (28.0) | 94.8 (16.7) | 0.16 | 86.0 (28.8) | 0.65 |
| HDL cholesterol (mg/dl) (sd) | 54.8 (14.3) | 57.7 (11.3) | 0.38 | 58.5 (13.7) | 0.14 |
| Glucose (mg/dl) (sd) | 93.7 (25.3) | 96.2 (19.0) | 0.35 | 91.2 (20.8) | 0.37 |
| Insulin (μIU/ml) (sd) | 7.2 (6.0) | 7.3 (4.0) | 0.57 | 5.4 (2.3) | 0.32 |
| HbA1c (sd) | 5.6 (0.73) | 5.5 (0.65) | 0.72 | 5.5 (0.53) | 0.41 |
Discussion
Together these rodent and human studies indicate that signaling through the MC4R is necessary for the sustained and robust weight loss response to gastric bypass but that the presence of a single normal allele at this locus is sufficient to confer normal responsiveness to RYGB. This finding persists across multiple genetic backgrounds and multiple surgical procedures and appears to be conserved across species. These findings highlight the profound physiological effects of this operation and underscore its biological mechanism of action. We have previously demonstrated that RYGB leads to an increase in energy expenditure due primarily to an increase in feeding-related DIT (15). The central melanocortin system plays a critical role in both of these thermogenic processes and the regulation of food intake, with MC4R as a central mediator of these effects (25, 26, 34). Genetic or pharmacological blockade of MC4R induces profound hyperphagia (29) and impedes the up-regulation of uncoupling protein-1 in brown adipose tissue in response to a HFD (26). Thus, interference with MC4R signaling may interfere with the HFD-mediated induction of DIT by RYGB, either by blocking the central effects of signals from the gastrointestinal tract or by preventing melanocortin-mediated activation of efferent sympathetic activity. In the present study, MC4R−/− mice consuming normal chow exhibited a response to RYGB-C, but when challenged with a HFD, these mice rapidly gained weight, indicating that the effect of MC4R on weight loss after RYGB may involve complex gene-environment interactions. Notably, mice heterozygous for MC4R remained fully responsive to surgery, demonstrating that although loss of even one copy of the MC4R gene is sufficient to disrupt energy balance and cause obesity, such single-copy mutations do not impair the powerful effects of RYGB.
Based on these observations in mice, we would expect that humans heterozygous for pathogenic mutations in MC4R, having a single normal allele at this locus, would respond normally to RYGB. Indeed, patients heterozygous for pathogenic mutations in MC4R display a normal distribution of weight loss after RYGB. These individuals, representing the most common monogenic form of severe, early-onset obesity (27), are particularly refractory to weight loss by diet and exercise (35), and no targeted pharmacotherapy is available for them. The present study indicates that these patients, despite their severe obesity, are normally responsive to RYGB. These findings extend a recent report of four cases of RYGB patients with MC4R mutations (36) and a recent analysis of the two most common nonpathogenic mutations, I251L and V103I (37), indicating that patients heterozygous for these mutations in MC4R respond normally to RYGB. In the latter study, patients heterozygous for the nonpathogenic I251L mutation lost slightly but significantly more weight than those without MC4R mutations, a difference that we did not observe in our cohort. Thus, all of the available evidence indicates that patients heterozygous for mutations in MC4R, even those with pathogenic mutations, should be considered as appropriate candidates for RYGB. In contrast, based on our observations in mice, the rare patient homozygous for loss-of-function mutations in MC4R would be expected to respond poorly to gastric bypass. Despite differences in gastric anatomy between humans and mice that could potentially affect circulating levels of ghrelin or other gastrointestinal hormones, the effect of surgery appears to be conserved between mice and humans. Homozygous loss-of-function mutations in humans are extremely rare, with fewer than 10 individuals reported worldwide, none of whom have undergone RYGB (38). In view of the outcomes of patients with single-copy MC4R mutations and the rarity of homozygous MC4R defects, there is no evidence to support routine screening for MC4R mutations as part of the RYGB care pathway.
In conclusion, MC4R signaling appears to play a critical role in promoting weight loss after RYGB. The specific mechanisms of signaling from the surgically manipulated gut to the MC4R system remain to be determined because MC4R expression and signaling are widely distributed in the central and autonomic nervous systems (18, 39). The requirement for an intact melanocortin system for the weight loss effects of RYGB underscores the physiological mechanism of action of this procedure and demonstrates that RYGB both influences and is dependent on the normal pathways that regulate energy balance.
Supplementary Material
Acknowledgments
I.J.H., N.S., D.P.Y., K.L.J.E., R.D.C., and L.M.K. received research support from the National Institute of Diabetes and Digestive and Kidney Diseases. D.P.Y., K.L.J.E., and L.M. received support from the Vanderbilt Mouse Metabolic Phenotyping Center (National Institute of Diabetes and Digestive and Kidney Diseases). I.J.H., N.S., and L.M.K. received support from Merck Research Laboratories and Ethicon Endo-Surgery. I.S.F. received support from the Wellcome Trust, the Medical Research Council Centre for Obesity and Related Disorders and the National Institute for Health Research Cambridge Biomedical Research Centre.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- Body mass index
- DIT
- diet-induced thermogenesis
- HbA1c
- glycosylated hemoglobin
- HDL
- high-density lipoprotein
- Het
- heterozygous
- HFD
- high-fat diet
- KO
- knockout
- LDL
- low-density lipoprotein
- MC4R
- melanocortin-4 receptor
- MC4R−/−
- MC4R null
- MC4R+/−
- MC4R heterozygous
- NC
- normal chow
- RYGB
- Roux-en-Y gastric bypass
- RYGB-C
- mouse RYGB that completely bypasses the stomach
- RYGB-H
- mouse RYGB procedure that includes a proximal gastric pouch
- WT
- wild type
- WT-SO
- WT animals randomized to sham operation.
References
- 1. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. 2006. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 2. Sturm R. 2007. Increases in morbid obesity in the USA: 2000–2005. Public Health 121:492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawley J, Meyerhoefer C. 2012. The medical care costs of obesity: an instrumental variables approach. J Health Econ 31:219–230 [DOI] [PubMed] [Google Scholar]
- 4. Wing RR. 2010. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. 2007. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 297:969–977 [DOI] [PubMed] [Google Scholar]
- 6. Bray GA. 2008. Medications for weight reduction. Endocrinol Metab Clin North Am 37:923–942 [DOI] [PubMed] [Google Scholar]
- 7. Kaplan LM. 2010. Pharmacologic therapies for obesity. Gastroenterol Clin North Am 39:69–79 [DOI] [PubMed] [Google Scholar]
- 8. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 9. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM. 2007. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752 [DOI] [PubMed] [Google Scholar]
- 10. Stylopoulos N, Hoppin AG, Kaplan LM. 2009. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity 17:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjostrom CD. 2005. Systematic review of bariatric surgery. JAMA 293:1726; author reply 1726 [DOI] [PubMed] [Google Scholar]
- 12. Romanova IV, Ramos EJ, Xu Y, Quinn R, Chen C, George ZM, Inui A, Das U, Meguid MM. 2004. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J Am Coll Surg 199:887–895 [DOI] [PubMed] [Google Scholar]
- 13. Shin AC, Zheng H, Pistell PJ, Berthoud HR. 2011. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35:642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. 2011. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc 25:1176–1181 [DOI] [PubMed] [Google Scholar]
- 15. Stylopoulos N, Zhang XB, Brownell A-L, Kaplan LM. 2010. Roux-en-Y gastric bypass activates brown adipose tissue and increases energy expenditure in obese mice. Gastroenterology 138:S754 [Google Scholar]
- 16. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 17. Spiegelman BM, Flier JS. 2001. Obesity and the regulation of energy balance. Cell 104:531–543 [DOI] [PubMed] [Google Scholar]
- 18. Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. 2003. Expression of melanocortin-4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235 [DOI] [PubMed] [Google Scholar]
- 19. Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. 1992. The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- 20. Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. 1993. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90:8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- 22. Ramachandrappa S, Farooqi IS. 2011. Genetic approaches to understanding human obesity. J Clin Invest 121:2080–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 24. Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. 2000. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97:12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 26. Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. 2007. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- 27. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. 2003. Clinical spectrum of obesity and mutations in the melanocortin-4 receptor gene. N Engl J Med 348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 28. Lubrano-Berthelier C, Le Stunff C, Bougnères P, Vaisse C. 2004. A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J Clin Endocrinol Metab 89:2028–2032 [DOI] [PubMed] [Google Scholar]
- 29. Srisai D, Gillum MP, Panaro BL, Zhang XM, Kotchabhakdi N, Shulman GI, Ellacott KL, Cone RD. 2011. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology 152:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin DP, Gao Q, Ma LL, Yan W, Williams PE, McGuinness OP, Wasserman DH, Abumrad NN. 2011. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg 254:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatoum IJ, Stein HK, Merrifield BF, Kaplan LM. 2009. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity 17:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. 2000. Dominant and recessive inheritance of morbid obesity associated with melanocortin-4 receptor deficiency. J Clin Invest 106:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S, Farooqi IS, Froguel P, Meyre D. 2008. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57:2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. 2007. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117:3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reinehr T, Hebebrand J, Friedel S, Toschke AM, Brumm H, Biebermann H, Hinney A. 2009. Lifestyle intervention in obese children with variations in the melanocortin-4 receptor gene. Obesity (Silver Spring) 17:382–389 [DOI] [PubMed] [Google Scholar]
- 36. Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. 2011. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 21:930–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. 2011. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab 96:E2088–E2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C. 2011. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 35:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. 1994. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8:1298–1308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.