Abstract
Context:
The pathogenetic mechanisms of kidney stone formation are complex and involve both metabolic and environmental risk factors. Over the past decade, major advances have been made in the understanding of the pathogenesis, diagnosis, and treatment of kidney stone disease.
Evidence Acquisition and Synthesis:
Both original and review articles were found via PubMed search reporting on pathophysiology, diagnosis, and management of kidney stones. These resources were integrated with the authors' knowledge of the field.
Conclusion:
Nephrolithiasis remains a major economic and health burden worldwide. Nephrolithiasis is considered a systemic disorder associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and the metabolic syndrome. Further understanding of the pathophysiological link between nephrolithiasis and these systemic disorders is necessary for the development of new therapeutic options.
The increased prevalence of kidney stone disease is pandemic (1). The lifetime risk of kidney stones is currently at 6–12% in the general U.S. population (2). Nephrolithiasis has become increasingly recognized as a systemic disorder (3) that is associated with chronic kidney disease, nephrolithiasis-induced bone disease (4), increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and the metabolic syndrome (MS) (5). Without medical treatment, nephrolithiasis is a chronic illness with a recurrence rate greater than 50% over 10 yr (6). Given that the annual expenditure in the United States exceeds $5 billion, the economic and social burden of nephrolithiasis is immense (7).
Epidemiology
The prevalence of nephrolithiasis in the United States has doubled over the past three decades. This increase has also been noted in most European countries and Southeast Asia (1). Racial and ethnic differences are seen in kidney stone disease, primarily occurring in Caucasian males and least prevalent in young African-American females. The prevalence in Asian and Hispanic ethnicities is intermediate (1).
The incidence of nephrolithiasis is highest in Caucasian males (2), where the incidence of kidney stones rises after age 20, peaks between 40 and 60 yr of age (at approximately 3 per 1000 per year), and then declines (8). In females, the incidence rate is higher in the late 20s, decreases by age 50, and remains relatively constant thereafter (2, 8).
Pathophysiological Mechanism(s) of Calcium Stones
Approximately 80% of calcium kidney stones are calcium oxalate (CaOx) (9), with a small percentage (15%) of calcium phosphate (CaP) (10). The pathophysiological mechanisms for calcium kidney stone formation are complex and diverse and include low urine volume, hypercalciuria, hyperuricosuria, hypocitraturia, hyperoxaluria, and abnormalities in urine pH (Table 1) (11).
Table 1.
Causes and treatment of kidney stone formation
| Etiology | Prevalence | Physiological mechanism(s) | Pharmacological treatment | Potential side effects |
|---|---|---|---|---|
| Calcium kidney stones | ||||
| Hypercalciuria | 30–60% | 1,25(OH)2D-dependent | Hydrochlorothiazide (50 mg/d) | Hypokalemia |
| 1,25(OH)2D-independent | Chlorthalidone (25–50 mg/d) | Glucose intolerance | ||
| Intrinsic renal calcium leak | Indapamide (1.2–2.5 mg/d) | Hypomagnesemia | ||
| Intrinsic renal phosphorus leak Resorptive hypercalciuria: PTH-dependent (primary hyperparathyroidism); PTH-independent |
Amiloride + hydrochlorothiazide (5 mg/d + 50 mg/d) | Hypertriglyceridemia | ||
| Hyperuricosuria | 10–40% | Exogenous: diet-induced (purine-rich food) | Allopurinol (100–300 mg/d) | Rare, severe skin hypersensitivity |
| Endogenous: urate overproduction | ||||
| Hypocitraturia | 20–60% | Low extracellular fluid pH: chronic diarrhea; exercise-induced lactic acidosis; dRTA; drug-induced (acetazolamide, topiramate) | Alkali treatment (30–60 mEq/d) | Alkali treatment is generally safe |
| Normal extracellular fluid pH: potassium deficiency; excess dietary protein; urinary tract infection | Potassium alkali is preferred to avert complications with calcium stone formation Although it has not been proven, high doses of alkali may increase the risk of CaP stones |
|||
| Hyperoxaluria | 10–50% | Intestinal hyperabsorption of oxalate: imbalance between intestinal calcium and oxalate content; diet-induced high oxalate intake (chocolate, brewed tea, spinach, nuts; vitamin C, >2 g/d); role of Oxalobacter formigenes | Alkali treatment (30–60 mEq/d) to avert metabolic acidosis | Both alkali and pyridoxine treatments are generally safe |
| Primary hyperoxaluria: enzymatic disturbances (types I, II, or undefined primary hyperoxaluria) | Pyridoxine (25–50 mg/d) in type I primary hyperoxaluria | Potassium alkali is preferred to avert complications with calcium stone formation Although it has not been proven, high doses of alkali may increase the risk of CaP stones |
||
| Urine pH | Unknown | Acidic urine: diet, diarrhea, low urine ammonium | Alkali treatment (30–60 mEq/d) | Alkali treatment is generally safe |
| Alkaline urine: infection; drug-induced (alkali overtreatment, topiramate, acetazolamide); defective renal acid excretion | Potassium alkali is preferred to avert complications with calcium stone formation Although it has not been proven, high doses of alkali may increase the risk of CaP stones |
|||
| Non-calcium kidney stones | ||||
| UA | 5–10% | Low urine volume | Alkali treatment (30–60 mEq/d) | Alkali treatment is generally safe |
| Hyperuricosuria | Allopurinol (100–300 mg/d) | Potassium alkali is preferred to avert complications with calcium stone formation | ||
| Unduly acidic urine | Although it has not been proven, high doses of alkali may increase the risk of CaP stones | |||
| Allopurinol rarely causes severe skin hypersensitivity | ||||
| Cystine | <5% | Renal tubular defect in dibasic amino acid transport | Alkali treatment (30–60 mEq/d) | Alkali treatment is generally safe |
| d-Penicillamine (1000–2000 mg/d) | Potassium alkali is preferred to avert complications with calcium stone formation | |||
| α-Mercaptopropionylglycine (400–1200 mg/d) | Although it has not been proven, high doses of alkali may increase the risk of CaP stones | |||
| Both d-penicillamine and α-mercaptopropionylglycine may cause nausea, vomiting, diarrhea, fever, skin rashes, arthralgia, lupus-like syndrome, dysgeusia, insomnia, leukopenia, thrombocytopenia, and proteinuria | ||||
| α-Mercaptopropionylglycine has generally less frequent severe side effects than d-penicillamine | ||||
| Infection | Unknown | Urease-producing bacteria | Acetohydroxamic acid (10–15 mg/kg/d) | This treatment should only be used if surgical removal of infectious stone followed by eradication of infection with antibiotics is ineffective |
| Severe side effects of acetohydroxamic acid include intractable headache, hemolytic anemia, and thrombophlebitis |
Hypercalciuria
Hypercalciuria is the most prevalent abnormality in calcium kidney stone formers. It is detected in 30–60% of adults with nephrolithiasis (12). In 1939, Flocks (13) initially described the link between hypercalciuria and nephrolithiasis. In 1958, Albright and Henneman (14, 15) applied the term “idiopathic hypercalciuria.” The pathophysiological mechanisms for hypercalciuria are numerous and may involve increased intestinal calcium absorption, decreased renal calcium reabsorption, and enhanced calcium mobilization from bone (4, 16–18). However, intestinal calcium hyperabsorption is the most common abnormality in this population (19). Hence, some have adopted the term “absorptive hypercalciuria” (19). Nevertheless, all the aforementioned physiological defects may coexist in individual patients, leading to decreased bone mineral density and bone fracture (4).
Hypercalciuria is a heterogeneous disorder in which intestinal calcium hyperabsorption may be dependent (20, 21) or independent (19, 22–25) of 1,25-dihydroxyvitamin D [1,25(OH)2D]. Classically, hypercalciuria is classified into two different groups. The most severe variant is characterized by normocalcemia, hypercalciuria, intestinal hyperabsorption of calcium, and normal or suppressed serum PTH and/or urinary cAMP. However, a less severe form shares many of the same biochemical characteristics, but hypercalciuria normalizes after a restricted calcium diet (<400 mg/d) (26).
1,25(OH)2D-dependent hypercalciuria
In two studies, increased serum 1,25(OH)2D concentration was reported in the majority of kidney stone formers with hypercalciuria (20, 21). Insogna et al. (21) demonstrated increased 1,25(OH)2D production rather than disturbed clearance in well-characterized patients with hypercalciuria. The underlying mechanism(s) of enhanced 1,25(OH)2D production have yet to be elucidated. However, in the majority of well-defined hypercalciuric stone formers, the main regulators of 1,25(OH)2D production, namely serum PTH, phosphorus, and tubular maximum renal phosphorus reabsorption, were all at comparable levels to those of normal non-stone-forming subjects (24). Few studies have suggested a link between renal tubular phosphorus abnormalities and serum 1,25(OH)2D levels (20, 27). Further supporting the role of 1,25(OH)2D-mediated hypercalciuria, several studies have shown excessive urinary calcium excretion in normal subjects challenged with a large dose of 1,25(OH)2D (20, 28). However, a disagreement has arisen between the origin of hypercalciuria in which one study supports the intestinal origin (20) and the other suggests calcium mobilization from bone (28).
1,25(OH)2D-independent hypercalciuria
Despite reports of high circulating 1,25(OH)2D in hypercalciuric stone formers (20, 21), several studies have shown that hyperabsorption of calcium is independent of vitamin D, with over two thirds of idiopathic hypercalciuric patients exhibiting increased intestinal calcium absorption with normal prevailing serum 1,25(OH)2D concentration (19, 22–25). To probe this possibility, short-term administration of ketoconazole, an antimycotic agent known to reduce serum 1,25(OH)2D, was shown to significantly lower serum 1,25(OH)2D concentration without a significant alteration in intestinal calcium absorption in hypercalciuric subjects (29). Similarly, treatment with thiazide, glucocorticoids, and phosphate was not shown to influence intestinal calcium absorption in this population, suggesting that calcitriol has a limited pathophysiological role in hypercalciuria (25, 30, 31).
Several studies have exhibited similar phenotypic characteristics in a model of hypercalciuric stone-forming rat (GHS rat). These studies demonstrated normal serum calcium and calcitriol concentrations, increased intestinal calcium absorption with the presence of CaP and CaOx stones (32, 33), enhanced bone resorption, and diminished renal tubular calcium reabsorption (32, 34, 35). In this rat model, an increased abundance of vitamin D receptor (VDR) was shown with normal circulating calcitriol levels and increased VDR protein in the intestine, kidney, and bone that was attributed to increased VDR half-life (34, 36). These results support the notion that prolonged VDR half-life increases VDR tissue expression, resulting in hypercalciuria mediated via VDR-regulated genes controlling vitamin D-regulated calcium transport mechanisms (36). The biological action of the VDR-1,25(OH)2D complex was supported by increased expression of both 9- and 28-kDa calbindin levels in the duodenum and renal cortical tissue of the GHS rat (36).
In human subjects with hypercalciuria, peripheral blood mononuclear cells (PBMC) were used as an organ model to further explore the functionality of the VDR-1,25(OH)2D complex (37). When the PBMC were activated with phytohemagglutinin, these patients showed a significantly greater concentration of VDR in the presence of normal serum calcitriol levels, suggesting 1,25(OH)2D-independent VDR up-regulation (37). In addition, higher VDR levels were detected in hypercalciuric subjects in PBMC without stimulation (38).
Renal leak hypercalciuria
Renal leak hypercalciuria is a second, less common variety of hypercalciuria in which defective renal tubular calcium reabsorption is accompanied by enhanced PTH, calcitriol, and net intestinal calcium absorption (39). Studies using a single dose of hydrochlorothiazide in both unselected and selected groups of calcium stone formers have linked defective renal calcium reabsorption to a proximal renal tubular defect (16, 17).
Resorptive hypercalciuria
The most common prototype of resorptive hypercalciuria is primary hyperparathyroidism. However, due to the more frequent early diagnosis of primary hyperparathyroidism, the prevalence of nephrolithiasis in this condition is nowadays approximately 2–8% (11). Hypercalciuria is perceived as a cause of kidney stones in this population. Nevertheless, the exact relationship between hypercalciuria and the risk of nephrolithiasis with primary hyperparathyroidism is not fully agreed upon (40, 41). It has been disputed whether hypercalciuria originates from increased calcium mobilization from bone or reflects increased intestinal calcium absorption (40, 42, 43). It is suggested that kidney stones are more prevalent in younger populations with primary hyperparathyroidism due to enhanced synthesis of 1,25(OH)2D with intact kidney function, and consequent increased intestinal calcium absorption (42). Bone disease may occur in older subjects due to their lower serum 1,25(OH)2D levels and consequently diminished intestinal calcium absorption.
Hyperuricosuria
Hyperuricosuria as an isolated abnormality is detected in 10% of calcium stone formers. However, in combination with other metabolic abnormalities it may be present in 40% of this population (44). The pathophysiological mechanism underlying hyperuricosuria is attributed to a high purine diet (45). However, in approximately one third of patients, endogenous uric acid (UA) overproduction prevails, and dietary restriction does not significantly alter urinary UA excretion (46). The physicochemical basis involved in this process has not been well established. Although one study has attributed the physicochemical process to urinary supersaturation with colloidal monosodium urate-induced CaOx crystallization (47), another study has shown a lack of effect of monosodium urate and attributes CaOx stone formation to decreased solubility of CaOx in solution, a process described as “salting out” (48). Additionally, a retrospective population-based study in a large number of patients has not shown a relationship between urinary UA and CaOx stone formation (49).
Hypocitraturia
Citrate is an endogenous inhibitor of calcium stone formation, and low urine citrate excretion (hypocitraturia) is encountered in 20–60% of calcium nephrolithiasis (50). The major determinant of urinary citrate excretion is acid-base balance (51). Hypocitraturia commonly occurs with metabolic acidosis or acid loading mediated through up-regulation of proximal renal tubular reabsorption of citrate (52). The main conditions include distal renal tubular acidosis (dRTA) (53), carbonic anhydrase inhibitors (54, 55), and normal bicarbonatemic states (56), including incomplete dRTA (57), thiazide treatment with hypokalemia (58), primary aldosteronism (59), high protein consumption (60), excessive salt intake (61), and converting enzyme inhibitors (62). The physicochemical basis for the inhibitory role of citrate involves the formation of soluble complexes and reduction of urinary saturation with respect to calcium salts in addition to direct inhibition of CaOx crystallization processes (63).
Hyperoxaluria
Urinary oxalate and calcium are equally important in raising urinary CaOx supersaturation (5). Hyperoxaluria is detected in 10–50% of calcium stone formers (5). The underlying mechanisms of hyperoxaluria can be divided into: 1) oxalate overproduction as a result of an inborn error in metabolism; 2) increased dietary intake and bioavailability (64); and 3) increased intestinal oxalate absorption. Inborn errors in metabolism include type I hyperoxaluria resulting from a deficiency or mistargeting of hepatic alanine glyoxylate transferase, type II primary hyperoxaluria due to a deficiency in glyoxylate reductase/hydroxypyruvate reductase, and the rare type III hyperoxaluria as a result of the gain of function of hepatic or renal mitochondrial 4-hydroxy-2-oxoglutarate aldolase (65–67). Subsequent investigation substantiated such a mutation; however, it remains unknown how changes in enzyme activity result in hyperoxaluria (68). Recently, Oxalobacter formigenes in humans have been proposed to participate in intestinal oxalate metabolism (69). Although a putative anion exchange transporter SLC26A6 has been shown to play a key role in intestinal oxalate absorption in mice, phenotypic and functional analysis has excluded a significant effect of identified variants in the corresponding human gene on oxalate excretion in humans (70, 71).
The most important circumstances in clinical practice are intestinal malabsorptive disorders including chronic diarrhea, inflammatory bowel diseases, and intestinal resection as occurs post-gastric bypass surgery (72) leading to “enteric hyperoxaluria.” Normally, oxalate absorption takes place throughout the small intestine and, to a certain extent, in the colon (73). In enteric hyperoxaluria, the underlying mechanisms are purported to be increased permeability to oxalate with unabsorbed bile acid and fatty acids interacting with divalent cations in the lumen of the intestine, thereby raising intestinal luminal oxalate content and resulting in excessive urinary oxalate excretion (74). In addition to hyperoxaluria, these disorders are associated with multiple other kidney stone risk factors including low urine volume, hypocitraturia, hypomagnesuria, and highly acidic urine.
Disturbances in urinary pH
Both highly acidic urine (pH ≤ 5.5) and highly alkaline urine (pH ≥ 6.7) predispose patients to calcium kidney stone formation. With unduly acidic pH, urine becomes supersaturated with undissociated UA that participates in CaOx crystallization (47). Significantly alkaline urine increases the abundance of monohydrogen phosphate [dissociation constant (pKa) ∼ 6.7], which, in combination with calcium, transforms to thermodynamically unstable brushite (CaHPO4.2H2O) and finally to hydroxyapatite [Ca10(PO4)6(OH)2]. In clinical practice, conditions associated with CaP stone formation include dRTA, primary hyperparathyroidism, and use of carbonic anhydrase inhibitors (75, 76).
Histopathological mechanisms of calcium kidney stone formation
One suggested mechanism for the formation of calcium stones is increased urinary supersaturation of stone-forming salts, which leads to homogeneous nucleation in the lumen of the nephron, followed by crystal growth and consequent obstruction in the distal nephron (5). However, over the past decade it has become apparent that CaOx stone formation differs histologically from that of CaP (77). CaOx stones have been shown to anchor on and grow from an interstitial apatite plaque (Randall's plaque) that covers the renal papillary surface (77). The extent of plaque in the renal papilla has been positively correlated with urinary calcium excretion and negatively correlated with urinary volume (77). The decreased proximal tubular reabsorption of calcium and enhanced renal tubular calcium reabsorption at the thick ascending limb have been purported as the potential pathophysiological mechanism resulting in interstitial plaque formation. Unlike CaOx, in CaP stone formers there is apatite crystal deposition in the inner medullary collecting duct, producing plugs associated with interstitial scarring (77).
Genetic basis of calcium stone formation
A higher percentage of kidney stones has been reported in first-degree relatives and family members with kidney stones (78). This genetic link was further documented in a study showing a greater concordance with renal stone incidence in monozygotic than dizygotic twins (79). However, due to the complex nature of idiopathic hypercalciuria, many putative candidate genes have been identified that participate in this polygenic illness. Genome-wide linkage approach in three families with absorptive hypercalciuria discovered polymorphisms in the putative soluble adenylyl cyclase (ADCY10) gene on chromosome 1q23.4–1q24 (80). Another genome-wide screening in a large population from Iceland and The Netherlands with documented radio-opaque kidney stones found polymorphisms in sequence variants in the Claudin 14 (CLDN14) gene, which encodes for the tight junction protein in the kidney, liver, and inner ear (81). Another study has suggested an association between the calcium sensor receptor (CASR) gene polymorphism and recurrent nephrolithiasis (82). However, none of these instances established the functional significance of these polymorphisms. Future phenotype-genotype studies are needed to identify the associated gene defect.
Pathophysiological Mechanism(s) of Non-Calcium Stones
UA stone formation and its link to the MS
The etiological causes of UA stone formation are genetic, acquired, or a combination of both (60, 83). Over the past decade, the MS has been characterized as the most prevalent cause of UA stone formation (Fig. 1A) (84, 85). The underlying pathophysiological mechanisms responsible for UA nephrolithiasis are: 1) low urine volume; 2) hyperuricosuria; and 3) unduly acidic urine.
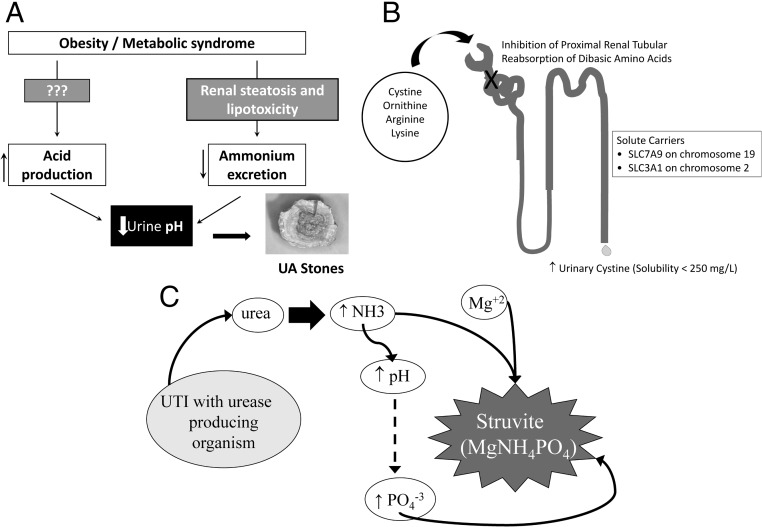
Fig. 1.
Causes of non-calcium kidney stone formation. A, UA stones; B, cystine stones; C, infectious stones.
Unduly acidic urine (urinary pH ≤ 5.5) is an invariable feature in UA nephrolithiasis (5). In such an acidic milieu, the urinary environment becomes supersaturated with sparingly soluble undissociated UA (84). The two principal causes for acidic urine are: 1) impaired ammonium (NH4+) excretion; and 2) increased endogenous acid production (84, 85). Impaired NH4+ excretion in this population is shared with patients with the MS and type II diabetes mellitus without kidney stones (85). Recent experimental evidence using an established rodent model of obesity (Zucher diabetic fatty rat) and a renal proximal tubular cell line have demonstrated a causal role of renal steatosis in the pathogenesis of disturbed urinary acidification (86, 87). Increased endogenous acid production has been shown in both UA stone formers and diabetic non-stone formers (84, 85). However, the nature and source of this putative organic anion has not been fully elucidated. Hyperuricosuria may be encountered in certain clinical circumstances and/or rare genetic disorders linked to the UA synthetic pathway and as a result of a genetic mutation in renal UA transporters (83, 88).
Genetic basis of UA stone formation
Many monogenic mutations are associated with hyperuricosuria, hyperuricemia, gout, renal failure, and kidney stone formation (83, 88). However, most UA stone formers have a low fractional excretion of urate and low urinary pH (84). In one study conducted in a Sardinian cohort, most subjects exhibited low urinary pH with high urinary titratable acidity, with only one third showing elevated urinary UA excretion (89). In this study, genetic analysis identified a locus on chromosome 10q21-22 associated with increased propensity to UA nephrolithiasis. A subsequent study identified the putative gene as zinc finger protein 365 (ZNF365) (90). However, the functional importance of this finding and the role of the protein encoded by this gene have not been fully established.
Cystinuria
Cystine nephrolithiasis comprises only a small fraction of kidney stones in adults but is more prevalent among children and adolescents with stones (91). Cystinuria is either autosomal recessive (obligate heterozygotes with normal urinary cystine excretion) or autosomal dominant with incomplete penetrance (obligate heterozygotes with increased urinary cystine excretion but typically not enough to cause cystine stones). It is characterized by an inherited defect in renal cystine reabsorption expressed as b0,+ (SLC3A1 and SLC7A9). Although the defective renal tubular reabsorption affects other dibasic amino acids including arginine, lysine, and ornithine, cystine stones are the main complication of this genetic defect due to the low solubility of cystine in the urinary environment (Fig. 1B) (92). In a recent genetic classification, cystinuria is defined as type A if mutations are found in both SLC3A1 alleles, type B if mutations are found in both SLC7A9 alleles, and type AB if one mutation is found in each gene (93). With digenic inheritance, one would expect 50% of AB individuals to be affected by nephrolithiasis. However, cystine stones are rarely encountered in this genotype (94).
Infection and rare stones
The most important factors for the formation of infectious stones are highly alkaline urine pH (>7.2) in the presence of urease-producing organisms and supersaturated urinary environment with respect to magnesium, ammonium, and phosphate ions (Fig. 1C) (95). Rare forms of kidney stones such as dihydroxyadanine, ammonium urate, and stones resulting from protease inhibitor drugs may also occur.
Diagnosis
Medical history
In the diagnosis of these patients, systemic and environmental influences must be carefully identified. Systemic abnormalities include intestinal disease, disorders of calcium homeostasis such as primary hyperparathyroidism, conditions accompanied by extra renal 1,25(OH)2D production such as granulomatous diseases, obesity, type II diabetes, recurrent urinary tract infection, bariatric surgery, medullary sponge kidney, and various drug treatments.
Diet plays a crucial role in the formation of kidney stones. High dietary salt and protein consumption are the two most common dietary aberrations that increase the risk of nephrolithiasis (61, 96). Epidemiological studies have shown an association between a higher risk of kidney stone formation and lower dietary calcium intake (97, 98). Although the exact pathophysiological mechanism has not been established, it has been suggested to be due to lowered urinary oxalate excretion or possibly a rise in urinary antilithogenic factors with a high-calcium diet. In contrast, increased calcium and vitamin D supplementation is reportedly accompanied by a higher risk of nephrolithiasis (99). Aside from dietary risk factors, it has been suggested that kidney stone risk increases in hot climates as well as with frequent and/or intense exercise, largely due to extra renal fluid loss as a result of perspiration, resulting in a significant fall in urine volume (83). It has also been suggested that certain professions associated with decreased fluid intake and/or increased perspiration are at high risk for kidney stones.
Although hypercalciuric nephrolithiasis is typically a polygenetic complex trait, in rare instances it may be a monogenetic disorder. The occurrence of hypercalciuric nephrolithiasis among male subjects accompanied by renal impairment and low-molecular-weight proteinuria is suggestive of x-linked recessive disorder detected in patients with Dent's disease (100). The occurrence of kidney stones and nephrocalcinosis in male subjects with early cataracts, glaucoma, and neurological deficit is suggestive of Lowe syndrome (101). Aggressive nephrolithiasis, nephrocalcinosis, retarded growth, and deafness can be seen in patients with dRTA presenting with both autosomal dominant and autosomal recessive inheritance (102).
Laboratory diagnosis
Laboratory diagnosis includes stone analysis, imaging studies, blood profiles, and a urine metabolic evaluation (Table 2). Stone analysis plays a valuable role in the diagnosis of kidney stone patients, specifically in infrequently encountered kidney stones such as UA, cystine, infection-induced, drug-induced, and NH4+ urate stones. Imaging studies are valuable in the diagnosis of kidney stone disease. Despite numerous imaging methodologies, computed tomography is the most sensitive and specific mode of diagnosis (103). High fasting blood calcium, low phosphorus, and elevated PTH are suggestive of primary hyperparathyroidism. In that case, the patient must be considered for a noninvasive localization study followed by parathyroidectomy. Normal serum calcium, low serum phosphorus, elevated 1,25(OH)2D, and normal PTH are suggestive of renal phosphorus leak. The finding of low serum potassium and low CO2 is suggestive of dRTA. Hyperuricemia and high serum triglycerides are encountered in patients with UA stones.
Table 2.
Diagnostic evaluation and interpretation of laboratory profiles
| Simplified ambulatory metabolic evaluation | Extensive ambulatory metabolic evaluation | Expected daily values | Results interpretation |
|---|---|---|---|
| Random 24-h urinary profile | Random 24-h urine profile and 24-h urine profile after 1 wk of dietary restrictions | ||
| Total volume | Total volume | ≥2.5 liter | Indicative of daily fluid intake. This value diminishes with low fluid intake, sweating, and diarrhea |
| pH | pH | 5.9–6.2 | Values < 5.5 increase UA precipitation. Commonly found in UA stone patients, subjects with intestinal disease and diarrhea, and in those with intestinal bypass surgery. Values > 6.7 increase CaP precipitation. Commonly found in patients with dRTA, primary hyperparathyroidism, alkali overtreatment, and carbonic anhydrase treatment. Values > 7.0–7.5 indicate a urinary tract infection as a result of urease-producing bacteria |
| Creatinine | Creatinine | 15–25 mg/kg body weight | 15–20 mg/kg body weight in females; 20–25 mg/kg body weight in males |
| Sodium | Sodium | 100 mEq | Reflective of dietary sodium intake, given a lack of excessive sweating and/or diarrhea |
| Potassium | Potassium | 40–60 mEq | Reflective of dietary potassium intake, given a lack of diarrhea |
| Calcium | Calcium | ≤250–300 mg | There may be differences in male and female subjects. A higher value is expected in males |
| Magnesium | Magnesium | 30–120 mg | Low urinary magnesium is detected with low magnesium intake, intestinal malabsorption (small bowel disease), and after bariatric surgery |
| Oxalate | Oxalate | ≤45 mg | Commonly encountered with intestinal fat malabsorption and after bariatric surgery. Values > 100 mg/d may indicate primary hyperoxaluria |
| Phosphorus | Phosphorus | ≤1100 mg | Indicative of dietary phosphorus intake and absorption. A higher excretion may increase the risk of CaP stone formation |
| UA | UA | 600–800 mg | Hyperuricosuria is encountered with the overindulgence of purine-rich foods such as red meat, poultry, and fish |
| Sulfate | Sulfate | ≤25–30 mmol | Sulfate is a marker of an acid-rich diet that occurs as a result of increased oxidation of sulfur-rich amino acids (methionine) found in meat and meat products |
| Citrate | Citrate | ≥320 mg | An inhibitor of calcium stone formation. Hypocitraturia is commonly encountered in metabolic acidosis, dRTA, chronic diarrhea, excessive protein ingestion, strenuous physical exercise, hypokalemia, intracellular acidosis, with carbonic anhydrase inhibitor drugs (acetazolamide, topiramate, and zonisamide), and rarely with ACE-inhibitors |
| Ammonium | Ammonium | 30–40 mEq | Ammonium is a major buffer that neutralizes hydrogen protons secreted by the kidney. Its excretion corresponds with urinary sulfate (acid load). A higher ammonium:sulfate ratio indicates gastrointestinal alkali loss |
| Chloride | Chloride | 100 mEq | Chloride values also correspond with sodium intake |
| Cystine | Cystine | <30–60 mg | Cystine has a limited urinary solubility at 250 mg/liter |
| 2-h fasting Ca:Cr ratio | <0.11 mg/100 ml glomerular filtrate | Elevated fasting Ca:Cr, high serum calcium, and elevated PTH are suggestive of primary hyperparathyroidism. Elevated fasting Ca:Cr, normal serum calcium, and normal or suppressed PTH are suggestive of resorptive hypercalciuria. Elevated fasting Ca:Cr, normal serum calcium, and elevated PTH are suggestive of renal hypercalciuria | |
| 4-h Ca:Cr ratio after a 1-g oral calcium load | ≤0.20 mg/mg Cr | Elevated Ca:Cr after a 1-g oral calcium load is suggestive of absorptive hypercalciuria | |
| Simplified fasting blood chemistries | Extensive fasting blood chemistries | ||
| Complete metabolic panel | Complete metabolic panel | Variablea | Low serum potassium, high serum chloride, and low serum total CO2 content are suggestive of a diarrheal state of dRTA |
| PTH | PTH | 10–65 pg/mla | High serum calcium, low serum phosphorus, and high PTH are suggestive of primary hyperparathyroidism |
| 1,25(OH)2D | Variablea | Normal serum calcium, normal PTH, and elevated 1,25(OH)2D are suggestive of absorptive hypercalciuria. Normal serum calcium, normal PTH, low serum phosphorus, and elevated 1,25(OH)2D are suggestive of renal phosphorus leak | |
| Other evaluations | |||
| Bone mineral density measurements (DXA) | Z-score > −2; T-score > −2.5 | Z-score < −2 or T-score < −2.5 indicates bone loss. This finding may be more prevalent in hypercalciuric kidney stone formers |
These limits are mean + 2 sd (for calcium, oxalate, UA, pH, sodium, sulfate, and phosphorus) or mean − 2 sd (for citrate, pH, and magnesium) from normal. ACE, Angiotensin-converting enzyme; DXA, dual-energy x-ray absorptiometry.
Expected values should be cross-checked with reference laboratory recommendations because these values may differ.
Metabolic evaluation
A simplified metabolic evaluation starts with a random 24-h urinary profile (Table 2). However, there is disagreement whether a single collection or duplicate random 24-h urine collections are necessary to document kidney stone risk (104, 105). In some optional instances, 2-h fasting urinary calcium:creatinine ratio (Ca:Cr) and fasting urinary phosphorus are obtained to establish the diagnosis of renal leak calcium, excessive calcium mobilization from bone, and renal phosphorus leak. A 4-h urinary Ca:Cr after 1 g oral calcium load may follow the 2-h fasting Ca:Cr for indirect assessment of intestinal calcium absorption (19). An extensive metabolic evaluation may also include bone density analysis because the prevalence of bone fracture has been shown to be higher in kidney stone formers than in the general population (4). Although it is opinion-based, extensive metabolic evaluation may be performed in recurrent kidney stone formers, those with a family history of kidney stones, a history of bone fractures, dRTA, and chronic diarrheal state.
Urinary supersaturation
The utility of urinary supersaturation measurement as a surrogate of kidney stone incidence has not been fully studied. To date, only a single study has provided evidence that a reduction in CaOx supersaturation is associated with a fall in stone incidence (106). However, urinary supersaturation is reported in stone risk profiles by most commercial laboratories. Two methods applied for urinary supersaturation measurements are relative supersaturation (RS) ratio and urinary RS (106, 107). With RS ratio, values greater than 1 indicate supersaturation for all stone types. With RS, the upper limit of normal for oxalate, brushite, monosodium urate, and UA is defined as 2.
Treatment
Acute treatment for symptomatic stone passage is beyond the scope of this review and has previously been extensively described (108).
Conservative management
High oral fluid intake must be considered in all stone formers. A prospective controlled study has shown that increasing water intake to ensure a urinary volume of approximately 2.5 liters/d was associated with reduced urinary supersaturation with CaOx and a significant reduction in stone recurrence (109). Another study suggests that fluid intake as fruit juice, specifically orange juice, is also effective in reducing urinary CaOx saturation and increasing urinary citrate excretion (110). This effectiveness is not shared with apple juice, grapefruit juice, cola, and some sport drinks due to their elevated oxalate and fructose content (110–112). Based on one study in Italian men with hypercalciuria, great emphasis has been placed on low dietary sodium (<100 mEq/d) and animal protein consumption (50–60 g/d) as well as normal calcium intake (1200 mg/d) in the reduction of calcium stone recurrence (113). However, another study using only a low-fiber, low-protein diet in a cohort in the United States did not show decreased stone recurrence (114). Dietary oxalate restriction (<100 mg/d) is also useful in lowering urinary oxalate excretion. Foods that are known to raise urinary oxalate excretion include fruits such as raspberries, figs, and plums; vegetables such as spinach, rhubarb, and beets; and most nuts, tea, wheat bran, chocolate, and high amounts of vitamin C (115, 116). There is no specific report detailing the amount of vitamin D intake for these subjects. However, 800 IU/d is generally recommended.
Pharmacological treatment
Pharmacological treatment is needed in most recurrent calcium kidney stone formers as well as in specific stone-forming populations such as UA, cystine, and infection-induced stones due to the lack of availability and/or consensus regarding the effectiveness of dietary restrictions (Tables 1 and 3) (113, 114).
Table 3.
Major clinical trials in pharmacotherapy of calcium and non-calcium nephrolithiasis
| First author (Ref.) | Treatment | No. of patients | Design | Outcome |
|---|---|---|---|---|
| Thiazide diuretics | ||||
| Laerum (117) | Hydrochlorothiazide vs. placebo | 50 | RCT | Decreased new stone formation and prolonged stone-free interval |
| Ettinger (118) | Chlorthalidone vs. Mg hydroxide vs. placebo | 124 | RCT | Chlorthalidone more effective than Mg hydroxide or placebo in reducing stone events |
| Ohkawa (119) | Trichlormethiazide vs. no treatment | 175 | RCT | Decreased calciuria and stone formation rate |
| Borghi (120) | Diet vs. diet + indapamide vs. diet + indapamide + allopurinol | 75 | RCT | Diet + pharmacotherapy better than diet alone |
| Yendt (121) | Hydrodiuril | 33 | NNT | Decreased number of stone events or invasive and noninvasive procedures |
| Coe (122) | Trichlormethiazide | 37 | NNT | Decreased new stone formation |
| Coe (123) | Trichlormethiazide vs. allopurinol vs. both | 222 | NNT | Decreased new stone formation |
| Yendt (124) | Hydrochlorothiazide | 139 | NNT | Decreased new stone formation or stone growth |
| Backman (125) | Bendroflumethiazide | 44 | NNT | Decreased new stone formation |
| Maschio (126) | Hydrochlorothiazide + amiloride vs. both + allopurinol | 519 | NNT | Decreased new stone formation |
| Pak (127) | Hydrochlorothiazide | 37 | NNT | Decreased new stone formation |
| Alkali treatment | ||||
| Pak (129) | Potassium citrate vs. pretreatment in calcium and UA stone formers | 89 | NNT | Decreased stone events |
| Preminger (57) | Potassium citrate | 9 | NNT | Decreased new stone formation |
| Pak (130) | Potassium citrate | 18 | NNT | Decreased stone events |
| Barcelo (131) | Potassium citrate vs. placebo | 57 | RCT | Decreased new stone formation and increased urinary citrate |
| Hofbauer (132) | Diet + sodium potassium citrate vs. diet | 50 | RCT | No difference in stone formation |
| Ettinger (133) | Potassium magnesium citrate vs. placebo | 64 | RCT | Decreased new stone formation |
| Soygür (134) | Potassium citrate vs. no treatment after shock wave lithotripsy | 110 | RCT | Decreased stone recurrence |
| Kang (135) | Mix of potassium citrate, thiazide, allopurinol vs. no treatment after percutaneous nephrolithotomy | 226 | NCT | Decreased stone recurrence |
| Allopurinol treatment | ||||
| Ettinger (137) | Allopurinol vs. placebo | 60 | RCT | Decreased stone events |
| Coe (123) | Thiazide vs. allopurinol vs. both | 202 | RCT | Decreased stone events vs. pretreatment |
| Other treatment | ||||
| Dahlberg (139) | d-Penicillamine | 89 | R | Decreased stone event and dissolution of stones |
| Pak (140) | d-Penicillamine or α-mercaptopropionylglycine vs. conservative Rx | 66 | R | Both drugs equally effective in reducing stone events |
| Chow (141) | d-Penicillamine or α-mercaptopropionylglycine vs. conservative Rx | 16 | NNT | Decreased stone event |
| Barbey (138) | d-Penicillamine or α-mercaptopropionylglycine vs. conservative Rx | 27 | R | Decreased stone events |
| Williams (142) | Acetohydroxemic acid vs. placebo | 18 | RCT | Decreased stone size |
| Griffith (143) | Acetohydroxemic acid vs. placebo | 210 | RCT | Decreased stone growth |
| Griffith (144) | Acetohydroxemic acid vs. placebo | 94 | RCT | Decreased stone growth |
R, Retrospective; RCT, randomized controlled trial; NCT, nonrandomized controlled trial; NNT, nonrandomized, non-placebo controlled trial.
Thiazide diuretic treatment
Thiazide diuretics and their analogs are commonly used medical treatments for lowering calcium excretion in recurrent calcium stone formers (108). In several randomized controlled trials, thiazide diuretics were effective in significantly reducing kidney stone recurrence (117–120). These results were consistent with a number of open studies showing reduced kidney stone formation with thiazides (121–127). Thiazides are effective in treating hypercalciuria and reducing stone recurrence regardless of the underlying pathophysiological mechanism (127). The optimal effect of thiazides is achieved with a low-salt diet that attenuates urinary calcium excretion and the provision of sufficient potassium supplementation to avoid hypocitraturia (58). Potassium citrate holds an advantage over potassium chloride (128).
Alkali treatment
Potassium citrate is used either alone or in combination with thiazide treatment in recurrent calcium or UA stone formers. In four randomized controlled trials, three nonrandomized nonplacebo controlled studies, and one nonrandomized controlled trial, this treatment was shown to reduce the risk of kidney stone events (57, 129–135). Alkali treatment is effective in lowering urinary calcium excretion, raising urinary citrate, and reducing urinary CaOx, CaP, and undissociated UA supersaturation (136). Because bone loss and fracture are prevalent in patients with nephrolithiasis, both alkali and thiazide treatments have been shown to increase bone mineral density in the kidney stone-forming population (4). However, no randomized data have been obtained showing decreased fracture rate.
Allopurinol treatment
In a randomized controlled trial in hyperuricosuric calcium stone formers, treatment with allopurinol was shown to reduce urinary UA excretion as well as stone recurrence (137). Because multiple metabolic abnormalities may coexist in hyperuricosuric patients, one study has shown that combined thiazide and allopurinol treatment is more effective in reducing stone events compared with either treatment alone (123).
Other drug treatment for stone prevention
Although urinary cystine solubility is pH dependent, alkali treatment alone has limited effectiveness in the management of cystine stone formers. This is due to the high pKa of cystine at 8.0, requiring a large dose of alkali that is difficult to achieve. Furthermore, highly alkaline urine can predispose the patient to CaP stones. The main treatments used in those who suffer from severe cystinuria (>500 mg/d) are thiol-derivatives that split cystine molecules into two cysteines and produce a highly soluble disulfide compound (92). Two such drugs are d-penicillamine and α-mercaptopropionylglycine. In four retrospective, nonrandomized, nonplacebo, controlled trials, both drugs were shown to decrease stone events (138–141). Both drugs share many side effects; however, α-mercaptopropionylglycine may have lower incidence of side effects compared with d-penicillamine (92).
Acetohydroxamic acid is the only drug approved for the treatment of infectious kidney stones. This treatment should only be used if surgical removal of an infectious stone followed by eradication of infection with antibiotics is ineffective. This medication causes an irreversible inhibition of the enzyme urease, therefore attenuating the rise in both urinary pH and NH4+. Three randomized controlled studies have found reduced stone growth with this treatment (142–144). However, compliance is very poor due to severe side effects.
Clinical Follow-Up
Follow-up treatment is typically indicated with an annual clinical visit. This evaluation includes medical history, physical examination, and laboratory examination for full serum chemistries and urine profiles.
Future Directions
The current management of nephrolithiasis lacks a reliable surrogate marker of kidney stone formation to correlate with stone incidence. The development of practical and novel techniques to easily assess the physicochemical processes involved in crystal growth, aggregation, agglomeration, and attachment will immensely benefit the field. Further effort must also be aimed at understanding the molecular and genetic basis of both calcium and non-calcium kidney stones. Such an effort is necessary for the development of targeted therapy based on the underlying pathophysiological mechanisms of nephrolithiasis. To accomplish these goals, a close interaction between bed and bench investigation is crucial.
Acknowledgments
The authors acknowledge Ms. Hadley Palmer for her primary role in the preparation and review of this manuscript.
The authors were supported by the National Institutes of Health (R01 DK081423, P01 DK20543, K23 RR021710).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ca:Cr
- Calcium:creatinine (ratio)
- CaOx
- calcium oxalate
- CaP
- calcium phosphate
- dRTA
- distal renal tubular acidosis
- MS
- metabolic syndrome
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- PBMC
- peripheral blood mononuclear cells
- RS
- relative supersaturation
- UA
- uric acid
- VDR
- vitamin D receptor.
References
- 1. Romero V, Akpinar H, Assimos DG. 2010. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 12:e86–e96 [PMC free article] [PubMed] [Google Scholar]
- 2. Lieske JC, Peña de la Vega LS, Slezak JM, Bergstralh EJ, Leibson CL, Ho KL, Gettman MT. 2006. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int 69:760–764 [DOI] [PubMed] [Google Scholar]
- 3. Sakhaee K. 2008. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens 17:304–309 [DOI] [PubMed] [Google Scholar]
- 4. Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. 2011. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 79:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakhaee K. 2009. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 75:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uribarri J, Oh MS, Carroll HJ. 1989. The first kidney stone. Ann Intern Med 111:1006–1009 [DOI] [PubMed] [Google Scholar]
- 7. Saigal CS, Joyce G, Timilsina AR. 2005. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 68:1808–1814 [DOI] [PubMed] [Google Scholar]
- 8. Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. 1982. Frequency of urolithiasis in a prepaid medical care program. Am J Epidemiol 115:255–265 [DOI] [PubMed] [Google Scholar]
- 9. Mandel NS, Mandel GS. 1989. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J Urol 142:1516–1521 [DOI] [PubMed] [Google Scholar]
- 10. Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M. 2005. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 67:576–591 [DOI] [PubMed] [Google Scholar]
- 11. Pak CY. 1991. Etiology and treatment of urolithiasis. Am J Kidney Dis 18:624–637 [DOI] [PubMed] [Google Scholar]
- 12. Pak CY, Britton F, Peterson R, Ward D, Northcutt C, Breslau NA, McGuire J, Sakhaee K, Bush S, Nicar M, Norman DA, Peters P. 1980. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 69:19–30 [DOI] [PubMed] [Google Scholar]
- 13. Flocks RH. 1939. Calcium and phosphorus excretion in the urine of patients with renal or ureteral calculi. JAMA 113:1466–1471 [Google Scholar]
- 14. Albright F, Henneman P, Benedict PH, Forbes AP. 1953. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med 46:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henneman PH, Benedict PH, Forbes AP, Dudley HR. 1958. Idiopathic hypercaicuria. N Engl J Med 259:802–807 [DOI] [PubMed] [Google Scholar]
- 16. Sutton RA, Walker VR. 1980. Responses to hydrochlorothiazide and acetazolamide in patients with calcium stones. Evidence suggesting a defect in renal tubular function. N Engl J Med 302:709–713 [DOI] [PubMed] [Google Scholar]
- 17. Sakhaee K, Nicar MJ, Brater DC, Pak CY. 1985. Exaggerated natriuretic and calciuric responses to hydrochlorothiazide in renal hypercalciuria but not in absorptive hypercalciuria. J Clin Endocrinol Metab 61:825–829 [DOI] [PubMed] [Google Scholar]
- 18. Worcester EM, Coe FL. 2008. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28:120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pak CY, Oata M, Lawrence EC, Snyder W. 1974. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest 54:387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broadus AE, Insogna KL, Lang R, Mallette LE, Oren DA, Gertner JM, Kliger AS, Ellison AF. 1984. A consideration of the hormonal basis and phosphate leak hypothesis of absorptive hypercalciuria. J Clin Endocrinol Metab 58:161–169 [DOI] [PubMed] [Google Scholar]
- 21. Insogna KL, Broadus AE, Dreyer BE, Ellison AF, Gertner JM. 1985. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab 61:490–495 [DOI] [PubMed] [Google Scholar]
- 22. Birge SJ, Peck WA, Berman M, Whedon GD. 1969. Study of calcium absorption in man: a kinetic analysis and physiologic model. J Clin Invest 48:1705–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pak CY, East DA, Sanzenbacher LJ, Delea CS, Bartter FC. 1972. Gastrointestinal calcium absorption in nephrolithiasis. J Clin Endocrinol Metab 35:261–270 [DOI] [PubMed] [Google Scholar]
- 24. Kaplan RA, Haussler MR, Deftos LJ, Bone H, Pak CY. 1977. The role of 1α,25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest 59:756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zerwekh JE, Pak CY. 1980. Selective effects of thiazide therapy on serum 1α,25-dihydroxyvitamin D and intestinal calcium absorption in renal and absorptive hypercalciurias. Metabolism 29:13–17 [DOI] [PubMed] [Google Scholar]
- 26. Pak CY, Sakhaee K, Moe OW, Poindexter J, Adams-Huet B, Pearle MS, Zerwekh JE, Preminger GM, Wills MR, Breslau NA, Bartter FC, Brater DC, Heller HJ, Odvina CV, Wabner CL, Fordtran JS, Oh M, Garg A, Harvey JA, Alpern RJ, Snyder WH, Peters PC. 2011. Defining hypercalciuria in nephrolithiasis. Kidney Int 80:777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Den Berg CJ, Kumar R, Wilson DM, Heath H, 3rd, Smith LH. 1980. Orthophosphate therapy decreases urinary calcium excretion and serum 1,25-dihydroxyvitamin D concentrations in idiopathic hypercalciuria. J Clin Endocrinol Metab 51:998–1001 [DOI] [PubMed] [Google Scholar]
- 28. Maierhofer WJ, Gray RW, Cheung HS, Lemann J., Jr 1983. Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int 24:555–560 [DOI] [PubMed] [Google Scholar]
- 29. Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. 1992. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab 75:1446–1452 [DOI] [PubMed] [Google Scholar]
- 30. Barilla DE, Zerwekh J, Pak CY. 1979. A critical evaluation of the role of phosphate in the pathogenesis of absorptive hypercalciuria. Miner Electrolyte Metab 2:302–309 [Google Scholar]
- 31. Zerwekh JE, Pak CY, Kaplan RA, McGuire JL, Upchurch K, Breslau N, Johnson R., Jr 1980. Pathogenetic role of 1α,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: different response to prednisolone therapy. J Clin Endocrinol Metab 51:381–386 [DOI] [PubMed] [Google Scholar]
- 32. Bushinsky DA, Grynpas MD, Nilsson EL, Nakagawa Y, Coe FL. 1995. Stone formation in genetic hypercalciuric rats. Kidney Int 48:1705–1713 [DOI] [PubMed] [Google Scholar]
- 33. Bushinsky DA, Asplin JR, Grynpas MD, Evan AP, Parker WR, Alexander KM, Coe FL. 2002. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 61:975–987 [DOI] [PubMed] [Google Scholar]
- 34. Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. 1993. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest 91:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsuruoka S, Bushinsky DA, Schwartz GJ. 1997. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int 51:1540–1547 [DOI] [PubMed] [Google Scholar]
- 36. Karnauskas AJ, van Leeuwen JP, van den Bemd GJ, Kathpalia PP, DeLuca HF, Bushinsky DA, Favus MJ. 2005. Mechanism and function of high vitamin D receptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res 20:447–454 [DOI] [PubMed] [Google Scholar]
- 37. Zerwekh JE, Yu XP, Breslau NA, Manolagas S, Pak CY. 1993. Vitamin D receptor quantitation in human blood mononuclear cells in health and disease. Mol Cell Endocrinol 96:1–6 [DOI] [PubMed] [Google Scholar]
- 38. Favus MJ, Karnauskas AJ, Parks JH, Coe FL. 2004. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab 89:4937–4943 [DOI] [PubMed] [Google Scholar]
- 39. Coe FL, Bushinsky DA. 1984. Pathophysiology of hypercalciuria. Am J Physiol 247:F1–F13 [DOI] [PubMed] [Google Scholar]
- 40. Pak CY, Nicar MJ, Peterson R, Zerwekh JE, Snyder W. 1981. A lack of unique pathophysiologic background for nephrolithiasis of primary hyperparathyroidism. J Clin Endocrinol Metab 53:536–542 [DOI] [PubMed] [Google Scholar]
- 41. Rejnmark L, Vestergaard P, Mosekilde L. 2011. Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab 96:2377–2385 [DOI] [PubMed] [Google Scholar]
- 42. Patron P, Gardin JP, Paillard M. 1987. Renal mass and reserve of vitamin D: determinants in primary hyperparathyroidism. Kidney Int 31:1174–1180 [DOI] [PubMed] [Google Scholar]
- 43. Odvina CV, Sakhaee K, Heller HJ, Peterson RD, Poindexter JR, Padalino PK, Pak CY. 2007. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res 35:123–128 [DOI] [PubMed] [Google Scholar]
- 44. Preminger GM. 1992. Renal calculi: pathogenesis, diagnosis, and medical therapy. Semin Nephrol 12:200–216 [PubMed] [Google Scholar]
- 45. Coe FL. 1978. Hyperuricosuric calcium oxalate nephrolithiasis. Kidney Int 13:418–426 [DOI] [PubMed] [Google Scholar]
- 46. Coe FL, Parks JH. 1981. Hyperuricosuria and calcium nephrolithiasis. Urol Clin North Am 8:227–244 [PubMed] [Google Scholar]
- 47. Pak CY, Arnold LH. 1975. Heterogeneous nucleation of calcium oxalate by seeds of monosodium urate. Proc Soc Exp Biol Med 149:930–932 [DOI] [PubMed] [Google Scholar]
- 48. Grover PK, Ryall RL. 1994. Urate and calcium oxalate stones: from repute to rhetoric to reality. Miner Electrolyte Metab 20:361–370 [PubMed] [Google Scholar]
- 49. Curhan GC, Taylor EN. 2008. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73:489–496 [DOI] [PubMed] [Google Scholar]
- 50. Pak CY. 1994. Citrate and renal calculi: an update. Miner Electrolyte Metab 20:371–377 [PubMed] [Google Scholar]
- 51. Hamm LL. 1990. Renal handling of citrate. Kidney Int 38:728–735 [DOI] [PubMed] [Google Scholar]
- 52. Aruga S, Wehrli S, Kaissling B, Moe OW, Preisig PA, Pajor AM, Alpern RJ. 2000. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int 58:206–215 [DOI] [PubMed] [Google Scholar]
- 53. Backman U, Danielson BG, Johansson G, Ljunghall S, Wikström B. 1980. Incidence and clinical importance of renal tubular defects in recurrent renal stone formers. Nephron 25:96–101 [DOI] [PubMed] [Google Scholar]
- 54. Gordon EE, Sheps SG. 1957. Effect of acetazolamide on citrate excretion and formation of renal calculi. N Engl J Med 256:1215–1219 [DOI] [PubMed] [Google Scholar]
- 55. Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. 2006. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis 48:555–563 [DOI] [PubMed] [Google Scholar]
- 56. Alpern RJ, Sakhaee K. 1997. The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29:291–302 [DOI] [PubMed] [Google Scholar]
- 57. Preminger GM, Sakhaee K, Skurla C, Pak CY. 1985. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol 134:20–23 [DOI] [PubMed] [Google Scholar]
- 58. Pak CY, Peterson R, Sakhaee K, Fuller C, Preminger G, Reisch J. 1985. Correction of hypocitraturia and prevention of stone formation by combined thiazide and potassium citrate therapy in thiazide-unresponsive hypercalciuric nephrolithiasis. Am J Med 79:284–288 [DOI] [PubMed] [Google Scholar]
- 59. Shey J, Cameron MA, Sakhaee K, Moe OW. 2004. Recurrent calcium nephrolithiasis associated with primary aldosteronism. Am J Kidney Dis 44:e7–e12 [DOI] [PubMed] [Google Scholar]
- 60. Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. 2002. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40:265–274 [DOI] [PubMed] [Google Scholar]
- 61. Sakhaee K, Harvey JA, Padalino PK, Whitson P, Pak CY. 1993. The potential role of salt abuse on the risk for kidney stone formation. J Urol 150:310–312 [DOI] [PubMed] [Google Scholar]
- 62. Melnick JZ, Preisig PA, Haynes S, Pak CY, Sakhaee K, Alpern RJ. 1998. Converting enzyme inhibition causes hypocitraturia independent of acidosis or hypokalemia. Kidney Int 54:1670–1674 [DOI] [PubMed] [Google Scholar]
- 63. Kok DJ, Papapoulos SE, Bijvoet OL. 1986. Excessive crystal agglomeration with low citrate excretion in recurrent stone-formers. Lancet 1:1056–1058 [DOI] [PubMed] [Google Scholar]
- 64. Holmes RP, Goodman HO, Assimos DG. 2001. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 59:270–276 [DOI] [PubMed] [Google Scholar]
- 65. Danpure CJ, Jennings PR. 1986. Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett 201:20–24 [DOI] [PubMed] [Google Scholar]
- 66. Giafi CF, Rumsby G. 1998. Kinetic analysis and tissue distribution of human D-glycerate dehydrogenase/glyoxylate reductase and its relevance to the diagnosis of primary hyperoxaluria type 2. Ann Clin Biochem 35:104–109 [DOI] [PubMed] [Google Scholar]
- 67. Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y. 2010. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Monico CG, Rossetti S, Belostotsky R, Cogal AG, Herges RM, Seide BM, Olson JB, Bergstrahl EJ, Williams HJ, Haley WE, Frishberg Y, Milliner DS. 2011. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 6:2289–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H. 2006. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70:1305–1311 [DOI] [PubMed] [Google Scholar]
- 70. Monico CG, Weinstein A, Jiang Z, Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ, Milliner DS, Aronson PS. 2008. Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am J Kidney Dis 52:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. 2006. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38:474–478 [DOI] [PubMed] [Google Scholar]
- 72. Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K. 2010. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol 183:1026–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lindsjö M, Danielson BG, Fellström B, Ljunghall S. 1989. Intestinal oxalate and calcium absorption in recurrent renal stone formers and healthy subjects. Scand J Urol Nephrol 23:55–59 [DOI] [PubMed] [Google Scholar]
- 74. Dobbins JW, Binder HJ. 1976. Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology 70:1096–1100 [PubMed] [Google Scholar]
- 75. Pak CY, Poindexter JR, Adams-Huet B, Pearle MS. 2003. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med 115:26–32 [DOI] [PubMed] [Google Scholar]
- 76. Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. 2004. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int 66:777–785 [DOI] [PubMed] [Google Scholar]
- 77. Coe FL, Evan A, Worcester E. 2011. Pathophysiology-based treatment of idiopathic calcium kidney stones. Clin J Am Soc Nephrol 6:2083–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ljunghall S, Danielson BG, Fellström B, Holmgren K, Johansson G, Wikström B. 1985. Family history of renal stones in recurrent stone patients. Br J Urol 57:370–374 [DOI] [PubMed] [Google Scholar]
- 79. Goldfarb DS, Fischer ME, Keich Y, Goldberg J. 2005. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 67:1053–1061 [DOI] [PubMed] [Google Scholar]
- 80. Reed BY, Heller HJ, Gitomer WL, Pak CY. 1999. Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24. J Clin Endocrinol Metab 84:3907–3913 [DOI] [PubMed] [Google Scholar]
- 81. Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d'Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. 2009. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41:926–930 [DOI] [PubMed] [Google Scholar]
- 82. Shakhssalim N, Kazemi B, Basiri A, Houshmand M, Pakmanesh H, Golestan B, Eilanjegh AF, Kashi AH, Kilani M, Azadvari M. 2010. Association between calcium-sensing receptor gene polymorphisms and recurrent calcium kidney stone disease: a comprehensive gene analysis. Scand J Urol Nephrol 44:406–412 [DOI] [PubMed] [Google Scholar]
- 83. Maalouf NM, Cameron MA, Moe OW, Sakhaee K. 2004. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens 13:181–189 [DOI] [PubMed] [Google Scholar]
- 84. Sakhaee K, Adams-Huet B, Moe OW, Pak CY. 2002. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int 62:971–979 [DOI] [PubMed] [Google Scholar]
- 85. Sakhaee K. 2010. Uric acid metabolism and uric acid stones. In: Rao NP, Preminger G, Kavanaugh J, eds. Urinary tract stone disease. London: BC Decker Publisher, Springer-Verlag [Google Scholar]
- 86. Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. 2008. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol 294:F1315–F1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. 2009. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297:F1419–F1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. 2004. Clinical and molecular analysis of patients with renal hypouricemia in Japan—influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15:164–173 [DOI] [PubMed] [Google Scholar]
- 89. Ombra MN, Forabosco P, Casula S, Angius A, Maestrale G, Petretto E, Casu G, Colussi G, Usai E, Melis P, Pirastu M. 2001. Identification of a new candidate locus for uric acid nephrolithiasis. Am J Hum Genet 68:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gianfrancesco F, Esposito T, Ombra MN, Forabosco P, Maninchedda G, Fattorini M, Casula S, Vaccargiu S, Casu G, Cardia F, Deiana I, Melis P, Falchi M, Pirastu M. 2003. Identification of a novel gene and a common variant associated with uric acid nephrolithiasis in a Sardinian genetic isolate. Am J Hum Genet 72:1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chillarón J, Font-Llitjós M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacín M. 2010. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6:424–434 [DOI] [PubMed] [Google Scholar]
- 92. Sakhaee K. 1996. Pathogenesis and medical management of cystinuria. Semin Nephrol 16:435–447 [PubMed] [Google Scholar]
- 93. Dello Strologo L, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, Ponzone A, Gallucci M, Bisceglia L, Zelante L, Jimenez-Vidal M, Font M, Zorzano A, Rousaud F, Nunes V, Gasparini P, Palacín M, Rizzoni G. 2002. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol 13:2547–2553 [DOI] [PubMed] [Google Scholar]
- 94. Font-Llitjós M, Jiménez-Vidal M, Bisceglia L, Di Perna M, de Sanctis L, Rousaud F, Zelante L, Palacín M, Nunes V. 2005. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet 42:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bichler KH, Eipper E, Naber K, Braun V, Zimmermann R, Lahme S. 2002. Urinary infection stones. Int J Antimicrob Agents 19:488–498 [DOI] [PubMed] [Google Scholar]
- 96. Maalouf NM, Moe OW, Adams-Huet B, Sakhaee K. 2011. Hypercalciuria associated with high dietary protein intake is not due to acid load. J Clin Endocrinol Metab 96:3733–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. 1993. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328:833–838 [DOI] [PubMed] [Google Scholar]
- 98. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. 1997. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 126:497–504 [DOI] [PubMed] [Google Scholar]
- 99. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. 2006. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683 [DOI] [PubMed] [Google Scholar]
- 100. Knohl SJ, Scheinman SJ. 2004. Inherited hypercalciuric syndromes: Dent's disease (CLC-5) and familial hypomagnesemia with hypercalciuria (paracellin-1). Semin Nephrol 24:55–60 [DOI] [PubMed] [Google Scholar]
- 101. Bockenhauer D, Bokenkamp A, van't Hoff W, Levtchenko E, Kist-van Holthe JE, Tasic V, Ludwig M. 2008. Renal phenotype in Lowe syndrome: a selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3:1430–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Karet FE. 2002. Inherited distal renal tubular acidosis. J Am Soc Nephrol 13:2178–2184 [DOI] [PubMed] [Google Scholar]
- 103. Shokeir AA, Abdulmaaboud M. 2001. Prospective comparison of nonenhanced helical computerized tomography and Doppler ultrasonography for the diagnosis of renal colic. J Urol 165:1082–1084 [PubMed] [Google Scholar]
- 104. Pak CY, Peterson R, Poindexter JR. 2001. Adequacy of a single stone risk analysis in the medical evaluation of urolithiasis. J Urol 165:378–381 [DOI] [PubMed] [Google Scholar]
- 105. Parks JH, Goldfisher E, Asplin JR, Coe FL. 2002. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol 167:1607–1612 [PubMed] [Google Scholar]
- 106. Parks JH, Coe FL. 1996. The financial effects of kidney stone prevention. Kidney Int 50:1706–1712 [DOI] [PubMed] [Google Scholar]
- 107. Werness PG, Brown CM, Smith LH, Finlayson B. 1985. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol 134:1242–1244 [DOI] [PubMed] [Google Scholar]
- 108. Moe OW, Pearle MS, Sakhaee K. 2011. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int 79:385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. 1996. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 155:839–843 [PubMed] [Google Scholar]
- 110. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. 1998. Beverage use and risk for kidney stones in women. Ann Intern Med 128:534–540 [DOI] [PubMed] [Google Scholar]
- 111. Taylor EN, Curhan GC. 2008. Fructose consumption and the risk of kidney stones. Kidney Int 73:207–212 [DOI] [PubMed] [Google Scholar]
- 112. Goodman JW, Asplin JR, Goldfarb DS. 2009. Effect of two sports drinks on urinary lithogenicity. Urol Res 37:41–46 [DOI] [PubMed] [Google Scholar]
- 113. Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. 2002. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346:77–84 [DOI] [PubMed] [Google Scholar]
- 114. Hiatt RA, Ettinger B, Caan B, Quesenberry CP, Jr, Duncan D, Citron JT. 1996. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol 144:25–33 [DOI] [PubMed] [Google Scholar]
- 115. Traxer O, Huet B, Poindexter J, Pak CY, Pearle MS. 2003. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol 170:397–401 [DOI] [PubMed] [Google Scholar]
- 116. Meschi T, Maggiore U, Fiaccadori E, Schianchi T, Bosi S, Adorni G, Ridolo E, Guerra A, Allegri F, Novarini A, Borghi L. 2004. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int 66:2402–2410 [DOI] [PubMed] [Google Scholar]
- 117. Laerum E, Larsen S. 1984. Thiazide prophylaxis of urolithiasis. A double-blind study in general practice. Acta Med Scand 215:383–389 [PubMed] [Google Scholar]
- 118. Ettinger B, Citron JT, Livermore B, Dolman LI. 1988. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol 139:679–684 [DOI] [PubMed] [Google Scholar]
- 119. Ohkawa M, Tokunaga S, Nakashima T, Orito M, Hisazumi H. 1992. Thiazide treatment for calcium urolithiasis in patients with idiopathic hypercalciuria. Br J Urol 69:571–576 [DOI] [PubMed] [Google Scholar]
- 120. Borghi L, Meschi T, Guerra A, Novarini A. 1993. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol 22(Suppl 6):S78–S86 [PubMed] [Google Scholar]
- 121. Yendt ER, Guay GF, Garcia DA. 1970. The use of thiazides in the prevention of renal calculi. Can Med Assoc J 102:614–620 [PMC free article] [PubMed] [Google Scholar]
- 122. Coe FL, Kavalach AG. 1974. Hypercalciuria and hyperuricosuria in patients with calcium nephrolithiasis. N Engl J Med 291:1344–1350 [DOI] [PubMed] [Google Scholar]
- 123. Coe FL. 1977. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 87:404–410 [DOI] [PubMed] [Google Scholar]
- 124. Yendt ER, Cohanim M. 1978. Prevention of calcium stones with thiazides. Kidney Int 13:397–409 [DOI] [PubMed] [Google Scholar]
- 125. Backman U, Danielson BG, Johansson G, Ljunghall S, Wikström B. 1979. Effects of therapy with bendroflumethiazide in patients with recurrent renal calcium stones. Br J Urol 51:175–180 [DOI] [PubMed] [Google Scholar]
- 126. Maschio G, Tessitore N, D'Angelo A, Fabris A, Pagano F, Tasca A, Graziani G, Aroldi A, Surian M, Colussi G, Mandressi A, Trinchieri A, Rocco F, Ponticelli C, Minetti L. 1981. Prevention of calcium nephrolithiasis with low-dose thiazide, amiloride and allopurinol. Am J Med 71:623–626 [DOI] [PubMed] [Google Scholar]
- 127. Pak CY, Peters P, Hurt G, Kadesky M, Fine M, Reisman D, Splann F, Caramela C, Freeman A, Britton F, Sakhaee K, Breslau NA. 1981. Is selective therapy of recurrent nephrolithiasis possible? Am J Med 71:615–622 [DOI] [PubMed] [Google Scholar]
- 128. Nicar MJ, Peterson R, Pak CY. 1984. Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J Urol 131:430–433 [DOI] [PubMed] [Google Scholar]
- 129. Pak CY, Fuller C, Sakhaee K, Preminger GM, Britton F. 1985. Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol 134:11–19 [DOI] [PubMed] [Google Scholar]
- 130. Pak CY, Sakhaee K, Fuller C. 1986. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int 30:422–428 [DOI] [PubMed] [Google Scholar]
- 131. Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. 1993. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 150:1761–1764 [DOI] [PubMed] [Google Scholar]
- 132. Hofbauer J, Höbarth K, Szabo N, Marberger M. 1994. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis—a prospective randomized study. Br J Urol 73:362–365 [DOI] [PubMed] [Google Scholar]
- 133. Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A. 1997. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 158:2069–2073 [DOI] [PubMed] [Google Scholar]
- 134. Soygür T, Akbay A, Küpeli S. 2002. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol 16:149–152 [DOI] [PubMed] [Google Scholar]
- 135. Kang DE, Maloney MM, Haleblian GE, Springhart WP, Honeycutt EF, Eisenstein EL. 2007. Effect of medical management on recurrent stone formation following percutaneous nephrolithotomy. J Urol 177:1785–1788; discussion 1788–1789 [DOI] [PubMed] [Google Scholar]
- 136. Sakhaee K, Nicar M, Hill K, Pak CY. 1983. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int 24:348–352 [DOI] [PubMed] [Google Scholar]
- 137. Ettinger B, Tang A, Citron JT, Livermore B, Williams T. 1986. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 315:1386–1389 [DOI] [PubMed] [Google Scholar]
- 138. Barbey F, Joly D, Rieu P, Méjean A, Daudon M, Jungers P. 2000. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 163:1419–1423 [DOI] [PubMed] [Google Scholar]
- 139. Dahlberg PJ, van den Berg, Kurtz SB, Wilson DM, Smith LH. 1977. Clinical features and management of cystinuria. Mayo Clin Proc 52:533–542 [PubMed] [Google Scholar]
- 140. Pak CY, Fuller C, Sakhaee K, Zerwekh JE, Adams BV. 1986. Management of cystine nephrolithiasis with α-mercaptopropionylglycine. J Urol 136:1003–1008 [DOI] [PubMed] [Google Scholar]
- 141. Chow GK, Streem SB. 1996. Medical treatment of cystinuria: results of contemporary clinical practice. J Urol 156:1576–1578 [DOI] [PubMed] [Google Scholar]
- 142. Williams JJ, Rodman JS, Peterson CM. 1984. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med 311:760–764 [DOI] [PubMed] [Google Scholar]
- 143. Griffith DP, Khonsari F, Skurnick JH, James KE. 1988. A randomized trial of acetohydroxamic acid for the treatment and prevention of infection-induced urinary stones in spinal cord injury patients. J Urol 140:318–324 [DOI] [PubMed] [Google Scholar]
- 144. Griffith DP, Gleeson MJ, Lee H, Longuet R, Deman E, Earle N. 1991. Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. Eur Urol 20:243–247 [DOI] [PubMed] [Google Scholar]