Abstract
Context:
GH secretion declines rapidly after glucose ingestion and then recovers to higher-than-baseline levels (rebound GH release).
Hypothesis:
Selective metabolic markers predict the magnitude of glucose-suppressed GH release and postglucose rebound-like GH secretion.
Design:
Prospectively randomized crossover study of GH secretion after glucose vs. water ingestion.
Setting:
The study was conducted at a clinical translational research center.
Participants:
Sixty-nine healthy men aged 19–78 yr with a body mass index of 18–39 kg/m2 participated in the study.
Outcomes:
Outcomes included nadir vs. peak GH concentrations and basal vs. pulsatile GH secretion.
Results:
Mean nadir GH concentrations were determined positively by sex hormone binding globulin (SHBG) after glucose administration (R2 = 0.088, P = 0.0077). Peak rebound GH concentrations were related positively to adiponectin and negatively to computed tomography-estimated abdominal visceral fat (AVF) (R2 = 0.182, P = 0.00049) after glucose ingestion. Deconvolution analysis showed that SHBG specifically predicted basal (nonpulsatile) GH secretion after glucose exposure (R2 = 0.153, P = 0.00052). In contrast, together exercise history and adiponectin (both positively) and AVF (negatively) predicted pulsatile GH escape after glucose suppression (R2 = 0.206, P = 0.00043). Moreover, adiponectin uniquely determined the size (mass), and AVF the mode (duration), of GH secretory bursts after glucose exposure (both P < 0.006).
Conclusion:
Glucose ingestion provides a clinical model for investigating complementary metabolic surrogates that determine suppression and recovery of basal and pulsatile GH secretion in healthy men.
In clinical studies, a rebound-like pattern of GH release begins several hours after oral glucose administration [oral glucose tolerance test (OGTT)] (1, 2). A similar time delay applies to exogenous glucagon-induced GH release, which also transiently elevates blood glucose concentrations (3, 4). The mechanisms that control delayed rebound-like GH secretion after glucose ingestion are not known, although the ratio of limb fat to trunk fat negatively predicts this response in HIV-infected patients (5). Other plausible determinants are age, body mass index (BMI), abdominal visceral fat (AVF), free fatty acids, exercise history, fasting plasma glucose, insulin, IGF-I, testosterone (T), estradiol (E2), and cortisol concentrations (6–9). Moreover, adipokines like resistin, adiponectin, and leptin regulate GH secretion in laboratory studies (10, 11), but their exact roles in the human are not known (9).
GH secretion is supervised by an ensemble of hypothalamic peptides and systemic hormones (8). Somatostatin is a noncompetitive inhibitor of GH release, whereas GHRH stimulates both GH synthesis and secretion (9). The GH-releasing peptide (GHRP), ghrelin, amplifies the amount of GH secreted per burst (12, 13). GHRP's primary action in vivo is via the regulation of GHRH and somatostatin, although strong GH release is observable in vitro also in primary primate pituitary cell cultures (14). Systemic hormones and metabolites may modulate GH secretion directly at the pituitary level and/or indirectly by modifying the release and actions of somatostatin, GHRH, or ghrelin. For example, in the rat, hyperglycemia evokes somatostatin-dependent inhibition of pulsatile GH secretion (15). In humans, oral glucose administration also suppresses GH concentrations rapidly and markedly (2) inferentially by inhibition of hypothalamic GHRH and/or the stimulation of somatostatin release (16) because GHRP attenuates the inhibitory effect of glucose ingestion (17, 18).
Suppression of GH release by glucose or somatostatin is followed by rebound-like secretion, resulting in peak GH concentrations above the fasting baseline values (1, 2, 5, 8, 9, 19, 20). The mechanisms mediating postsomatostatin rebound-like GH peak may include GHRH release (21) and somatostatin withdrawal, which triggers discharge of newly synthesized GH granules (22). Given this framework, GH responses to glucose should provide an indirect measure of endogenous peptidyl regulation of somatotropes. Thus, the present goal is to identify the direction and extent of effect of key clinical metabolic factors regulating glucose-suppressed and postglucose-rebound GH secretion in men. The expectation is to provide novel insights into distinct metabolic factors modulating human GH secretion in the fed and fasting state. Metabolic factors are significant, given that they mediate or mirror changes in body composition (e.g. obesity, cachexia), puberty (via sex steroids), prediabetes (altered glycemic control), aging (impaired insulin secretion), and genetic syndromes (leptin deficiency).
Materials and Methods
Subjects
Sixty-nine healthy men were recruited to participate after providing voluntary written informed consent approved by the Salem Veterans Affairs Institutional Review Board. To test the impact of a wide range of ages and body composition, the admissible age range was 18–80 yr with a BMI of 20–40 kg/m2. Exclusion diagnoses were congestive heart failure, acute or chronic liver or renal disease, anemia, hypothalamopituitary disease, neuropsychiatric drug exposure, glucocorticoid use, systemic inflammatory process, malignancy, substance abuse, intracranial disease, sleep apnea, or diabetes mellitus. Inclusion criteria were community-dwelling, independently living, consenting adults with stable diurnal work habits, body weight (within 2 kg in 3 months), and recreational exercise patterns.
Protocol
Subjects undertook two 10-min sampling sessions each of a duration of 6.5 h, scheduled at least 10 d apart. Each session began after overnight fasting from 1800 h onward. Blood sampling started at 0800 h the next morning. A sample of 5 ml serum was obtained at 0800 h fasting during the first session for metabolic measures, which were used in a regression analysis. At 0830 h, glucose (75 g) or the same volume of water (10 oz = 296 ml) was administered orally. Blood sampling continued every 10 min thereafter for 6 more h until 1430 h. In three subjects, there was a delay (not exceeding 1 h) in starting the protocol. Volunteers continued their customary weight-maintaining diet between sessions.
Abdominal visceral fat
An abdominal computed tomography scan was performed at the L3-L4 interspace to estimate the AVF cross-sectional area, as described (23).
Assays
Serum concentrations GH were assayed at each time point over 6.5 h (in each 10 min sample yielding 108 samples/subject) by Immulite 2000 (Siemens Healthcare Diagnostics, Flanders, NJ). The assay has a detection range of 0.05–40 μg/liter GH with intra- and interassay coefficients of variation of 4.1 and 6.3%, respectively. Testosterone, estradiol, and cortisol were measured at the 0800 h time point in the first session in the Siemens assay, as described recently (24). Serum samples obtained at 0800 h also were used to measure glucose and insulin concentrations via SYNCHRON (Beckman, Chaska, MN) and Siemens Dimension Vista autoanlyzers (Deerfield, IL). Fasting (0800 h) SHBG was determined by the Siemens assay, which has a detection range of 0.02–180 nmol/liter with intra- and interassay coefficients of variation of 2.3–5.3% (range) and 4.0–6.6% (range), respectively (24).
Serum concentrations of adiponectin and leptin were assayed in the first 0800 h samples by RIA, using reagents from Millipore (Billerica, MA). The adiponectin assay measures the 30-kDa human adipokine. The assay has respective intra- and interassay coefficients of variation of 1.8–6.2 and 6.3–9.2% at peptide concentrations of 1.5–7.5 mg/liter. The leptin assay has respective intra- and interassay coefficients of variation of 3.4–8.3 and 3.0–6.2% at concentrations of 4.9–25.6 μg/liter. The exercise history was by recall over the preceding week and graded as minutes per week total exercise (walking, bicycling, and/or resistance exercising or jogging). No exercise was assigned a value of 10 min/wk arbitrarily. The homeostasis model assessment insulin resistance index (HOMA-IR) was calculated as described to provide an index of fasting insulin resistance (25).
Model-free analysis
GH suppression was defined by the single lowest GH concentration observed after glucose/water ingestion (GH minimum) and the mean nadir GH concentration (averaged over 80–170 min after the start of blood sampling). The last 5 h of the 6.5-h sampling session was used to assess GH rebound because all GH minima occurred within the first 90 min of sampling. Model-independent measures of GH rebound were the mean, the peak (single maximal), and the incremental (peak minus nadir) postglucose/postwater GH concentrations (time window 100–400 min).
Deconvolution analysis
GH concentration-time series were subjected to automated deconvolution analysis using a validated maximum likelihood estimation methodology (26). The two-component GH elimination model comprised a fast half-life of 3.5 min and a slow (63% of total) half-life of 20.8 min (27). Candidate pulse-time sets were determined by the heat equation. Deconvolution models were compared statistically using the Akaike information criterion (28). Outcome variables were basal time-invariant (nonpulsatile) and pulsatile (burst-like) GH secretion (units of micrograms per liter sampling session) and the mass (micrograms per liter), number of bursts (per sampling session), and shape over time (mode in minutes) of GH secretory bursts. The total GH secretion is the algebraic sum of basal and pulsatile secretion (micrograms per liter per sampling session), as described fully elsewhere (8, 9, 28).
Statistics
A paired Student's t test was used to evaluate the effect of oral glucose compared with water ingestion on deconvolution and model-free measures of GH suppression and rebound. Significance was confirmed by the (paired) signed-rank nonparametric test. Uni- and multivariate linear regression analyses were used to assess the effects of adiponectin, leptin, SHBG, T, E2, glucose, HOMA-IR, insulin, IGF-I, age, exercise history, cortisol, BMI, and AVF on primary outcomes (29). The stepwise forward-selection model assumes the following: 1) some input variables may not have a significant explanatory effect and 2) multicollinearity exists (input variables themselves may be correlated). The procedure entails adding the most statistically significant term to the model and reassessing the overall R2 and the overall P value and individual slopes, t-statistics and P values. R2 is adjusted to reflect the residual degrees of freedom in the model. MATLAB 7.8.0 (The MathWorks Inc., Natick, MA) was the software platform. Data are given as the geometric mean ± sem and/or the median (range).
Results
Epidemiological, body compositional, and metabolic measures in the 69 men are summarized in Table 1. For the purposes of regression analysis, wide ranges of age (19–78 yr), BMI (18–39 kg/m2), exercise history (10–525 min/wk), HOMA-IR (0.4–6.0), nondiabetic fasting plasma glucose (73–119 mg/dl), and other subject characteristics were represented, as intended.
Table 1.
Subject characteristics (n = 69)
| Mean | ±sem | Median | Range | |
|---|---|---|---|---|
| Age (yr) | 38 | 2.1 | 42 | 19–78 |
| BMI (kg/m2) | 27 | 0.49 | 27 | 18–39 |
| AVF (cm2) | 89 | 12 | 105 | 12–410 |
| Exercise (min/wk) | 246 | 22 | 225 | 10–525 |
| Insulin (mU/liter) | 4.6 | 0.49 | 4.2 | 2–22 |
| FPG (mg/dL) | 93 | 1.1 | 94 | 73–119 |
| HOMA-IR | 1.06 | 0.14 | 1.00 | 0.4–6.0 |
| SHBG (nm) | 31.5 | 1.7 | 34 | 7.3–78 |
| T (ng/dl) | 445 | 22 | 466 | 181–1026 |
| Cortisol (μg/dl) | 11.7 | 0.41 | 12.2 | 5.2–20 |
| Estradiol (pg/ml) | 32 | 2.1 | 32 | 8–105 |
| Adiponectin (mg/liter) | 8.8 | 0.61 | 10 | 3.1–33 |
| Leptin (μg/liter) | 4.7 | 0.49 | 5.2 | 1.0–16 |
| GH (μg/liter) | 1.2 | 0.24 | 0.27 | 0.055–10 |
| IGF-I (μg/liter) | 159 | 8.5 | 157 | 63–403 |
Measurements were made in each subject using the first 0800 h fasting blood sample (n = 69 subjects). Data are the geometric mean, sem, median, and range as noted by column heads. To convert T (nanograms per deciliter), cortisol (micrograms per liter), and E2 (picograms per milliliter) to System International units, multiply by 0.0347, 27.6, and 3.68, respectively. FPG, Fasting plasma glucose.
Minimal, nadir, and peak GH concentrations
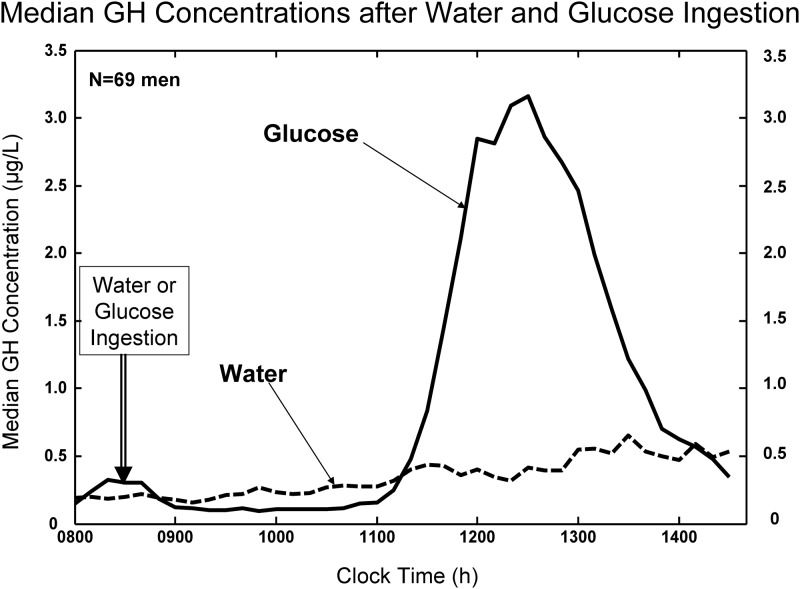
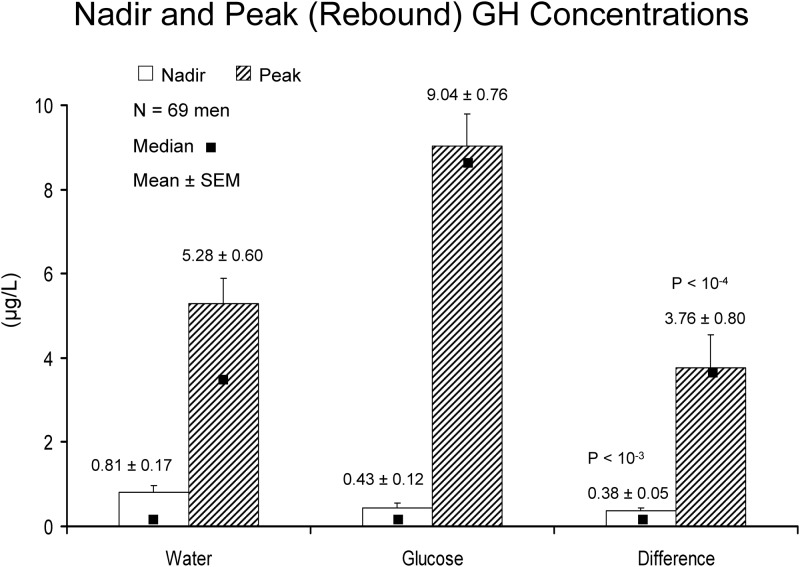
After water ingestion, fasting GH concentrations rose gradually over the 6.5 h of the 10-min sampling (Fig. 1, interrupted line). After 75 g glucose ingestion, GH concentrations decreased to a median absolute minimum of 0.061 (range 0.05–0.77) μg/liter [P = 0.014 vs. water associated value of 0.071 (0.05–1.13) by signed-ranks test]. Minima occurred at mean (median) times of 80 (65) min (water) and 120 (100) min (glucose) after sampling onset (P > 0.10). The (mean ± sem) GH nadir averaged over a 90-min window beginning 50 min after water or glucose ingestion was 0.81 ± 0.17 (water) and 0.43 ± 0.12 (glucose) [P < 10−4, mean difference 0.38 (95% confidence interval [CI] 0.16–0.61) μg/liter by paired t test; Fig. 2 (open bars)]. Thus, both the absolute minimum and the (90 min mean) nadir GH concentrations were reduced significantly by glucose compared with water ingestion.
Fig. 1.
Median serum GH concentrations sampled every 10 min for 6.5 h (40 samples) beginning at 0800 h in a cohort of 69 men studied after overnight fasting. Subjects were given water or glucose (75 g) orally after the third blood sample (double arrow).
Fig. 2.
Mean (bar graph ± sem) and median (dark square) GH concentrations in 69 men after water and glucose ingestion. Data are 90-min nadir (open bars) and postnadir (solid bars) peak (maximal) rebound GH concentrations. Comparisons are by Student's t test.
The decrease in GH concentrations after an OGTT was followed by a marked rebound-like GH peak (Fig. 1, continuous line). The mean ± sem time of the maximal GH concentration (peak rebound) was 310 ± 70 (340 median) min for water and 277 ± 51 (280 median) min for glucose ingestion (P = 0.0023 by paired t test and P = 0.0018 by signed rank test). Peak GH concentrations averaged 5.28 ± 0.60 μg/liter (water) and 9.04 ± 0.76 μg/liter (glucose) [P < 10−4 by t test; Fig. 2 (solid bars)]. Median values were 3.72 (water) and 8.44 (glucose) μg/liter (P < 10−4 by signed rank test). The mean (95% CI) intraindividual difference in rebound GH peaks between glucose and water ingestion was 3.76 (2.17–5.34) μg/dl [(median 3.73 μg/liter); Fig. 2]. Thus, a marked and unequivocal rebound in GH release occurs after glucose vs. water ingestion after a time delay of 4–5 h.
The question of whether water- and glucose-associated absolute minima or mean nadir GH concentrations are determined by different metabolic surrogates was tested by multivariate analysis. For single-point GH minima, leptin was the dominant (negative) correlate after both water (P = 0.0031) and glucose (P = 0.0034) exposure (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Nadir GH concentrations on the control (water) day correlated jointly with AVF (negatively) and SHBG (positively) (overall R2 = 0.252, P < 10−4). GH nadir values on the OGTT day were associated with only SHBG (positively) (R2 = 0.088, P = 0.0077).
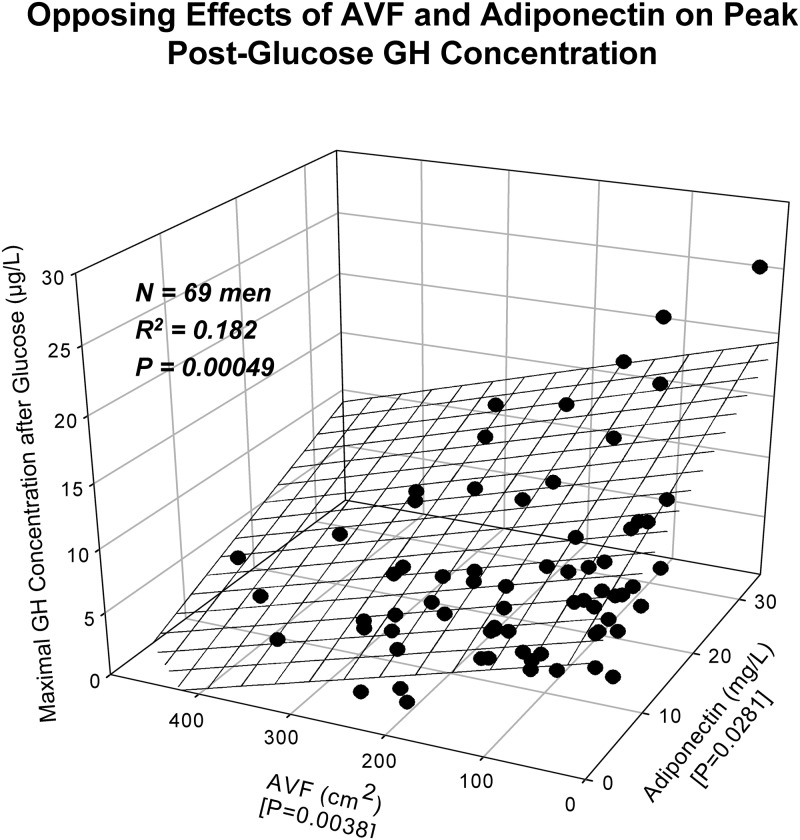
Absolute maximal (peak) GH concentrations after water ingestion correlated positively with IGF-I and negatively with AVF at joint (R2 = 0.305 and P < 10−5; Supplemental Table 1). Peak GH rebound after glucose ingestion was determined jointly by AVF (negatively) and adiponectin (positively) (R2 = 0.182, P = 0.00049; Fig. 3). Mean (100–400 min window) postnadir rebound GH concentrations after water ingestion were determined by AVF and leptin negatively (joint R2 = 0.386, P < 10−7), and after glucose by all three of AVF (negatively), SHBG (positively), and exercise history (positively) (joint R2 = 0.362, P < 10−6).
Fig. 3.
Multivariate determination of peak (maximal) postnadir rebound GH concentrations by AVF (negative) and adiponectin (positively) after glucose administration in 69 healthy men. Overall R2 and P values (bold face) and individual-factor P values (brace parentheses on x and y axes) are given.
The algebraic difference between the rebound GH peak and the nadir GH concentrations (δ or increment) in individual subjects averaged 3.62 ± 0.54 and 7.77 ± 0.76 μg/liter after water and glucose administration, respectively (P = 0.00021). Increments after water ingestion were correlated with leptin (negatively) and IGF-I (positively) (P < 10−4) and after glucose ingestion with AVF (negatively) and adiponectin (positively) (P = 0.0024]; Supplemental Table 1, bottom).
Pulsatile and basal (nonpulsatile) GH secretion
A deconvolution analysis was used to appraise the mechanisms underlying GH rebound (Table 2). Glucose exposure doubled pulsatile GH secretion during the rebound interval (last 5 h of sampling), viz. from 11 ± 2.2 to 25 ± 3.3 μg/liter per 5 h [mean difference 14 (8–21) μg/liter per 5 h, P < 10−4]. Total rebound GH secretion (P = 0.0002), but not basal (nonpulsatile) GH secretion, rose comparably. Rebound selectively augmented the mass of GH released per burst [mean difference for glucose-water 8.7 (4–13) μg/liter, P = 0.0002]. The number of GH secretory bursts and their shape did not change.
Table 2.
Deconvolution analysis of rebound GH profiles after water or glucose ingestion
| Parameter | Water | Glucose | Difference | P value |
|---|---|---|---|---|
| GH bursts per 5 h, n | 2.2 ± 0.12 (2.0) | 2.0 ± 0.13 (2.0) | −0.25 [−0.5 to 0.1] | 0.16 |
| Mode (min) | 15 ± 0.56 (16) | 14 ± 0.50 (15) | −1.4 [−2.8 to 0.11] | 0.069 |
| Basal sec (μg/liter per 5 h) | 2.1 ± 0.76 (2.0) | 2.5 ± 0.47 (2.7) | −0.14 [−1.7 to 1.4] | 0.85 |
| Pulsatile sec (μg/liter per 5 h) | 11 ± 2.2 (16) | 25 ± 3.3 (28) | 14 [8–21] | <10−4 |
| Total sec (μg/liter per 5 h) | 14 ± 2.7 (18) | 29 ± 3.5 (29) | 14 [7–22] | 0.0002 |
| Mass/burst (μg/liter) | 4.8 ± 1.6 (7.2) | 13 ± 1.9 (14) | 8.7 [4–13] | 0.0002 |
Data are geometric mean ± sem (median). Brackets define 95% CI of difference. Boldface denotes P < 0.01. Diff, Paired difference (glucose minus water value).
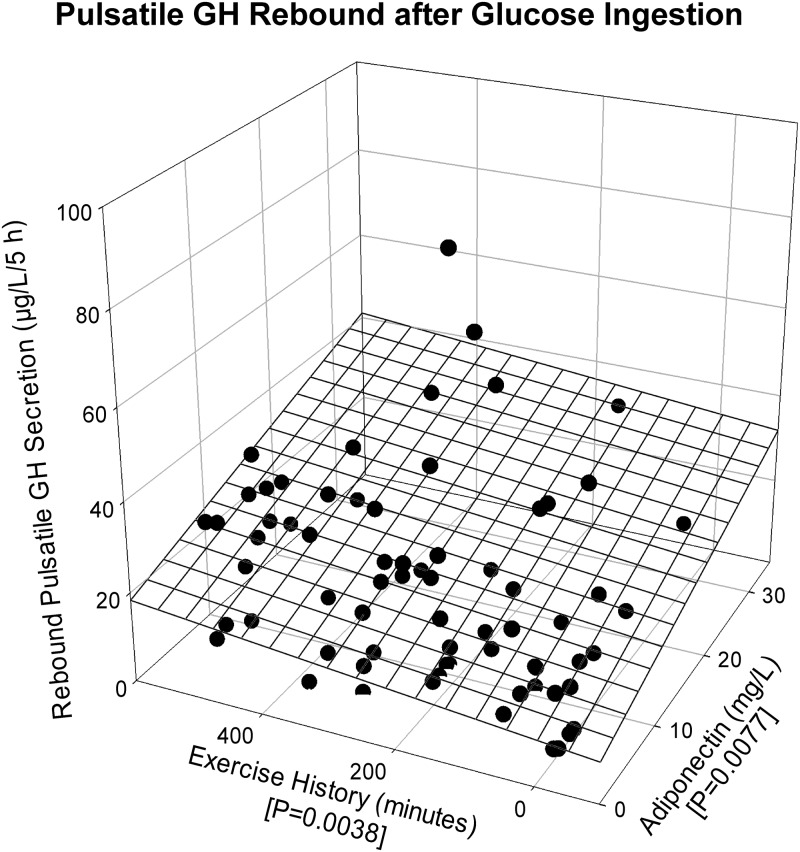
Multivariate correlation analysis at protected P < 0.01 revealed that basal (nonpulsatile) GH secretion was correlated with leptin negatively (P = 0.00049) after water and with SHBG positively (P = 0.00052) after glucose administration (Supplemental Table 2). After water ingestion, AVF (negatively) and E2 (positively) determined pulsatile GH secretion (joint R2 = 0.309, P < 10−5) (Supplemental Fig. 1). The inclusion of IGF-I (positive correlate) increased the joint R2 value to 0.332 (P < 10−5), yielding a strong three-variable model. In contrast, after glucose absorption, AVF (−), exercise history (+), and adiponectin (+) together determined pulsatile GH secretion (R2 = 0.206, P = 0.00043) (Supplemental Table 2). Figure 4 shows the joint effects of the positive predictors, exercise history (+) and adiponectin (+), on the pulsatile GH secretion after an OGTT (R2 = 0.195, P = 0.00078). Consistent with the associations for pulsatile GH secretion, the mass of GH secreted per burst was related to AVF (−) after water (P < 10−4) and to adiponectin (+) after glucose (P = 0.0081). GH secretory-burst mode (duration) was predicted by fasting plasma glucose (−) after water (P = 0.0080) and by AVF (−) after glucose (P = 0.0059) administration. Total (basal plus pulsatile) GH secretion after water ingestion was influenced by AVF (−) (P < 10−5) and after glucose ingestion jointly by AVF (−) and SHBG (+) (P = 0.00082).
Fig. 4.
Exercise history (positively) and adiponectin (positively) jointly determine after an OGTT rebound pulsatile GH secretion. The graphical format is that of Fig. 3.
Discussion
Little has been known about the metabolic factors that predict glucose-suppressed and postglucose rebound-like basal and pulsatile GH release. By using deconvolution, we found that oral administration of glucose markedly suppresses basal (nonpulsatile) GH secretion. Basal secretion is thought to reflect constitutive (unregulated) GH release driven by the total mass of somatotrope cells, each releasing a small finite fraction of its GH content (9). The present data challenge the notion of unregulated basal GH secretion because basal GH secretion evinced a negative association with leptin concentrations after water ingestion and a positive association with SHBG concentrations after glucose ingestion. These outcomes suggest that metabolically distinct mechanisms regulate fasting and glucose-suppressed basal GH secretion in healthy men.
Based on deconvolution analysis, rebound-like pulsatile GH secretion after glucose exposure was positively determined by exercise history and adiponectin concentrations and negatively by AVF. To our knowledge, no prior post-OGTT data exist on these correlations. In contrast, in the fasting state (water only), pulsatile GH secretion was determined by IGF-I and E2 concentrations positively and AVF negatively. The positive correlations of IGF-I and E2 with pulsatile GH secretion probably reflect the recognized feed-forward (stimulatory) effect of GH pulses on IGF-I production and the GH pulse-amplifying actions of estrogen (8, 9). AVF is itself a major negative correlate of GH pulse size via unknown mechanisms. Candidate inhibitory mechanisms would include free fatty acids, insulin, free IGF-I, and/or selected adipocytokines. Together metabolic surrogates explained up to 39% of the interindividual variance in endogenously regulated GH secretion after water and glucose absorption. Whether adding ghrelin or somatostatin measurements would increase this joint correlation further is not known (30).
Why rebound-like GH release occurs after glucose administration is not known. By deconvolution analysis, the rebound phase of GH release was due to doubling of the mass of GH secreted per burst, with no detectable change in burst number or basal GH secretion. This insight is important, inasmuch as the peptidyl determinants of GH secretory-burst mass are relatively well established (introductory text). In this context, we hypothesize that rebound GH secretion after glucose ingestion reflects delayed endogenous GHRH and ghrelin drive under waning somatostatin restraint. This postulate would be consistent with the following facts: 1) that hyperglycemia per se evokes somatostatin release in animal models, and 2) that acute somatostatin withdrawal elicits a burst of rebound GHRH and GH release (8, 9). The positive correlation between the mass of GH rebound and adiponectin suggests that this antiinflammatory and insulin-sparing adipokine is a key metabolic surrogate of GHRH/ghrelin release and/or somatostatin withdrawal in nondiabetic men.
An unexpected outcome was that total GH secretion as well as peak and mean GH concentrations correlate positively with SHBG levels after an OGTT. The precise bases for these strong SHBG associations are not known. However, SHBG is negatively regulated by insulin, GH, AVF, BMI, and androgen and positively regulated by age, estrogen, T4, and exercise (31, 32). For example, low SHBG is a major biological marker of hyperinsulinemia in the metabolic syndrome (33, 34), which is associated with low GH secretion (8, 9). Despite this association, the precise degree to which insulin represses GH secretion in humans in not established (9). Indeed, in the present analysis, neither insulin concentrations nor HOMA-IR correlated with rebound GH secretion. However, fasting plasma glucose predicted GH secretory-burst duration negatively. This result introduces a novel mechanism for shorter GH-concentration peaks in fasting individuals with higher glucose values.
In experimental animals the adipocytokines leptin, resistin, and adiponectin stimulate GH secretion (35, 36), whereas free fatty acids, insulin, glucose, and IGF-I suppress GH secretion (37, 38). In contrast, the negative GH-leptin relationship observed here and elsewhere in humans likely reflects the known inverse correlation between total body fat (and hence leptin also) and GH secretion (8, 9). Nonetheless, in one earlier clinical study, exogenous leptin failed to inhibit fasting-stimulated GH secretion (39).
Sex steroids alone were not predictive of postglucose rebound GH secretion, but E2 was a positive determinant of fasting pulsatile GH secretion. The estrogen correlation coexisted with that of AVF (negative) and IGF-I (positive) (overall R2 = 0.332). Estradiol, putatively acting via the estrogen receptor-α, is a major agonist of GH secretion in both men and women (8, 9). The present data show that E2, AVF, and IGF-I are triple joint regulators of pulsatile GH secretion in fasting men.
The role of endogenous somatostatin in mediating biphasic inhibition and stimulation is probable but not directly ascertainable here. In particular, somatostatin, at least at low concentrations in vitro, can directly stimulate GH release from primary cultures of primate pituitary cells, acting via the type 5 somatostatin receptor and cAMP (40). Thus, glucose or somatostatin-induced rebound GH release could also arise, in principle, from the degradation of systemic or hypothalamo-pituitary somatostatin to yield lower stimulatory concentrations. However, neither systemic nor pituitary portal-vessel somatostatin concentrations have been quantified to our knowledge in the OGTT model presented here (8, 9). We did not have sufficient peripheral serum to make such measurements.
Caveats include the relatively small cohort size (n = 69), occasional undetectability of GH after glucose (GH < 0.050 μg/liter), and the need for comparable data in women. Many indices (in this study) point to insulin resistance (hyperinsulinemia) being involved in alterations in GH output. However, there was no correlation with fasting insulin. The latter evidence may prematurely discount the role of insulin, whereas if glucose-stimulated insulin release and subsequent clearance were examined, the picture could be clearer. A clinical implication of these outcomes is that determining the 95% CI of peak rebound GH concentrations (here 2.17–5.34 μg/liter, median 3.73 μg/liter) in large and diverse cohorts may provide a low-risk means of evaluating endogenously regulated GH secretion without requiring the injection of insulin, GHRH, GHRP, or l-arginine in childhood, pregnancy, or frail older adults. To this end, future studies would require comparisons between OGTT and a more accepted test in the same population. The correlations observed in these analyses also suggest a possible means by which to predict the GH output using a single fasting sample estimate of the metabolic end point(s). Further appraisal of this idea in larger cohorts of healthy individuals will be needed to test this idea.
In conclusion, glucose-suppressed nadir GH concentrations and postglucose rebound-like peak GH release in men are strongly determined by selective metabolic surrogates, including especially AVF, adiponectin, leptin, and SHBG. These outcomes distinguish putative metabolic factors modulating basal and pulsatile GH outflow in the overnight fasting and postglucose recovery states.
Supplementary Material
Acknowledgments
We thank Jill Smith for the support of the manuscript preparation; Sandra Cabral for graphics assistance; Barbara Dunn of the Salem Veterans Affairs Medical Center research nursing staff for implementing the protocol; Patricia Roberts and Mary Buchy of the Salem Veterans Affairs Medical Center for performing the assays. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Matlab versions of the deconvolution methodology are available from J.D.V. (veldhuis.johannes@mayo.edu). Supplemental material is available.
This work was supported in part by Grant 1 UL 1 RR024150 from the Center for Translational Science Activities from the National Center for Research Resources (Rockville, MD), Grants AG19695 and DK050456 from the Metabolic Studies Core of the Minnesota Obesity Center and Grant DK073148 from the National Institutes of Health (Bethesda, MD). The work was also supported by the Salem Veterans Affairs Medical Center and the Salem Research Institute (Salem, VA).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVF
- Abdominal visceral fat
- BMI
- body mass index
- CI
- confidence interval
- E2
- estradiol
- GHRP
- GH-releasing peptide
- HOMA-IR
- homeostasis model assessment insulin resistance index
- OGTT
- oral glucose tolerance test
- T
- testosterone.
References
- 1. Frystyk J, Ritzel RA, Maubach J, Büsing M, Lück R, Klempnauer J, Schmiegel W, Nauck MA. 2008. Comparison of pancreas-transplanted type 1 diabetic patients with portal-venous versus systemic-venous graft drainage: impact on glucose regulatory hormones and the growth hormone/insulin-like growth factor-I axis. J Clin Endocrinol Metab 93:1758–1766 [DOI] [PubMed] [Google Scholar]
- 2. Grottoli S, Procopio M, Maccario M, Zini M, Oleandri SE, Tassone F, Valcavi R, Ghigo E. 1997. In obesity, glucose load loses its early inhibitory, but maintains its late stimulatory, effect on somatotrope secretion. J Clin Endocrinol Metab 82:2261–2265 [DOI] [PubMed] [Google Scholar]
- 3. Berg C, Meinel T, Lahner H, Yuece A, Mann K, Petersenn S. 2010. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol 162:477–482 [DOI] [PubMed] [Google Scholar]
- 4. Giuffrida FM, Berger K, Monte L, Oliveira CH, Hoff AO, Maciel RM, Vieira JG. 2009. Relationship between GH response and glycemic fluctuations in the glucagon stimulation test. Growth Horm IGF Res 19:77–81 [DOI] [PubMed] [Google Scholar]
- 5. Andersen O, Haugaard SB, Hansen BR, Orskov H, Andersen UB, Madsbad S, Iversen J, Flyvbjerg A. 2004. Different growth hormone sensitivity of target tissues and growth hormone response to glucose in HIV-infected patients with and without lipodystrophy. Scand J Infect Dis 36:832–839 [DOI] [PubMed] [Google Scholar]
- 6. Luque RM, Gahete MD, Valentine RJ, Kineman RD. 2006. Examination of the direct effects of metabolic factors on somatotrope function in a non-human primate model, Papio anubis. J Mol Endocrinol 37:25–38 [DOI] [PubMed] [Google Scholar]
- 7. Van Dam PS, Smid HE, de Vries WR, Niesink M, Bolscher E, Waasdorp EJ, Dieguez C, Casanueva FF, Koppeschaar HP. 2000. Reduction of free fatty acids by acipimox enhances the growth hormone (GH) responses to GH-releasing peptide 2 in elderly men. J Clin Endocrinol Metab 85:4706–4711 [DOI] [PubMed] [Google Scholar]
- 8. Giustina A, Veldhuis JD. 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- 9. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. 2006. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castaño JP, Malagon MM. 2007. Regulation of pituitary cell function by adiponectin. Endocrinology 148:401–410 [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez-Pacheco F, Vázquez-Martínez R, Martínez-Fuentes AJ, Pulido MR, Gahete MD, Vaudry H, Gracia-Navarro F, Diéguez C, Castaño JP, Malagón MM. 2009. Resistin regulates pituitary somatotrope cell function through the activation of multiple signaling pathways. Endocrinology 150:4643–4652 [DOI] [PubMed] [Google Scholar]
- 12. Tannenbaum GS, Epelbaum J, Bowers CY. 2003. Interrelationship between the novel peptide ghrelin, somatostatin and growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 144:967–974 [DOI] [PubMed] [Google Scholar]
- 13. Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. 2004. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab 89:2290–2300 [DOI] [PubMed] [Google Scholar]
- 14. Kineman RD, Luque RM. 2007. Evidence that ghrelin is as potent as GHRH in releasing GH from primary pituitary cell cultures of a non-human primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 148:4440–4449 [DOI] [PubMed] [Google Scholar]
- 15. Lewis BM, Dieguez C, Ham J, Page MD, Creagh FM, Peters JR, Scanlon MF. 1989. Effects of glucose on thyrotrophin-releasing hormone, growth hormone-releasing hormone, somatostatin and luteinizing hormone-releasing hormone release from rat hypothalamus in vitro. J Neuroendocrinol 1:437–441 [DOI] [PubMed] [Google Scholar]
- 16. Maccario M, Procopio M, Grottoli S, Oleandri SE, Razzore P, Camanni F, Ghigo E. 1995. In obesity the somatotrope response to either growth hormone-releasing hormone or arginine is inhibited by somatostatin or pirenzepine but not by glucose. J Clin Endocrinol Metab 80:3774–3778 [DOI] [PubMed] [Google Scholar]
- 17. Maccario M, Arvat E, Procopio M, Gianotti L, Grottoli S, Imbimbo BP, Lenaerts V, Deghenghi R, Camanni F, Ghigo E. 1995. Metabolic modulation of the growth hormone-releasing activity of hexarelin in man. Metab Clin Exp 44:134–138 [DOI] [PubMed] [Google Scholar]
- 18. Bowers CY, Laferrere B, Hurley DL, Veldhuis JD. 2008. The role of growth hormone and ghrelin in feeding and body composition. In: Donahoe PA, ed. Energy metabolism and obesity: research and clinical applications. Totowa, NJ: The Humana Press; 125–154 [Google Scholar]
- 19. Valcavi R, Zini M, Volta C, Ghizzoni L, Azzarito C, Bernasconi S, Portioli I. 1994. Effects of oral glucose administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH release in children and adults. J Clin Endocrinol Metab 79:1152–1157 [DOI] [PubMed] [Google Scholar]
- 20. Valcavi R, Zini M, Dieguez C, Portioli I, Scanlon MF. 1990. Effect of oral glucose on the late growth hormone rise and growth hormone responses to GHRH in normal subjects. Clin Endocrinol (Oxf) 32:539–543 [DOI] [PubMed] [Google Scholar]
- 21. Clark RG, Carlsson LM, Rafferty B, Robinson IC. 1988. The rebound release of growth hormone (GH) following somatostatin infusion in rats involves hypothalamic GH-releasing factor release. J Endocrinol 119:397–404 [DOI] [PubMed] [Google Scholar]
- 22. Stachura ME, Tyler JM, Farmer PK. 1988. Combined effects of human growth hormone (GH)-releasing factor-44 (GRF) and somatostatin (SRIF) on post-SRIF rebound release of GH and prolactin: a model for GRF-SRIF modulation of secretion. Endocrinology 123:1476–1482 [DOI] [PubMed] [Google Scholar]
- 23. Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. 2005. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab 90:6006–6013 [DOI] [PubMed] [Google Scholar]
- 24. Iranmanesh A, Lawson D, Dunn B, Veldhuis JD. 2011. Glucose ingestion selectively amplifies ACTH and cortisol secretory-burst mass and enhances their joint synchrony in healthy men. J Clin Endocrinol Metab 96:2882–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace TM, Matthews DR. 2002. The assessment of insulin resistance in man. Diabet Med 19:527–534 [DOI] [PubMed] [Google Scholar]
- 26. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. 2009. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faria AC, Veldhuis JD, Thorner MO, Vance ML. 1989. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab 68:535–541 [DOI] [PubMed] [Google Scholar]
- 28. Veldhuis JD, Keenan DM, Pincus SM. 2008. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29:823–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher LD, van Belle G. 1996. Descriptive statistics. Biostatistics: a methodology for the health sciences. 3rd ed New York: John Wiley, Sons; 58–74 [Google Scholar]
- 30. Yang I, Woo J, Kim S, Kim J, Kim Y, Choi Y. 1996. Suppression of TRH-stimulated TSH secretion by glucose-induced hypothalamic somatostatin release. Horm Metab Res 28:553–557 [DOI] [PubMed] [Google Scholar]
- 31. Peter A, Kantartzis K, Machann J, Schick F, Staiger H, Machicao F, Schleicher E, Fritsche A, Häring HU, Stefan N. 2010. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes 59:3167–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gascón F, Valle M, Martos R, Ruz FJ, Ríos R, Montilla P, Cañete R. 2000. Sex hormone-binding globulin as a marker for hyperinsulinemia and/or insulin resistance in obese children. Eur J Endocrinol 143:85–89 [DOI] [PubMed] [Google Scholar]
- 33. Lormeau C, Soudan B, d'Herbomez M, Pigny P, Duquesnoy B, Cortet B. 2004. Sex hormone-binding globulin, estradiol, and bone turnover markers in male osteoporosis. Bone 34:933–939 [DOI] [PubMed] [Google Scholar]
- 34. Pugeat M, Crave JC, Tourniaire J, Forest MG. 1996. Clinical utility of sex hormone-binding globulin measurement. Horm Res 45:148–155 [DOI] [PubMed] [Google Scholar]
- 35. Meier U, Gressner AM. 2004. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem 50:1511–1525 [DOI] [PubMed] [Google Scholar]
- 36. Luque RM, Gahete MD, Cordoba-Chacon J, Childs GV, Kineman RD. 2011. Does the pituitary somatotrope play a primary role in regulating GH output in metabolic extremes? Ann NY Acad Sci 1220:82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romero CJ, Ng Y, Luque RM, Kineman RD, Koch L, Bruning JC, Radovick S. 2010. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol Endocrinol 24:1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luque RM, Kineman RD. 2006. Impact of obesity on the growth hormone (GH)-axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 147:2754–2763 [DOI] [PubMed] [Google Scholar]
- 39. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. 2003. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Córdoba-Chacón J, Gahete MD, Culler MD, Castaño JP, Kineman RD, Luque RM. 2012. Somatostatin dramatically stimulates growth hormone release from primate somatotropes acting at low doses via sst5 and cAMP. J Neuroendocrinol 24:453–463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.