Abstract
Context:
It is unclear whether variation in insulin resistance mediates the positive association of fat mass with bone mass in children/adolescents.
Objective:
Our objective was to examine whether markers linked to insulin resistance [fasting insulin, glucose, triglycerides, and high-density lipoprotein cholesterol (HDLc)] are associated with bone mass in adolescents, and if they are, to examine whether they mediate the fat mass-bone mass association.
Design and Setting:
We conducted a cross-sectional analysis in participants from the Avon Longitudinal Study of Parents and Children.
Participants:
Participants included 2305 (1100 male) individuals of mean age 15.5 yr.
Outcome Measures:
We evaluated total body less head bone mineral content (BMC) (grams), bone area (BA) (square centimeters), and bone mineral density (BMD) (grams per square centimeter) from a dual-energy x-ray absorptiometry scan.
Results:
Fat mass, fasting insulin, and triglycerides were positively associated with BMD, BMC, and BA; HDLc was inversely associated with these outcomes. For example, the adjusted mean difference in BMC per 1 sd fasting insulin was 45 g (95% confidence interval = 17–73 g) in males and 50 g (95% confidence interval = 28–72 g) in females. When the associations of fat mass with outcomes were adjusted for markers of insulin resistance, they were largely unchanged. Associations of triglycerides and HDLc with outcomes were attenuated to the null when they were adjusted for fat mass, whereas those of insulin changed direction; i.e. with adjustment for fat mass, higher fasting insulin was associated with lower BMD, BMC, and BA.
Conclusions:
Fasting insulin, glucose, and lipids do not appear to mediate the positive association of fat mass with bone mass in children/adolescents. The inverse association of fasting insulin with BMD, BMC, and BA once fat mass has been controlled for needs further study.
There is increasing evidence that greater adiposity in childhood is associated with increased bone mass accrual (1). In the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, we have previously shown that fat mass at mean age 9.9 yr is positively associated with both bone mass measured concurrently and with subsequent gains in bone mass over the following 2 yr (1). Furthermore, a Mendelian randomization study in the same cohort, in which genetic variation at FTO and MC4R (loci associated with adiposity) (2, 3) were used as instrumental variables, confirmed likely causal effects of greater fat mass resulting in greater bone mass (4). Although increased weight is expected to increase bone mass as a consequence of greater mechanical strain, fat mass was also related to bone mass of the upper limb, suggesting that systemic factors contribute to this association (4). By age 15–16 in this cohort, fat mass was more strongly related to cortical bone mass in females compared with males (5).
The mechanisms underlying these associations are unclear, but it may involve insulin resistance. Obesity is associated with insulin resistance and hyperinsulinemia, (6, 7), which might contribute to associated increments in bone mass (8). Both insulin and IGF-I exert trophic effects on bone in animal studies, (9, 10), and in cross-sectional studies in human adults, insulin resistance and hyperglycemia are associated with increased bone density (11–14). Two of these human studies explicitly examined whether indices of insulin resistance/hyperglycemia mediated the association of adiposity with bone mass. Among a combined sample of 55 male coronary heart disease patients and 40 similarly aged healthy men, insulin resistance/hyperglycemia did seem to mediate most of the positive association of adiposity with bone mass (11). However, in a study of 317 healthy pre- and postmenopausal women, there was little evidence of such mediation (12).
To our knowledge, no previous study has examined the association of insulin resistance/fasting insulin with bone mass in children/adolescents and explored whether any association then explains (mediates) the association of fat mass with bone mass in early life. The aim of this paper was to examine whether markers that are linked to insulin resistance [fasting insulin, glucose, triglycerides, and high-density lipoprotein cholesterol (HDLc)] are associated with bone mass in adolescents and, if so, whether these associations mediate the association of fat mass with bone mass.
Subjects and Methods
Participants
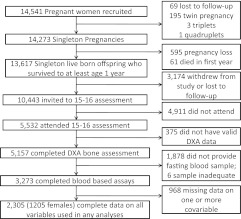
The ALSPAC is a longitudinal, population-based birth cohort that recruited 14,541 pregnant women resident in Avon, UK, with expected dates of delivery April 1, 1991, to December 31, 1992 (http://www.alspac.bris.ac.uk) (15). There were 13,988 children who were live births and still alive at 1 yr of age who are the core cohort participants. Since age 7, surviving offspring participants have been invited to regular follow-up clinics. In the current analysis, we use data from the 15- to 16-yr follow-up clinic at which fasting blood samples were taken. Figure 1 shows the participant flow through the study and how the study sample for this paper (n = 2305) was obtained. Ethical approval was obtained from the ALSPAC Law and Ethics Committee (IRB 00003312) and the Local Research Ethics Committee.
Fig. 1.
Participant flow.
Assessment of fat mass and bone mass outcomes
Height was measured to the nearest 0.1 cm using a Harpenden stadiometer with the participant unshod. A Lunar prodigy narrow fan beam densitometer was used to perform a whole-body dual-energy x-ray absorptiometry (DXA) scan where total body less head bone mineral content (BMC) and lean and fat mass are measured (grams). Additional DXA bone measurements comprised bone area (BA) (square centimeters) and bone mineral density (BMD) (grams per square centimeter). Additional details of these measurements, including reproducibility, are described elsewhere (16).
Assessment of fasting insulin, glucose, and lipids
Insulin, glucose, triglycerides, and HDLc were assessed on venous blood samples taken after participants had fasted overnight (for those attending in the morning) or for a minimum of 6 h for those attending after lunch. Blood samples were immediately spun and frozen at −80 C (7). Measurements were assayed shortly (3–9 months) after samples were taken with no previous freeze-thaw cycles. Insulin was measured by an ELISA (Mercodia, Uppsala, Sweden) automated microparticle enzyme immunoassay that does not cross-react with proinsulin, and plasma glucose was measured by automated enzymatic assay (hexokinase). Plasma lipids (total cholesterol, triglycerides, and HDLc) were performed by modification of the standard Lipid Research Clinics Protocol using enzymatic reagents for lipid determination. It has been established in children and adults that fasting insulin is very highly correlated with formulae that attempt to more accurately measure insulin resistance, such as homeostasis model assessment of insulin resistance (HOMA-IR) or Quantitative Insulin Sensitivity Check Index (QUICKI), by combining fasting glucose and insulin (17, 18). A recent consensus statement recommends that fasting insulin be used (rather than HOMA-IR or QUICKI) in epidemiological studies of children/adolescents (19). In our cohort in both males and females, fasting insulin was very highly correlated with HOMA-IR (Pearson's correlation coefficient 0.98 in both genders) and QUICKI (0.97 in females and 0.98 males), and all results were identical if either of these were used in place of fasting insulin. Our hypothesis was concerned with insulin resistance, but we did also consider insulin secretion, because the two are related. We generated HOMA-β (HOMA of pancreatic beta cell function) from fasting glucose and insulin (20). However, as with HOMA-IR, and consistent with other published studies, (21) this was also strongly correlated with fasting insulin (0.76 in both genders) and when we used this in place of fasting insulin in any of our analyses, the results were essentially the same as those using fasting insulin. We therefore present results with fasting insulin only.
Covariables
The following were considered potential confounding factors: age, gender, pubertal status, height and height squared (for analyses with fat mass), physical activity, head of household social class, maternal and paternal educational attainment, ethnicity, and maternal and paternal smoking.
All participants attending the clinic were asked to wear an Actigraph accelerometer (Actigraph LLC, Fort Walton Beach, FL) for 7 d. Data from participants who had worn the Actigraph for at least 10 h/d for at least 3 d were considered valid (22). From the accelerometer data, we calculated the time spent per valid day of measurement in moderate or vigorous physical activity (MVPA). All minutes of recording with a total of more than 3600 accelerometer counts were classified as MVPA. This threshold was derived from a calibration study conducted in a subsample of 246 ALSPAC children and represents an activity intensity of four times resting metabolic rate (four metabolic equivalents), approximate to brisk walking (23). Because physical activity earlier in life might be a stronger determinant of the key exposures (fasting insulin, glucose, and lipids) and outcomes (bone density) than that measured at the same time, we also examined the potential confounding by MVPA measured at younger ages (11.8 and 13.4 yr). The same methods were used at all three ages for assessing physical activity.
Questionnaires to the mother at approximately 18 and 32 wk gestation asked about socioeconomic position, parental education, parental smoking, and ethnicity. Mother's and her partner's occupations were used to generate a measure of highest household social class, using the 1991 United Kingdom Office of Population Censuses and Surveys classification (classes I–V, with class III split into manual and nonmanual). Educational attainment for the mother and her partner (separately) was categorized as below O-Level (Ordinary Level; exams taken in different subjects usually at age 15–16 at the completion of legally required school attendance, equivalent to today's General Certificate of Secondary Education), O-Level only, A-Level (Advanced Level; exams taken in different subjects usually at age 18), or university degree or above. Mother's smoking was categorized as never, prepregnancy or early pregnancy only, and throughout pregnancy and fathers as ever or never. Ethnicity of the child was recorded at the time of the clinic and is categorized as white or other because the vast majority described themselves as white (a similar proportion of mothers and fathers describing themselves as white at the time of enrollment to the cohort). Puberty was assessed at the clinic by asking participants to complete Tanner's questionnaire, which asks (using pictures) about breast and scrotal development and about pubic hair; because of apparent reporting bias, we used the stages defined by pubic hair only here. Girls were also asked about timing of menarche and additional adjustment for this was used in the females.
Statistical analysis
Fasting insulin and triglycerides and time spent in MVPA were positively skewed, and therefore, median (interquartile range) are presented in descriptive statistics, and logged values were used in regression analyses. For all exposure variables, including fat mass, we generated internally standardized age (in 1-month categories) z-(i.e., standardized sd) scores. This means that age is controlled for in all analyses and that all associations are mean change in the bone outcomes per 1 sd of exposure, and thus, the magnitudes of association for each exposure can be compared with each other.
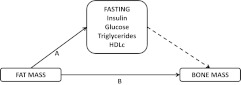
Figure 2 shows the proposed associations that we analyze in this study. The initial steps in our analyses were to quantify the associations between 1) each marker of insulin resistance and 2) fat mass with each measure of bone mass. Second, we wished to explore whether markers of insulin resistance mediated the association of fat mass with bone mass. For one variable to mediate the association of an exposure with an outcome, it has to fulfill the following criteria: 1) be causally influenced by the exposure of interest, 2) causally influence the outcome, and 3) attenuate the association of the exposure with the outcome (24, 25). With respect to our question, Mendelian randomization evidence suggests a causal association of adiposity with markers of insulin resistance, including all of the markers we have examined here (26). As noted in the introductory section, there is evidence that greater insulin resistance/fasting insulin is associated with greater bone mass in adults, but whether this is causal or not is unclear. In this paper, exploration of its possible role as a mediator of the fat mass-bone mass association is explored by first examining whether insulin resistance markers (model 1 with control for age only; model 2 including all other covariables except fat mass) are associated with bone mass and then by examining whether inclusion of markers of insulin resistance in a model of the association of fat mass with each outcome attenuated these associations in comparison with the associations obtained only from the confounder-adjusted model (model 3). Lastly, we examined whether fat mass confounded any associations of markers of insulin resistance with our outcomes by adjusting for fat mass in the association of each marker of insulin resistance with each outcome (model 3). If the association of any of these markers with outcomes attenuates upon adjustment for fat mass, this suggests that their association with bone mass is not causal but is largely explained by fat mass being related to fasting insulin and associated markers and also to bone mass.
Fig. 2.
Hypothesized causal relationships being examined. Solid lines represent associations for which there is good evidence of causality (see text). The dashed line represents the key associations being tested in this paper. If associations are found between fasting insulin and associated markers and bone mass, these may mediate some or all of the association between fat mass and bone mass. That is the total association of fat mass with bone mass (A plus B) would be attenuated upon adjustment for fasting insulin and associated markers. If all of the association was mediated (i.e. all via A), then attenuation would be to the null. If it was only partially mediated, then some association would remain (represented by B). Furthermore, an association of fasting insulin and associated markers with bone mass might be confounded by fat mass, because this is related both to fasting insulin and associated markers (A) and bone mass (B). If this association is fully confounded, adjustment for fat mass would result in attenuation to the null.
All associations were examined using multivariable linear regression, and results are the mean difference in bone outcome per 1-sd increase in exposure (fat mass or marker of insulin resistance). Any model that included fat mass was also adjusted for height and height squared so that the exposure reflects adiposity rather than greater fat mass relative to greater height. All analyses were conducted separately for males and females. A likelihood ratio test (comparing a model without a gender interaction to one with a gender interaction) was used to formally test whether associations differed by gender (i.e. a gender × exposure interaction).
Main analyses were conducted on the 2305 participants with complete data on exposures, outcomes, and all covariables except mean time per day spent in MVPA (Fig. 1). Of these 2305 participants, 1154 (50%) had valid Actigraph data for assessment of MVPA. We conducted a sensitivity analysis in these 1154 to examine whether further adjustment for physical activity altered any findings. All analyses were conducted in Stata/MP version 11.0 (Stata Corp., College Station, TX).
Results
Table 1 shows the distributions of all characteristics used in any analyses in males and females. Table 2 shows the correlations of the main exposures. These demonstrate the expected weak to modest associations between fat mass and the markers of insulin resistance with evidence that associations were generally stronger in females than in males. Tables 3–5 show the multivariable associations of fat mass and markers of insulin resistance with BMD (Table 3), BMC (Table 4), and BA (Tables 5) in males and females separately.
Table 1.
Characteristics of cohort (n = 2305)
| Characteristic | Male, n = 1100 | Female, n = 1205 |
|---|---|---|
| Age (months) | 185 (3) | 185 (3) |
| BMD (g/cm2) | 1.12 (0.09) | 1.10 (0.07) |
| BMC (kg) | 2.65 (0.49) | 2.37 (0.37) |
| BA (cm2) | 2342 (273) | 2143 (231) |
| Height (cm) | 174 (8) | 165 (6) |
| Fat mass (kg) [median (IQR)] | 8.4 (5.9–12.7) | 17.1 (13.4–22.1) |
| Fasting insulin (IU /liter) [median (IQR)] | 8.08 (5.91–10.70) | 9.64 (7.27–12.51) |
| Fasting glucose (mmol/liter) | 5.28 (0.37) | 5.12 (0.34) |
| Fasting triglycerides (mmol/liter) [median (IQR)] | 0.72 (0.57–0.96) | 0.76 (0.61–0.98) |
| Fasting HDLc (mmol/liter) | 1.22 (0.27) | 1.36 (0.30) |
| Time spent in MVPA (min/d) [median (IQR)]a | 25.7 (16.2–41.0) | 15.1 (7.6–26.0) |
| Maternal smoking [n (%)] | ||
| Never | 941 (86) | 1022 (85) |
| Pre/early pregnancy | 44 (4) | 53 (4) |
| Through pregnancy | 115 (10) | 130 (11) |
| Paternal smoking [n (%)] | ||
| Never | 764 (70) | 842 (70) |
| Ever | 336 (30) | 363 (30) |
| Head of household social class [n (%)] | ||
| I | 213 (19) | 217 (18) |
| II | 535 (49) | 564 (47) |
| III nonmanual | 240 (22) | 264 (22) |
| III manual | 78 (7) | 110 (9) |
| IV/V | 34 (3) | 50 (4) |
| Maternal education [n (%)] | ||
| Less than degree | 891 (81) | 963 (80) |
| University degree | 209 (19) | 242 (20) |
| Paternal education [n (%)] | ||
| Less than degree | 808 (73) | 899 (75) |
| University degree | 292 (27) | 306 (25) |
| Ethnicity [n (%)] | ||
| White | 1077 (97.9) | 1190 (98.8) |
| Non-White | 23 (2.1) | 15 (1.2) |
| Puberty (Tanner stage of pubic hair) [n (%)] | ||
| 1/2 | 16 (1.5) | 4 (0.3) |
| 3 | 109 (9.9) | 115 (9.5) |
| 4 | 577 (52.5) | 645 (53.5) |
| 5 | 398 (36.2) | 441 (36.6) |
Results are shown as mean (sd) or median [interquartile range (IQR)] for continuous variables and n (percent) for categorical variables.
Valid data available only on a subsample: 507 males and 647 females.
Table 2.
Correlation of fat mass and markers of insulin resistance in females (n = 1100) and males (n = 1205)
| Fat mass |
Insulin |
Glucose |
Triglycerides |
HDLc |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | |
| Fat mass | 1 | 1 | ||||||||
| Insulin | 0.38 | 0.29 | 1 | 1 | ||||||
| Glucose | 0.14 | −0.04b | 0.39 | 0.30 | 1 | 1 | ||||
| Triglycerides | 0.22 | 0.06a | 0.33 | 0.20 | 0.12 | 0.05b | 1 | 1 | ||
| HDLc | −0.22 | −0.23 | −0.12 | −0.12 | 0.13 | 0.07a | −0.37 | −0.29 | 1 | 1 |
Results are all Pearson correlation coefficients with standardization for age, height, and height squared. There was statistical evidence (gender interaction) that the correlation of fat mass with insulin, glucose, and triglycerides; of insulin with glucose and triglycerides; of glucose with triglycerides and HDLc; and of triglycerides with HDLc were stronger in females than in males (all P values for interaction > 0.05). All P values are <0.001 except those marked.
P values > 0.001 but <0.05.
P values > 0.1.
Table 3.
Multivariable associations of fat mass and markers of insulin resistance with BMD at age 15–16 yr (n = 2305)
| Exposurea | Mean difference in BMD (g/cm2) per 1-sd of exposure (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||
| Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | |
| Fat mass | 0.017 (0.012, 0.022) | 0.030 (0.026, 0.034) | 0.01 | 0.017 (0.012, 0.022) | 0.027 (0.024, 0.032) | 0.04 | 0.019 (0.013, 0.025) | 0.028 (0.024, 0.033) | 0.08 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Insulin | 0.002 (−0.003, 0.009) | 0.006 (0.002, 0.010) | 0.23 | 0.002 (−0.003, 0.007) | 0.005 (0.001, 0.009) | 0.48 | −0.007 (−0.012, −0.002) | −0.004 (−0.008, 0.000) | 0.64 |
| P value | 0.57 | 0.007 | 0.38 | 0.03 | 0.003 | 0.05 | |||
| Glucose | −0.002 (−0.008, 0.002) | −0.002 (−0.006, 0.003) | 0.75 | −0.001 (−0.006, 0.004) | −0.001 (−0.005, 0.003) | 0.85 | −0.003 (−0.008, 0.002) | 0.000 (−0.004, 0.003) | 0.51 |
| P value | 0.49 | 0.41 | 0.73 | 0.57 | 0.21 | 0.93 | |||
| Triglycerides | 0.004 (0.001, 0.010) | −0.001 (−0.005, 0.003) | 0.14 | 0.003 (−0.002, 0.009) | −0.001 (−0.005, 0.003) | 0.12 | 0.000 (−0.004, 0.005) | −0.001 (−0.005, 0.003) | 0.61 |
| P value | 0.04 | 0.33 | 0.09 | 0.22 | 0.84 | 0.62 | |||
| HDLc | −0.016 (−0.021, −0.011) | −0.008 (−0.012, −0.004) | 0.03 | −0.013 (−0.018, −0.008) | −0.008 (−0.012, −0.004) | 0.06 | −0.005 (−0.010, 0.000) | −0.002 (−0.006, 0.001) | 0.41 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | 0.23 | |||
Results are mean difference in BMD (grams per square centimeter) per 1-sd greater exposure value (95% confidence intervals) and P value. Model 1 controls for age by standardizing exposure on age and is adjusted for height and height squared in models with fat mass as exposure. Model 2 is the same as model 1 plus additional adjustment for confounders head of household social class, maternal and paternal education, ethnicity, maternal and paternal smoking, pubertal stage, and age of menarche (females). Model 3 assesses whether indicators of insulin resistance (insulin, glucose, triglyceride, and HDLc) mediate associations of fat mass and for other exposures whether fat mass confounds their association with outcomes. This model is identical to model 2 but also adjusts fat mass for all other exposures listed in the first column and adjusts each of these exposures for fat mass (height and height squared).
All exposures are age-standardized sd (z-) scores; hence, outcomes are mean difference per 1-sd exposure.
P interaction tests the null hypothesis that the association in males and females is the same.
Table 4.
Multivariable associations of fat mass and markers of insulin resistance with BMC at age 15–16 yr (n = 2305)
| Exposurea | Mean difference in BMC (g) per 1-sd of exposure (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||
| Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | |
| Fat mass | 153 (131, 174) | 226 (211, 242) | 0.001 | 153 (132, 174) | 219 (203, 235) | 0.04 | 161 (138, 184) | 223 (206, 240) | 0.06 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Insulin | 42 (12, 71) | 57 (35, 79) | 0.42 | 45 (17, 73) | 50 (28, 72) | 0.78 | −29 (−50, −8) | −18 (−33, −3) | 0.54 |
| P value | 0.006 | <0.001 | 0.002 | <0.001 | 0.006 | 0.02 | |||
| Glucose | −7 (−36, 23) | −13 (−36, 10) | 0.73 | 2 (−26, 30) | −11 (−33, 12) | 0.64 | −16 (−36, 3) | −3 (−18, 12) | 0.69 |
| P value | 0.69 | 0.33 | 0.88 | 0.41 | 0.12 | 0.72 | |||
| Triglycerides | 30 (1, 60) | −2 (−23, 20) | 0.08 | 29 (1, 59) | −6 (−27, 15) | 0.05 | −1 (−21, 18) | 2 (−11, 16) | 0.81 |
| P value | 0.04 | 0.94 | 0.03 | 0.63 | 0.90 | 0.68 | |||
| HDLc | −107 (−134, −79) | −53 (−74, −32) | 0.002 | −90 (−116, −63) | −49 (−69, −28) | 0.008 | −20 (−40, −1) | −8 (−21, 6) | 0.07 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | 0.32 | |||
Results are mean difference in BMC (grams) per 1-sd greater exposure value (95% confidence intervals) and P value. Model 1 controls for age by standardizing exposure on age and is adjusted for height and height squared in models with fat mass as exposure. Model 2 is the same as model 1 plus additional adjustment for confounders head of household social class, maternal and paternal education, ethnicity, maternal and paternal smoking, pubertal stage, and age of menarche (females). Model 3 assesses whether indicators of insulin resistance (insulin, glucose, triglyceride, and HDLc) mediate associations of fat mass and for other exposures whether fat mass confounds their association with outcomes. This model is identical to model 2 but also adjusts fat mass for all other exposures listed in the first column and adjusts each of these exposures for fat mass (height and height squared).
All exposures are age-standardized sd (z-) scores; hence, outcomes are mean difference per 1-sd exposure.
P interaction tests the null hypothesis that the association in males and females is the same.
Table 5.
Multivariable associations of fat mass and markers of insulin resistance with BA at age 15–16 yr (n = 2305)
| Exposurea | Mean difference in BA (cm2) per 1-sd of exposure (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||
| Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | Males, n = 1100 | Females, n = 1205 | P interactionb | |
| Fat mass | 99 (89, 108) | 145 (137, 153) | <0.001 | 99 (89, 108) | 142 (134, 151) | <0.001 | 102 (92, 112) | 145 (136, 154) | <0.001 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Insulin | 33 (17, 49) | 40 (25, 52) | 0.58 | 35 (19, 50) | 35 (22, 49) | 0.92 | −12 (−22, −3) | −9 (−17, −2) | 0.69 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | 0.02 | |||
| Glucose | −2 (−18, 15) | −9 (−23, 5) | 0.51 | 2 (−13, 18) | −8 (−22, 6) | 0.45 | −9 (−17, 0) | −3 (−10, 5) | 0.51 |
| P value | 0.82 | 0.20 | 0.76 | 0.29 | 0.05 | 0.51 | |||
| Triglycerides | 19 (2, 35) | −2 (−15, 11) | 0.05 | 16 (0.2, 32) | −4 (−17, 9) | 0.04 | −2 (−11, 7) | 3 (−4, 10) | 0.71 |
| P value | 0.03 | 0.79 | 0.05 | 0.52 | 0.61 | 0.46 | |||
| HDLc | −64 (−79, −48) | −31 (−44, −19) | 0.001 | −55 (−70, −39) | −29 (−42, −16) | 0.006 | −9 (−17, 0) | −2 (−9, 5) | 0.28 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.05 | 0.55 | |||
Results are mean difference in BA (cm2) per 1-sd greater exposure value (95% confidence intervals) and P value. Model 1 controls for age by standardizing exposure on age and is adjusted for height and height squared in models with fat mass as exposure. Model 2 is the same as model 1 plus additional adjustment for confounders head of household social class, maternal and paternal education, ethnicity, maternal and paternal smoking, pubertal stage, and age of menarche (females). Model 3 assesses whether indicators of insulin resistance (insulin, glucose, triglyceride, and HDLc) mediate associations of fat mass and for other exposures whether fat mass confounds their association with outcomes. This model is identical to model 2 but also adjusts fat mass for all other exposures listed in the first column and adjusts each of these exposures for fat mass (height and height squared).
All exposures are age-standardized sd (z-) scores; hence, outcomes are mean difference per 1-sd exposure.
P interaction tests the null hypothesis that the association in males and females is the same.
Fat mass was positively associated with all three of BMD, BMC, and BA in simple analyses controlling only for age and height (model 1) and also with additional adjustment for social class, parental education, ethnicity, parental age, and pubertal stage (model 2). Associations were stronger in females compared with males, with strong statistical evidence for gender-fat mass interactions. Consistent with these positive associations, fasting insulin was positively associated with BMC and BA in both model 1 and model 2, but unlike the situation with fat mass, associations were similar in males and females. Fasting insulin was positively associated with BMD in females, but not males, although statistically, the associations were consistent with each other (i.e. there was no evidence of interaction with gender). Triglycerides were positively associated with BMD, BMC, and BA in males but were not associated with any outcomes in females, although there was no strong statistical evidence for an interaction of gender with triglycerides in these associations. HDLc was inversely associated with all three of BMD, BMC, and BA in both genders, but here the associations were stronger in males than females with statistical evidence for gender differences. Glucose was not associated with any outcomes in either gender.
When the associations of fat mass with BMD, BMC, and BA were further adjusted for all of the markers of insulin resistance (model 3 in Tables 2–4), the associations were essentially unchanged from those observed in the confounder-adjusted model (model 2). By contrast, adjustment of the observed associations of fasting insulin, triglycerides, and HDLc with each outcome for fat mass attenuated these markedly. For insulin, the associations changed direction, such that once fat mass was taken into account, insulin was inversely associated with each outcome (i.e. greater insulin resistance was associated with lower bone mass). Triglyceride associations were abolished and HDLc associations attenuated, although some inverse associations remained, with these remaining stronger in males compared with females.
To examine whether exclusion of participants because of lack of covariable data might have biased our findings, we repeated all of the age-adjusted analyses on the 3273 participants with exposure and outcome data, irrespective of whether they had covariable data. These associations were essentially identical to those presented for model 1 in Tables 2–4 (results available on request from authors). Finally, we explored whether physical activity might confound the associations. MVPA assessed at ages 11.8 and 13.4 yr and at the same time as all other measurements used in this study (15.4 yr) were positively correlated with each other: Pearson correlation coefficient between 11.8 and 13.4 yr measures 0.42, between 13.4 and 15.4 measures 0.44, and between 11.8 and 15.4 measures 0.38 (all three P < 0.0001). There were 1926 participants with valid Actigraph data at age 11.8 yr, 1670 at 13.4 yr, and 1154 at 15.4 yr. Simple age and gender (model 1) and fully confounder-adjusted, but without time spent in MVPA (equivalent to model 2 in Tables 2–4), associations were similar in these subgroups with Actigraph data to those presented in Tables 2–4. Further adjustment for time spent in MVPA, whether the 11.8-, 13.4-, or 15.4-yr data were used, did not importantly alter any associations. For example, among the 507 males with physical activity data at 15.4 yr, the mean difference in BMC per 1-sd fat mass was 138 g (95% confidence interval = 108–167) in the confounder-adjusted (minus physical activity) model; with additional adjustment for MVPA, this became 135 g (106–164). Similarly, among the 905 males with physical activity data at 11.8 yr, the mean difference in BMC per 1-sd fat mass was 137 g (95% confidence interval = 114–161) in the confounder-adjusted (minus physical activity) model; with additional adjustment for MVPA, this became 142 g (118–167).
Despite correlations between the main exposure variables, when these were all included together in multivariable models, there was no evidence of problems with collinearity; the variance inflation factors for all models were between 1.02 and 1.47.
Discussion
We have confirmed previous findings from this study of a positive association of fat mass with BMD, BMC, and BA in children/adolescents that are stronger in females compared with males (5). We have extended this earlier work by examining the associations of markers linked to insulin resistance with bone mass and further exploring whether insulin resistance potentially mediates any associations of fat mass with bone outcomes. Our results showed associations of fasting insulin, triglycerides, and HDLc with bone outcomes that were in line with (in terms of direction) the associations of fat mass with these outcomes but with no evidence that these mediate the association of fat mass with bone mass outcomes.
Strengths and limitations
We used data from a well-characterized general population study with fasting levels of insulin, glucose, and lipids and DXA-assessed fat mass, BMD, BMC, and BA. Our analyses are cross-sectional and therefore cannot determine temporality or the true direction of associations. That said, trial and Mendelian randomization evidence support the direction of association being from fat mass to insulin resistance and also from fat mass to bone mass (4, 26). Our cross-sectional findings of the association of fat mass with insulin resistance in this study [reported elsewhere (7)] are consistent with these causal findings from trials and Mendelian randomization (26). Similarly, the cross-sectional associations presented here between fat mass and bone mass are consistent with causal results from Mendelian randomization (4). Thus, the only parts of the pathway that we have examined that we cannot make strong claims about are those between fasting insulin, glucose, and lipids and bone mass. To our knowledge, no study to date has examined these associations prospectively, but we acknowledge further exploration of our findings in prospective cohort studies and Mendelian randomization studies is warranted. Consistent with other large epidemiological studies conducted in healthy general population samples, we are not able to directly measure insulin resistance using the gold standard euglycemic-hyperinsulinemic clamp. Fasting insulin has been shown to have modest to strong correlations with clamp-assessed insulin resistance (correlation coefficients 0.5–0.9) in children and adolescents (17, 18). Any measurement error is likely to be nondifferential and therefore would be expected to bias results toward the null. However, even if nondifferential measurement error resulted in our associations being weaker than they would be with a direct measure of insulin resistance, with correlations of 0.5–0.9, we would have expected some attenuation of the fat mass-bone mass association with adjustment for our markers if insulin resistance were an important mediator of this association. Consistent with other prospective birth cohorts, there has been attrition over time. Our results would be markedly biased if the associations that we have observed were all null or in the opposite direction to those observed in the group of participants who were lost to follow-up. It is impossible to test this, but we cannot think of plausible reasons why this would be the case. Missing data on covariables, among those who had exposure and outcome data, did not appear to result in biased estimates. Despite being able to adjust for a wide range of potential confounding factors, as with all observational studies, observed associations could be explained by residual confounding. We were unable to adjust for dietary intake, including use of vitamin D supplementation. However, our previous Mendelian randomization study results suggest that the positive associations of fat mass with bone mass are not explained by residual confounding (4). The ALSPAC cohort is largely of white European participants, and we cannot assume that our results would generalize to other populations.
Implications and possible mechanisms
There was no evidence that markers of insulin resistance mediated the associations of fat mass with BMD, BMC, and BA, suggesting that the influence of fat mass on bone health is via other pathways. Weight bearing is likely to make a contribution, in light of a previous report that fat mass is positively related to cortical bone parameters as assessed by peripheral quantitative computed tomography at the tibia but not the radius (27). However, we have previously observed positive relationships between fat mass and bone mass as assessed by total-body DXA at the upper as well as lower limb, suggesting this is not the sole explanation (4). Another possible pathway is adiponectin, which is inversely related to bone mass, bone accrual, and insulin resistance, with these associations being independent of fat mass in our cohort and present for bone outcomes assessed both prepubertally and postpubertally (the latter using the data from the clinic assessment used in this paper) (28). Other possible mechanisms, which are yet to be explored, include the secretion of other bone-active hormones from the adipocyte, such as estrogen and leptin, and the action on bone of novel hormones related to nutrition (8).
In general, much of the association of triglycerides and HDLc with outcomes was explained by confounding due to fat mass. Interestingly, once we controlled for fat mass, the positive association of insulin with bone mass was reversed and became inverse. Thus, our results suggest that given two adolescents who have similar fat mass, the one with higher fasting insulin levels will have lower BMD, BMC, and BA. These findings were unexpected. However, they are consistent with cross-sectional studies in adults (29–32) and also with results of a recent study of 143 overweight adolescents, in which an inverse association was seen between insulin resistance and bone mass (33). The inverse association of fasting insulin with bone mass after adjustment for fat mass may reflect an influence of osteoblast function on glucose homeostasis, in light of animal studies that suggest that osteoblast-derived uncarboxylated osteocalcin acts to improve insulin sensitivity (34), something that has recently been confirmed in studies in childhood in which uncarboxylated osteocalcin levels were found to be lower in those with prediabetes/insulin resistance (35). However, to the extent that bone and energy metabolism are related, how reduced levels of uncarboxylated osteocalcin in the context of hyperinsulinemia might affect osteoblast function and hence bone mass is currently unclear. However, because this emergence of an inverse association upon adjustment for fat mass was unexpected, possible chance or bias have to be considered as possible explanations. Collinearity was not a problem in analyses where fat mass and all markers of insulin resistance are included in the same model. Furthermore, we do not feel that the measurement of fat mass and insulin have marked and differing levels of measurement error such that this could introduce bias with the adjustment. If either of these had biased the results, we might have expected similar patterns of association with triglycerides and HDLc upon adjustment for fat mass, but this is not the case. However, until replicated in other studies, this novel finding of an inverse association of fasting insulin with bone mass after adjustment for fat mass should be treated with caution and may be a chance finding.
In conclusion, we do not find any evidence that fasting insulin, glucose, or lipids mediate the positive association of fat mass with BMD, BMC, and BA in adolescents. The finding of an inverse association of fasting insulin with bone mass once fat mass has been adjusted for needs further replication and exploration, including in prospective studies and by Mendelian randomization.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The Medical Research Council (MRC), the Wellcome Trust, and the University of Bristol provide core funding support for ALSPAC. The MRC and the University of Bristol provide core funding for the MRC Centre of Causal Analyses in Translational Epidemiology. Funding for completion of insulin, glucose, and lipid assays used in this publication came from a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK077659). DXA scans in ALSPAC were funded by Wellcome Grant 067100.
The views expressed in this paper are those of the authors and not necessarily those of any funding body or others whose support is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
D.A.L. had full access to the data and takes responsibility for the integrity of the data and its analysis.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- BA
- bone area
- BMC
- bone mineral content
- BMD
- bone mineral density
- DXA
- dual-energy x-ray absorptiometry
- HDLc
- high-density lipoprotein cholesterol
- HOMA-IR
- homeostasis model assessment of insulin resistance
- MVPA
- moderate or vigorous physical activity
- QUICKI
- Quantitative Insulin Sensitivity Check Index.
References
- 1. Clark EM, Ness AR, Tobias JH. 2006. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 91:2534–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, et al. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, et al. 2008. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. 2009. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res 24:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sayers A, Tobias JH. 2010. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab 95:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiarelli F, Marcovecchio ML. 2008. Insulin resistance and obesity in childhood. Eur J Endocrinol 159(Suppl 1):S67–S74 [DOI] [PubMed] [Google Scholar]
- 7. Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, Cherry L, Watt P, Ness AR, Davey Smith G, Sattar N. 2010. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ 341:c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reid IR. 2008. Relationships between fat and bone. Osteoporos Int 19:595–606 [DOI] [PubMed] [Google Scholar]
- 9. Cornish J, Callon KE, Reid IR. 1996. Insulin increases histomorphometric indices of bone formation In vivo. Calcif Tissue Int 59:492–495 [DOI] [PubMed] [Google Scholar]
- 10. Tobias JH, Chow JW, Chambers TJ. 1992. Opposite effects of insulin-like growth factor-I on the formation of trabecular and cortical bone in adult female rats. Endocrinology 131:2387–2392 [DOI] [PubMed] [Google Scholar]
- 11. Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H. 2000. Correlations between insulin sensitivity and bone mineral density in non-diabetic men. Diabet Med 17:124–129 [DOI] [PubMed] [Google Scholar]
- 12. Haffner SM, Bauer RL. 1993. The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women. Metabolism 42:735–738 [DOI] [PubMed] [Google Scholar]
- 13. Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J. 1993. Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol 265:E655–E659 [DOI] [PubMed] [Google Scholar]
- 14. Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhäger JC, Lamberts SW, Grobbee DE. 1996. Hyperinsulinemia and bone mineral density in an elderly population: the Rotterdam Study. Bone 18:545–549 [DOI] [PubMed] [Google Scholar]
- 15. Golding J, Pembrey M, Jones R. 2001. ALSPAC: the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 15:74–87 [DOI] [PubMed] [Google Scholar]
- 16. Tobias JH, Steer CD, Emmett PM, Tonkin RJ, Cooper C, Ness AR. 2005. Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos Int 16:1731–1741 [DOI] [PubMed] [Google Scholar]
- 17. Gungor N, Saad R, Janosky J, Arslanian S. 2004. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 144:47–55 [DOI] [PubMed] [Google Scholar]
- 18. Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. 2008. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care 31:783–788 [DOI] [PubMed] [Google Scholar]
- 19. Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, Chiarelli F. 2010. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 95:5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 21. Hermans MP, Levy JC, Morris RJ, Turner RC. 1999. Comparison of tests of β-cell function across a range of glucose tolerance from normal to diabetes. Diabetes 48:1779–1786 [DOI] [PubMed] [Google Scholar]
- 22. Mattocks C, Ness A, Leary S, Tilling K, Blair SN, Shield J, Deere K, Saunders J, Kirkby J, Smith GD, Wells J, Wareham N, Reilly J, Riddoch C. 2008. Use of accelerometers in a large field-based study of children: protocols, design issues, and effects on precision. J Phys Act Health 5:S98–S111 [DOI] [PubMed] [Google Scholar]
- 23. Mattocks C, Leary S, Ness A, Deere K, Saunders J, Tilling K, Kirkby J, Blair SN, Riddoch C. 2007. Calibration of an accelerometer during free-living activities in children. Int J Pediatr Obes 2:218–226 [DOI] [PubMed] [Google Scholar]
- 24. Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. 2001. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry 158:848–856 [DOI] [PubMed] [Google Scholar]
- 25. Hafeman DM, Schwartz S. 2009. Opening the Black Box: a motivation for the assessment of mediation. Int J Epidemiol 38:838–845 [DOI] [PubMed] [Google Scholar]
- 26. Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, Lindgren CM, Lango H, Melzer D, Ferrucci L, Paolisso G, Neville MJ, Karpe F, Palmer CN, Morris AD, Elliott P, Jarvelin MR, Smith GD, McCarthy MI, Hattersley AT, Frayling TM. 2008. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 57:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorentzon M, Landin K, Mellström D, Ohlsson C. 2006. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res 21:1871–1878 [DOI] [PubMed] [Google Scholar]
- 28. Sayers A, Timpson NJ, Sattar N, Deanfield J, Hingorani AD, Davey-Smith G, Tobias JH. 2010. Adiponectin and its association with bone mass accrual in childhood. J Bone Miner Res 25:2212–2220 [DOI] [PubMed] [Google Scholar]
- 29. Hwang DK, Choi HJ. 2010. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos Int 21:425–431 [DOI] [PubMed] [Google Scholar]
- 30. von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. 2007. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int 18:1337–1344 [DOI] [PubMed] [Google Scholar]
- 31. Kim HY, Choe JW, Kim HK, Bae SJ, Kim BJ, Lee SH, Koh JM, Han KO, Park HM, Kim GS. 2010. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif Tissue Int 86:350–358 [DOI] [PubMed] [Google Scholar]
- 32. Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. 2010. Men with metabolic syndrome have lower bone mineral density but lower fracture risk–the MINOS study. J Bone Miner Res 25:1446–1454 [DOI] [PubMed] [Google Scholar]
- 33. Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. 2011. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr 158:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee NK, Karsenty G. 2008. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 19:161–166 [DOI] [PubMed] [Google Scholar]
- 35. Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL. 2011. Lower Uncarboxylated Osteocalcin Concentrations in Children with Prediabetes Is Associated with {beta}-Cell Function. J Clin Endocrinol Metab 96:E1092–E1099 [DOI] [PMC free article] [PubMed] [Google Scholar]